Abstract
Targeted biologic therapies have revolutionised treatment of immune‐mediated inflammatory diseases (IMIDs) due to their efficacy, speed of onset and tolerability. The discovery that clinically unrelated conditions, such as rheumatoid arthritis and Crohn's disease, share similar immune dysregulation has led to a shift in the management of IMIDs from one of organ‐based symptom relief to mechanism‐based treatment. The fact that anticytokine therapy has been effective in treating multiple orphan inflammatory conditions confirms the IMID paradigm. In this review we examine the biologic agents currently licensed for use in the US and Europe: infliximab, etanercept, adalimumab, rituximab, abatacept, anakinra, alefacept and efalizumab. We also discuss the rationale behind the management of IMIDs using rheumatoid arthritis, Crohn's disease, psoriasis and psoriatic arthritis as examples. For the medical profession, IMID represents a breakthrough in the way pathology is classified. In this burgeoning era of biologic therapy the prospect of complete disease remission is conceivable.
Keywords: anti‐TNF, autoimmune disease, biologics, immune‐mediated inflammatory disease, rheumatoid arthritis
Immune‐mediated inflammatory disease (IMID) is a concept used to collectively describe a group of ostensibly unrelated conditions that share common inflammatory pathways. Encompassing disorders as diverse as ankylosing spondylitis (AS), type 1 diabetes and multiple sclerosis, the immune dysregulation may afflict any organ system and result in significant morbidity, reduced quality of life (QoL) and premature death. Although the aetiology of these conditions is unknown, advances in molecular research have revealed that an imbalance in inflammatory cytokines is central to their pathogenesis.1
The incidence of IMIDs in Western society approximates 5–7%. Genetic factors are crucial determinants of susceptibility and animal models have led to the identification of several genes that contribute to an autoimmune diathesis when deleted or overexpressed.2 Many of these diseases also share similar environmental precipitants such as infection and trauma. Frequently, multiple IMIDs co‐exist within the same patient, for example a large study of AS found that 39% of patients also developed iritis, 16% psoriasis and 8% inflammatory bowel disease.3 Various IMIDs may also co‐exist within the same family.
The most convincing evidence linking the pathophysiology and treatment of autoimmune diseases has been demonstrated with the tumour necrosis factor‐α (TNFα) inhibitors. Infliximab, the first anti‐TNFα agent licensed, has shown clinical benefit in a number of seemingly unrelated conditions including rheumatoid arthritis (RA), Crohn's disease (CD) and psoriasis. In addition, there have been anecdotal suggestions of efficacy with anti‐TNFα agents in a multitude of orphan inflammatory diseases. This, together with the observation that treatment of one condition is capable of improving multiple organ systems, further validates the IMID paradigm and has inspired additional research for common treatment strategies. IMID therapy truly epitomises the triumph of translational research (bench to bedside).
The therapeutic aims for IMIDs are identical: to gain rapid control of inflammation, prevent tissue damage, improve QoL and, if possible, achieve long‐term disease remission. As these goals are rarely met in each patient with traditional treatments, presumably due to failure to address the underlying immunopathology, targeted biologic therapy has been revolutionary. In this review we describe the molecular basis for the IMID concept, examine the immune dysregulation that many IMIDs share, and briefly focus on the evidence for biologic therapies that are currently licensed for use in immune‐mediated conditions.
