Abstract
Background
There is an increased prevalence of coeliac disease (CD) among relatives of those with the disease.
Aims
To compare the clinical features in patients with CD detected via family screening with those in patients diagnosed routinely.
Methods
Information on screening was provided to relatives of patients. Those who wished to be screened were tested for endomysial and/or tissue transglutaminase antibodies. Duodenal biopsy was performed in those with positive antibodies. The clinical details of the relative screening group were compared with those of 105 patients diagnosed routinely.
Results
183 relatives underwent screening, of whom 32 had positive serology, 24 had histology diagnostic of CD, six had normal biopsies and two declined duodenal biopsy. Patients in the relative screening group were younger with a median age of 33 years (range 17–72 years) compared to the routine group which had a median age of 54 years (range 25–88 years). In the relative screening group, there was a male preponderance (M:F ratio 16:8), anaemia at presentation was significantly less common (13% v 58%; p<0.001) and osteoporosis was less frequent (9% v 22%; p<0.244) compared with the routine group. 65% of the relative screening group had gastrointestinal symptoms or anaemia at diagnosis.
Conclusions
Patients detected by family screening are younger with a male preponderance, but fewer had anaemia and osteoporosis.
Keywords: clinical features, coeliac disease, complications, osteoporosis, relative screening
Coeliac disease (CD) is an immune mediated disorder characterised by small intestinal mucosal injury and malabsorption in genetically susceptible individuals.1,2 Clinical manifestations of CD are highly variable. They may present at any age and involve various organs. Intestinal symptoms include diarrhoea, weight loss, failure to grow, abdominal pain, bloating, anorexia and constipation. Extra‐intestinal features include osteoporosis, iron deficiency anaemia, short stature and infertility.3 The diagnosis is usually based on a combination of clinical features, serological tests and histopathological features. The main serological tests used are immunoglobulin A (IgA)‐antihuman tissue transglutaminase (TTG) and IgA‐endomysial antibody (EMA) immunofluorescence tests. The characteristic histological features of intestinal mucosa in CD include blunted or flat villi, hyperplastic crypts, loss of surface enterocyte cell height and lymphocytic infiltration of epithelium. The Marsh classification has been used to describe the histological changes in coeliac mucosa and thereby standardise pathology reports.4,5
CD is a major cause of malabsorption in Europe and its reported prevalence has increased in the past 20 years from 1 in 1800 to at least 1 in 100.6,7,8,9,10 Estimates of prevalence of undiagnosed and preclinical CD range from 0.7% to 2.0% in most populations in Europe and the United States.11,12,13,14,15,16 Also seroepidemiologic studies suggest that 1–3% of the general population in Europe and the United States become affected serologically at some point in their lives and there may be three to seven undiagnosed cases for each diagnosed case of CD.16 The prevalence is higher among first degree relatives, with a rate of 4–12% of those biopsied and a pooled prevalence of 7.6%.17,18 The serology based prevalence of CD among second degree relatives and first cousins varies from 2.6% to 19.5%.12,19,20
As well as presenting classically, CD may present with iron deficiency, osteoporosis, short stature and infertility, or silently when detected by serology in an asymptomatic person or when endoscopy is carried out for another reason.13 This implies that CD is often undiagnosed because it is either silent or atypical.10 Most symptomatic patients experience improvement on a gluten free diet, whereas if left untreated they may develop osteoporosis, iron deficiency anaemia, lymphoma, other gastrointestinal (GI) cancers, infertility, and vitamin and mineral deficiencies.21 The benefits of treatment are less clear in asymptomatic patients.15 Nevertheless, it may be important to screen for silent CD, for instance in family members, in order to treat them with a gluten free diet, which resolves iron deficiency, and so that problems can be treated and prevented.22,23,24,25
Since 1997, our CD clinic has offered a family screening programme to all relatives of patients with CD. Patients attending the CD clinic were provided with information to give to first and second degree relatives. We have compared the clinical presentation and incidence of complications in patients detected via family screening with those in patients diagnosed by routine referral.
Methods
Subjects
All adult relatives (aged 16 years or above) who wished to be screened were counselled regarding the implications of being diagnosed with CD prior to obtaining serological results. Details of symptoms, diet and associated medical conditions were obtained by questionnaire.