IMID pathogenesis
Over a decade ago, investigators discovered that cytokine dysregulation was pivotal to the pathophysiology of IMIDs. While these molecules are known to be indispensable mediators of normal immune function, an imbalance in their production can lead to chronic inflammatory conditions. Commonly, IMIDs are associated with a relative over‐expression of cytokines, such as TNFα in RA, yet a relative under‐expression of cytokines may be equally important in disease pathogenesis, for example interleukin 10 (IL10) deficiency in CD.4,5 Interestingly, cytokines may have different effects, either pro‐ or anti‐inflammatory, depending on the stage of disease.2
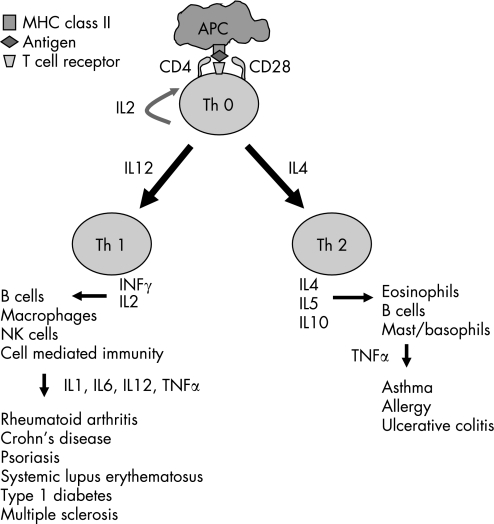
Although immunoregulatory cytokines are produced by many different cell types, CD4+ T lymphocytes are thought to be the main orchestrators of the immune response. These T helper cells are typically divided into two functionally heterogeneous subsets, Th 1 and Th 2, on the basis of the cytokines they produce (fig 1). Th 1 cells have predominantly pro‐inflammatory effects and have been implicated in the immunopathogenesis of IMIDs. Th 2 cells are thought to have anti‐inflammatory or protective functions in this context.6 Because of this central role in IMIDs, modulation of T cell function has became an attractive target for intervention.
Figure 1 Th 1 and Th 2 cell responses. APC, antigen presenting cell; IL, interleukin; MHC, major histocompatibility complex; Th, T helper cell; TNF, tumour necrosis factor.
Another area arousing considerable interest is the role of B lymphocytes in IMIDs. Early concepts focused on the ability of B cells to produce antibodies, however it is now known that B cells have much broader functions within the immune system, including T cell activation, cytokine synthesis, regulation of lymphoid architecture and maintenance of tolerance.7 Emerging evidence indicates that disruption of these tightly regulated B cell processes can potentially lead to autoimmune disease. Certainly in RA loss of B cell tolerance and inappropriate autoantibody production is a key pathological process, and this has motivated investigators to attempt B cell depletion as a novel therapeutic strategy.8 Preliminary studies with the anti‐CD20 monoclonal antibody, rituximab, have now been completed for a number of IMIDs with clear evidence of efficacy (table 1).
Table 1 IMIDs in which biologic therapy appears promising.
| Infliximab | Etanercept | Adalimumab | Rituximab |
|---|---|---|---|
| Sjogren's | Sjogren's | Crohn's disease | Polymyositis/dermatomyositis |
| Polymyositis/dermatomyositis | Polymyositis/dermatomyositis | UC | Wegener's granulomatosis |
| Wegener's vasculitis | Wegener's vasculitis | Psoriasis | GCA/PMR |
| Behcet's | Behcet's | JIA | Polyarteritis nodosa |
| GCA/PMR | GCA | Behcet's | JIA |
| Takayasu's arteritis | Takayasu's arteritis | Takayasu's arteritis | Graft versus host disease |
| Polyarteritis nodosa | Polyarteritis nodosa | Sarcoidosis | Cryoglobulinaemic vasculitis |
| Sarcoidosis | Sarcoidosis | Adult onset Still's disease | ITP |
| Adult onset Still's disease | Adult onset Still's disease | Hydradenitis supprativa | TTP |
| JIA | Cryoglobulinaemic vasculitis | Pyoderma gangrenosum | AIHA |
| Kawasaki disease | Relapsing polychondritis | Pemphigus | Anti‐phospholipid syndrome |
| Cryoglobulinaemic vasculitis | Hydradenitis supprativa | Idiopathic membranous GN | |
| Relapsing polychondritis | Pyoderma gangrenosum | Hep C associated GN | |
| Hydradenitis supprativa | Graft versus host disease | Multiple sclerosis | |
| Coeliac disease | Chronic hepatitis C | Myasthenia gravis | |
| Myelodysplastic syndrome | ITP | Pemphigus | |
| Pyoderma gangrenosum | Refractory asthma | Grave's disease | |
| Erythema nodosum | Amyloidosis | CIDP | |
| SAPHO syndrome | Multicentric reticulohistiocytosis | ||
| Graft versus host disease | Pemphigus | ||
| Chronic hepatitis B/C | Grave's disease | ||
| TTP | CIDP | ||
| Refractory asthma | |||
| SLE | |||
| Amyloidosis | |||
| Multicentric reticulohistiocytosis | |||
| Pemphigus |
This list is not exhaustive. AIHA, autoimmune haemolytic anaemia; CIDP, chronic inflammatory demyelinating polyneuropathy; GCA, giant cell arteritis; GN, glomerulonephritis; JIA, juvenile idiopathic arthritis; PMR, polymyalgia rheumatica; SAPHO, synovitis, acne, pustulosis, hyperostosis, osteitis; SLE, systemic lupus erythematosus; TTP/ITP, thrombotic/idiopathic thrombocytopaenic purpura; UC, ulcerative colitis.
An appraisal of the specific pathogenic mechanisms involved in every IMID is beyond the scope of this article, however this review will focus on four common IMIDs in which biologic agents have had a major impact, namely RA, CD, and psoriasis and psoriatic arthritis (PsA).
Rheumatoid arthritis
Rheumatoid arthritis (RA) is a systemic inflammatory condition that primarily affects the synovial membrane of affected joints. Extra‐articular manifestations are common and may involve any organ system. RA affects approximately 1% of the adult population and is associated with chronic pain, disability and increased mortality.9 The long‐term prognosis is generally poor with approximately 80% of patients experiencing significant functional disability within 20 years of diagnosis.10
Although the aetiology of RA remains unknown, it is postulated that an antigen, either exogenous or endogenous, triggers an abnormal immune response in a genetically susceptible individual.11 Th 1 cells, activated by antigen presenting cells and co‐stimulatory pathways, infiltrate the synovium and stimulate the production of pro‐inflammatory cytokines and destructive proteinases. While many cell types and mediators are involved in the pathogenesis of RA, the key drivers of inflammation are thought to be TNFα, interleukin 1 (IL1) and interleukin 6 (IL6).12 There is clear evidence from clinical trials that antibodies directed against TNFα and IL1 are capable of ameliorating disease activity. In contrast, the anti‐inflammatory cytokines IL4 and IL10 may play a protective role in RA.13 IL4, for example, is produced by Th 2 cells and has been shown, in vitro, to inhibit the activation of Th 1 cells and therefore decrease the production of IL1 and TNFα (fig 1).14
The exact role of B lymphocytes in the pathogenesis of RA requires elucidation, however several possible mechanisms have been proposed. Perhaps the most well described process is loss of B cell tolerance and the production of pathogenic autoantibodies, such as rheumatoid factor.15 Additional experiments in mice have demonstrated that T cell activation within the rheumatoid synovium is critically dependent on the presence of B cells.16
Biologics have significantly expanded the therapeutic options available for RA patients who remain refractory to traditional disease modifying anti‐rheumatic drugs (DMARDs). Agents currently licensed for use are the three TNFα inhibitors (infliximab, etanercept and adalimumab) and the two newer agents, rituximab (anti‐CD20) and abatacept (T cell co‐stimulation blocker). Anakinra, a recombinant IL1 receptor antagonist is also licensed, however its efficacy in clinical trials has been less impressive than with the TNFα blockers and thus the National Institute for Health and Clinical Excellence (NICE) does not recommend its use in the United Kingdom. The anti‐IL6 receptor antibody tocilizumab is however showing promise in clinical trials and a US Food and Drug Administration (FDA) licence application is anticipated for 2007.
Crohn's disease
Crohn's disease (CD) is a T cell mediated disorder characterised by a relapsing inflammatory process in the gut with extra‐intestinal manifestations typically involving the skin, eyes and joints.17 Although the cause of CD is unknown, the most prominent theory implicates a dysfunctional mucosal immune response to an otherwise innocuous luminal antigen in a genetically susceptible host.18 Complications such as perianal fistulae occur in up to 43% of patients, many of which require surgery.19 With a prevalence of around 0.1% in the developed world,20 CD places a major burden on health care resources.
Similar to RA, an imbalance in the cytokine milieu is central to its pathogenesis. Gut inflammation typically begins with mucosal infiltration of neutrophils and macrophages, which in turn activate T cells.18 By stimulating the production of pro‐inflammatory cytokines, Th 1 cells amplify the immune response and promote tissue destruction.21 Equally, a relative underproduction of regulatory cytokines (IL4 and IL10) has been observed in Crohn's affected mucosa.5 This finding has led to targeted drug therapies focusing on either inhibiting or augmenting cytokine action. IL10, for example, is thought to be a potent downregulator of the Th 1 response, and although animal models have shown a strong link between IL10 deficiency and gut inflammation, the use of IL10 therapy in clinical trials has been disappointing.22 Great success however, has been achieved with certain anti‐TNFα agents.