Serological tests
Immunoglobulin (Ig)A‐EMA was analysed in patients screened before 2000 and IgA‐TTG was analysed in samples from 2000 onwards. Since selective IgA deficiency is more common in CD26,27 and may give a false negative result on screening, the total IgA value was also measured. When a low level of IgA was found, IgG‐EMA or IgG‐TTG was measured.
Anti‐endomysial antibodies and TTG antibodies were analysed by standard techniques using human umbilical cord immunofluorescence slides and enzyme linked immunosorbent assay (ELISA) kits (Orgentec, Mainz, Germany) using human recombinant TTG antigen, respectively.
Small bowel biopsy
All subjects with positive serology were encouraged to undergo endoscopy and duodenal biopsies. At least four random biopsies from the second part of the duodenum were obtained. CD was diagnosed using the Marsh score to stage the histological changes in the mucosa of the small intestine.5
Clinical presentation and other details were obtained from the medical records of patients diagnosed with CD through relative screening. These details were compared with the information obtained from the medical records of 105 CD patients diagnosed during the same time period by routine referrals.
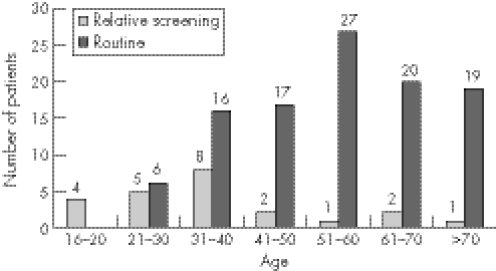
Figure 1 Age at diagnosis of patients with coeliac disease diagnosed by relative screening or by routine referral.
Other investigations
All patients had full blood count, thyroid function tests, serum glucose and haematinics (B12, folic acid and ferritin). Dual energy x ray absorptiometry (DEXA) scans were performed where indicated and the results were reviewed for osteoporosis and osteopenia by a consultant physician with a special interest in bone diseases. Anaemia was defined as haemoglobin <13.2 g/l in males and <11.3 g/l in females, respectively. Osteoporosis was diagnosed by DEXA bone scan by using T score and Z score. Osteoporosis was diagnosed when the T score value for bone mineral density was less than −2.5 or the Z score value was less than −1.5, and osteopenia when the T score value was between −1 and −2.5.
Statistical tests
Statistical analysis was performed using SPSS software. Fisher's exact test, χ2 tests and Mann Whitney non‐parametric tests were used to analyse our data.28,29 A p value of <0.05 was taken as the level of significance. The 95% confidence interval (95% CI) was calculated for the difference in various parameters between the two groups.
Ethics
The study was approved by the South East Wales Local Research Ethics committee.
Results
During the study period of November 1997 to April 2003, 183 relatives (77 males and 106 females) underwent screening. Participants were diagnosed with CD if they had positive duodenal biopsies. Thirty two had positive serology and 30 of these agreed to biopsy. Twenty four of these 30 had abnormal histology suggestive of CD according to Marsh scoring criteria: 14 grade IIIc, five grade IIIb, three grade IIIa and one grade II. Six had normal biopsies and two declined endoscopy. Of the 24 patients diagnosed with CD, six lived outside the area of South East Wales. No clinical information was available on one male patient who was therefore excluded, so 23 patients were included for analysis. During the same period 105 other patients were diagnosed with CD by routine referrals.
Age and sex
Patients in the relative screening group were younger with a median age of 33 years (range 17–72 years) compared to the routine group which had a median age of 54 years (range 25–88 years) (p<0.0001) (fig 1). There was a male preponderance in the relative screening group (M:F ratio 16:8) and a female preponderance in the routine group (M:F ratio 35:70), which was statistically significant (p<0.003).