Infliximab is currently the only biologic agent approved for the treatment of moderate‐severe and/or fistulising CD. Certolizumab (Cimzia, Nektar), a subcutaneous anti‐TNFα agent, has also demonstrated efficacy in CD and an application has been submitted to the FDA for appraisal.23 Interestingly, the TNF receptor fusion protein etanercept has failed to show significant benefit in clinical trials, presumably due to its different mechanism of action.24 Etanercept only binds soluble TNF, yet it is the transmembrane binding of TNF that is crucial for inducing apoptosis of T cells in CD.25
Psoriasis and psoriatic arthritis
Psoriasis is a chronic inflammatory disease of uncertain aetiology that affects approximately 2% of the population.26 Characterised by scaly erythematous plaques, the disease can lead to significant physical and psychological distress, and in up to 30% of patients a debilitating arthropathy may develop.27 A genetic predisposition for psoriasis is clear, with concordance of approximately 70% in monozygotic twins, however a complex interplay with environmental factors is likely.28
Akin to RA and CD, psoriasis and PsA are T cell‐mediated. Histologically, skin plaques and inflamed synovial tissue demonstrate an abundance of T cells and increased vascularity. The inflammatory cascade is thought to be triggered by the local activation of CD4+ T cells which generate a series of pro‐inflammatory cytokines such as TNFα that subsequently activate CD8+ T cells, the main effector cells.29 High concentrations of TNFα have been detected in psoriatic skin lesions and in the synovium of affected joints and it is believed that this cytokine plays a pivotal role in perpetuating inflammation in addition to stimulating the angiogenesis and keratinocyte proliferation.30
In many cases, conventional systemic therapies for both psoriasis and PsA are either ineffective, are poorly tolerated or are unable to maintain long‐lasting remission.31 Recently, however, enormous growth in our understanding of the disease pathogenesis has led to an expansion in therapeutic options.
Biologic agents that are currently licensed for plaque psoriasis include the TNFα inhibitors, etanercept and infliximab, as well as the T cell‐targeted therapies, efalizumab and alefacept (FDA only). NICE guidance has recently been issued for the use of etanercept in severe plaque psoriasis in patients who have failed conventional therapies, and for efalizumab as a second line agent to etanercept. For PsA, three anti‐TNFα agents (infliximab, etanercept and adalimumab) are licensed, however only infliximab and etanercept are NICE approved. Benefit in psoriasis trials has also been seen with the T cell costimulation blocker, abatacept.32
Biologics currently licensed for IMID treatment
Infliximab
Infliximab (Remicade, Schering‐Plough/Centocor), a mouse‐human chimeric monoclonal antibody directed against TNFα, is licensed for use in RA, AS, PsA, CD, ulcerative colitis (UC) and psoriasis (tables 2 and 3). To date, the only NICE‐approved indications for infliximab are RA, CD and PsA, although an appraisal is in development for AS. The drug is given as a 2 h intravenous infusion with a dose of 3–5 mg/kg at weeks 0, 2 and 6, and then every 8 weeks thereafter. In the event of waning efficacy, the dose may be increased up to 10 mg/kg, or the infusion frequency increased to four to six weekly.
Table 2 Properties of approved biologics.
| Biologic agent | Infliximab | Etanercept | Adalimumab | Rituximab | Abatacept | Anakinra |
|---|---|---|---|---|---|---|
| Proprietary name | Remicade | Enbrel | Humira | Rituxan | Orencia | Kineret |
| Mabthera | ||||||
| Construct | Chimeric mAb to | TNFα receptor | Fully humanised | Chimeric mAb to | CTLA4Ig | IL1 receptor antagonist |
| TNFα | fusion protein | mAb to TNFα | CD20 | |||
| Mode of action | Binds to soluble and | Binds to soluble | Binds to soluble | Binds to CD20 | Blocks T cell co‐ | Binds to IL1 receptor |
| membrane bound | TNFα and TNFβ | and membrane | molecule on B cells | stimulation via | ||
| TNFα | bound TNFα | CD28‐CD80/86 | ||||
| Half‐life | 9 days | 4 days | 14 days | Variable | 13 days | 6 h |
| Dose | 3–5 mg/kg of body | 25 mg twice | 40 mg every second | 1000 mg on day 1 | 10 mg/kg of body | 100 mg daily |
| weight at 0, 2 and | weekly | week. For incomplete | and day 15 | weight at 0, 2 and | ||
| 6 weeks, and then | response, dose may | 4 weeks, and then | ||||
| 8 weekly. Dose | be given weekly | monthly thereafter | ||||
| can be increased | Repeated every 6– | |||||
| to 10 mg/kg or | 9 months | |||||
| frequency of | ||||||
| injection increased | ||||||
| Administration | Intravenous | Subcutaneous | Subcutaneous | Intravenous | Intravenous | Subcutaneous |
CTLA4, cytotoxic T lymphocyte antigen‐4; IL, interleukin; mAb, monoclonal antibody; TNF, tumour necrosis factor.