Presenting symptoms
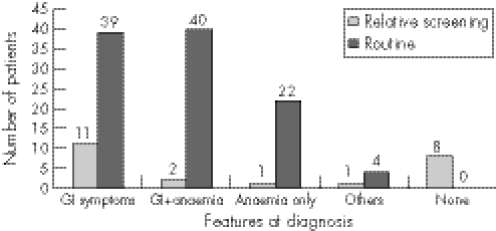
Thirteen of 23 patients in the relative screening group and 79 of 105 patients in the routine group had predominantly GI symptoms at diagnosis, which included diarrhoea, constipation, abdominal pain, vomiting and weight loss (fig 2). In the relative screening group, seven (30%) patients had abdominal pain and bloating, five (22%) had diarrhoea, two had constipation and three (13%) had weight loss. Also, one patient complained of tiredness and one patient had short stature. Among the routine group, 47 (45%) had abdominal pain and bloating, 48 (46%) had weight loss, 65 (62%) had diarrhoea and five patients had vomiting. Two patients had joint symptoms and four patients had short stature.
Figure 2 Symptoms at presentation in patients with coeliac disease diagnosed by relative screening or by routine referral.
Anaemia
Anaemia at presentation was significantly less common in the relative screened group (13%) compared to the routine group (58%; difference 45% (95% CI: 24% to 57%); p<0.001).
DEXA results
Osteoporosis was present in two (9%) patients in the relative screening group compared with 23 (22%) in the routine group (difference 13% (95% CI: 6% to 24%); p = 0.244), but the difference was not statistically significant. No patient in the relative screening group had osteopenia, but 12 patients in the routinely diagnosed group had osteopenia at the time of diagnosis of CD. Three patients in the relative screening group did not have (DEXA) bone scans as they were under 20 years of age and one patient declined a DEXA bone scan. In the routine group, eight patients declined DEXA and two patients died of other causes before undergoing a DEXA bone scan.
Prevalence of autoimmune diseases
In the routine group, 18% of the patients had associated autoimmune conditions, including diabetes mellitus, thyroid disease and pernicious anaemia, none of which were present in the relative screening group.
Malignancy
There were no malignancies in the relative screening group, while three patients in the routine group developed or presented with enteropathy associated GI lymphomas.
A comparison of the clinical features of the relative screening and routine groups is given in table 1.
Table 1 Comparison of clinical features between the relative screening and routinely diagnosed groups.
| Features | Screened patients | Routine patients | p value |
|---|---|---|---|
| Male:female | 16: 8 | 35: 70 | 0.003 |
| (number) | |||
| Age (median | 33 (17–72) | 54 (25–88) | 0.0001 |
| and range), years | |||
| % under 40 | 17 (74%) | 22 (21%) | 0.001 |
| years of age | |||
| GI symptoms | 13 (57%) | 79 (75%) | 0.071 |
| Anaemia | 3 (13%) | 61 (58%) | 0.001 |
| Asymptomatic | 8 (35%) | 0% | 0.000 |
| and no anaemia | |||
| Osteoporosis at | 2 (9%) | 23 (22%) | 0.244 |
| presentation | |||
| Other autoimmune | 0% | 19 (18%) | 0.024 |
| conditions | |||
| GI malignancy | 0% | 3 (3%) | 1.000 |
Gluten free diet follow‐up
Serology was only weakly positive in the six patients with positive serology and normal biopsies; of these, four declined further follow‐up and two will be followed up. Two patients with positive serology declined endoscopy because they were totally asymptomatic. Two patients diagnosed with CD on histopathology initially refused a gluten free diet. However, they developed diarrhoea 18 months and 4 years later, which subsequently resolved with a gluten free diet. In the relative screening group, after 6 months of gluten free diet, antibody titres became negative in 14 of 23 (61%) patients and nine (39%) patients continued to remain weakly positive. All routinely diagnosed patients went on a gluten free diet. After 6 months, the titres in patients with positive antibody titres became negative in 58% and remained weakly positive in 18%.
Discussion
Our study shows that the prevalence of CD among relatives was 13%, which is similar to the results of previous family studies of CD which reported an approximately 12% rate of CD in relatives of patients with CD.17 Of the screened subjects, 154 were first degree and 29 were second degree relatives. All relatives were taking a normal diet at the time of screening. Although more female relatives were screened, there was a male preponderance in the number of CD patients diagnosed, which is perhaps unusual. However, the number in the relative screening group was too small to allow definite conclusions. Patients diagnosed by relative screening were younger; 74% of our relative screening group were less than 40 years of age compared with 21% of our routine patients (difference 53% (95% CI: 31% to 68%); p<0.001). This could be explained by the fact that 62% of the relatives screened were equal to or less than 40 years of age (average 39 years; range 16–84 years).
GI symptoms or anaemia were present in 29.5% of all relatives who underwent screening. However, the prevalence was higher among relatives who were diagnosed with CD (61%) compared to the relatives who had negative serology (26.4%). From our study it was not possible to elicit whether these patients had sought medical advice for their symptoms before they were screened. Farre et al30 studied serological markers and clinical features suggestive of CD in first degree relatives and found that the most frequent clinical features (diarrhoea, anaemia and food intolerance) were present in two thirds of relatives with CD.
A family tree was recorded for all patients, but owing to confidentiality issues, we were unable to approach the patients' relatives directly. Patients were therefore given information to pass on to their relatives and those interested in taking part in the screening contacted us. Therefore, information on all relatives who were approached and those who declined screening is not available. There is likely to be a positive bias, as one would expect that relatives with symptoms may come forward for to be screened and asymptomatic CD may be undetected in relatives who did not come forward for screening.
Although there is evidence that a gluten free diet is beneficial in restoring bone density and resolving iron deficiency,22,23,24,25 reported morbidity is no greater in serologically positive individuals. In fact, there was a trend towards less cardiovascular morbidity.15 It is therefore important to inform relatives who consent for screening about the implications of having a positive or negative diagnosis. Owing to the sensitivities of coeliac serology, approximately 5% of patients with CD with negative serology may not have been picked up in our study. Patients with positive serology and normal histology require regular follow up and will need repeat biopsies if they become symptomatic.
Conclusion
Our study has shown that patients diagnosed with CD by relative screening are younger and fewer patients have osteoporosis, anaemia and other complications at diagnosis. However, gastrointestinal symptoms were common in these patients. Larger patient groups are required to confirm the differences we observed in the clinical presentations and incidence of complications between relative screening and routinely diagnosed CD patients.
Acknowledgements
We are grateful to Ms Rebecca Cannings, Statistician at Cardiff University, Department of General Practice, Wales College of Medicine for her help with statistical analysis of our study.
Abbreviations
CD - coeliac disease
CI - confidence interval
DEXA - dual energy x ray absorptiometry
ELISA - enzyme linked immunosorbent assay
EMA - endomysial antibody
GI - gastrointestinal
IgA - immunoglobulin A
TTG - tissue transglutaminase
Footnotes
Competing interests: None.
References
- 1.Kagnoff M F. Overview and pathogenesis of celiac disease. Gastroenterology 2005128s10–s11. [DOI] [PubMed] [Google Scholar]
- 2.Kagnoff M F. Celiac disease. A gastrointestinal disease with environmental, genetic and immunologic components. Gastroenterol Clin North Am 199221405–425. [PubMed] [Google Scholar]
- 3.Green P H. The many faces of celiac disease: clinical presentation of celiac disease in the adult population. Gastroenterology 2005128s74–s78. [DOI] [PubMed] [Google Scholar]
- 4.Dewar D H, Ciclitira P. Clinical features and diagnosis of celiac disease. Gastroenterology 2005128s19–s24. [DOI] [PubMed] [Google Scholar]
- 5.Marsh M N. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (celiac sprue). Gastroenterology 1992102(1)330–354. [PubMed] [Google Scholar]
- 6.Bingley P, Williams A, Norcross A.et al Undiagnosed coeliac disease at age seven: population based prospective birth cohort study. BMJ 2004328322–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnston S D, Watson R G, McMillan S A.et al Coeliac disease detected by screening is not silent‐‐simply unrecognized. QJM 199891(12)853–860. [DOI] [PubMed] [Google Scholar]
- 8.West J, Lloyd C A, Reader R.et al Prevalence of undiagnosed celiac disease in the general population of England. Gut 200148(Suppl 1)A237 [Google Scholar]
- 9.Not T, Hovarth K, Hill I D.et al Coeliac disease risk in the USA: high prevalence of antiendomysium antibodies in healthy blood donors. Scand J Gastroenterol 199833494–498. [DOI] [PubMed] [Google Scholar]
- 10.Ivarsson A, Persson L A, Juto P.et al High prevalence of undiagnosed celiac disease in adults: a Swedish population based study. J Intern Med 199924563–68. [DOI] [PubMed] [Google Scholar]
- 11.Murray J A, Van Dyke C, Plevak M F.et al Trends in the identification and clinical features of celiac disease in a North American Community, 1950–2001. Clin Gastroenterol Hepatol 2003119–27. [DOI] [PubMed] [Google Scholar]
- 12.Maki M, Mustalahti K, Kokkonen J.et al Prevalence of celiac disease among children in Finland. N Engl J Med 20033482517–2524. [DOI] [PubMed] [Google Scholar]
- 13.Fasano A, Berti I, Gerrarduzzi T.et al Prevalence of CD in at risk and non‐at‐risk groups in the United States: a large multi centre study. Arch Intern Med 2003163286–292. [DOI] [PubMed] [Google Scholar]
- 14.Mustalahti K, Reunanen A, Heuer M.et al Prevalence of coeliac disease in four European countries. Abstract P60. The 11th International Symposium: Coeliac Disease, Belfast, Northern Ireland 2004
- 15.West J, Logan R F A, Hill P G.et al Seroprevalence, correlates, and characteristics of undetected coeliac disease in England. Gut 200352960–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rewers M. Epidemiology of celiac disease: what are the prevalence, incidence and progression of celiac disease? Gastroenterology 2005128s47–s51. [DOI] [PubMed] [Google Scholar]
- 17.Pittscheiler K, Gentili L, Neiderhofer H. Onset of celiac disease: a prospective longitudinal study. Acta Paediatr 2003921149–1152. [DOI] [PubMed] [Google Scholar]
- 18.Dube C, Rostom A, Sy R.et al The prevalence of CD in average risk and at risk western European populations: a systematic review. Gastroenterology 2005128s57–s67. [DOI] [PubMed] [Google Scholar]
- 19.Book L, Zone J J, Neuhausen S L. Prevalence of celiac disease among relatives of sib pairs with celiac disease in U.S. families. Am J Gastroenterol 200398377–381. [DOI] [PubMed] [Google Scholar]
- 20.Korponay‐Szabo I, Kovacs J, Lorincz M.et al Families with multiple cases of gluten sensitive enteropathy. Z Gastroenterol 199836553–558. [PubMed] [Google Scholar]
- 21.Trier J S. Celiac sprue. N Engl J Med 19913251709–1719. [DOI] [PubMed] [Google Scholar]
- 22.Ogunji F, Saloum Y, Beharry S.et al Efficacy of gluten‐free diet alone on recovery from iron deficiency anaemia in adult celiac patients. Am J Gastroenterol 200196138. [DOI] [PubMed] [Google Scholar]
- 23.Mora S, Barera G, Ricotti A.et al Reversal of low bone density with a gluten free diet in children and adolescents with celiac disease. Am J Clin Nutr 199867477. [DOI] [PubMed] [Google Scholar]
- 24.McFarlane X A, Bhalla A K, Reeves D E.et al Osteoporosis in treated adult celiac disease. Gut 199536710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Catassi C, Bearzi I, Holmes G K T. Association of celiac disease and intestinal lymphomas and other cancers. Gastroenterology 2005128s79–s86. [DOI] [PubMed] [Google Scholar]
- 26.Collin P, Maki M, Keyrilainen O.et al Selective IgA deficiency and celiac disease. Scand J Gastroenterol 199227367–371. [DOI] [PubMed] [Google Scholar]
- 27.Cataldo F, Marino V, Ventura A.et al Prevalence and clinical features of selective immunoglobulin A deficiency in celiac disease. An Italian multicentre study. Italian Society of Paediatric Gastroenterology and Hepatology (SIGEP) and ‘Club del Tenue' Working Groups on Coeliac Disease. Gut 199842362–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armitage P, Berry G, Mathews J N S.Statistical methods in medical research. 4th edn. Oxford: Blackwell Science, 2002, 128–137, 277–82
- 29.Altman D G.Practical statistics for medical research. 1st edn. London: Chapman and Hall, 1992, 194–7, 241–5
- 30.Farre C, Humbert P, Vilar P.et al Serological markers and HLA–DQ2 haplotype among first degree relatives of celiac patients. Catalonian Coeliac Disease Study Group. Dig Dis Sci 199944(11)2344–2349. [DOI] [PubMed] [Google Scholar]