Table 3 Biologics approved for IMIDs: FDA, EMEA and NICE.
| Biologic agent | Action | FDA licence | EMEA licence | NICE approval |
|---|---|---|---|---|
| Infliximab | Anti‐TNFα | RA | RA | RA |
| AS | AS | PsA | ||
| PsA | PsA | CD | ||
| CD | CD | AS (appraisal) | ||
| UC | UC | UC (submitted) | ||
| Paediatric CD | Psoriasis | Psoriasis | ||
| Paediatric CD (appraisal) | ||||
| Etanercept | Anti‐TNFα | RA | RA | RA |
| JIA | JIA | JIA | ||
| AS | AS | PsA | ||
| PsA | PsA | Psoriasis | ||
| Psoriasis | Psoriasis | AS (appraisal) | ||
| Adalimumab | Anti‐TNFα | RA | RA | RA (appraisal) |
| PsA | PsA | PsA | ||
| AS | AS | AS | ||
| Rituximab | Anti‐CD20 | RA | RA | RA (submitted) |
| Abatacept | CTLA4Ig | RA | RA (submitted) | RA (submitted) |
| Efalizumab | Anti‐CD11a | Psoriasis | Psoriasis | Psoriasis |
| Alefacept | LFA‐3/IgG Fc construct | Psoriasis | None | None |
| Anakinra | Anti‐IL1 | RA | RA | None |
AS, ankylosing spondylitis; CD, Crohn's disease; CTLA4, cytotoxic T lymphocyte antigen‐4; EMEA, European Medicines Agency; FDA, US Food and Drug Administration; IL, interleukin; JIA, juvenile idiopathic arthritis; NICE, UK National Institute for Health and Clinical Excellence; PsA, psoriatic arthritis; RA, rheumatoid arthritis; TNFα, tumour necrosis factor‐α; UC, ulcerative colitis.
Infliximab is currently approved for active RA in those who have failed DMARDs, and for severe progressive RA in those not previously treated with DMARDs. The efficacy of infliximab has been well documented in randomised controlled trials (RCTs). In the ATTRACT trial, infliximab was compared with placebo in 428 RA patients taking methotrexate (MTX).33 The actively treated subjects showed significant improvements in all American College of Rheumatology (ACR) responder indices (box 1). Radiological progression was inhibited and QoL was improved in the infliximab‐MTX groups for up to 2 years.34 Interestingly, sub‐analysis showed reduced radiological progression even in the absence of clinical response.
The ASPIRE trial examined MTX naive patients with early active RA.35 In the group taking MTX alone, radiographic progression was associated with high inflammatory markers and persistent disease activity.36 Infliximab plus MTX, on the other hand, virtually halted radiographic progression of disease and improvement was seen in all ACR indices. These data suggested that early aggressive treatment of RA may avert joint destruction.
Approval for infliximab in psoriasis was based on two large RCTs. In the SPIRIT study, 88% of patients treated with infliximab met the primary end point of PASI 75 (75% improvement in Psoriasis Area and Severity Index) at week 10 versus 6% receiving placebo.37 Significant improvements were also seen in health related QoL. Similarly, in the EXPRESS study, 80% of patients on infliximab therapy achieved a PASI 75 response compared to 3% on placebo.38
For PsA, the efficacy of infliximab plus MTX has been studied in two RCTs.39,40 At week 16 in the IMPACT trial, almost two thirds of patients achieved an ACR20 response compared to 10% on MTX alone. Improvements in articular and dermatologic manifestations of PsA were sustained until study completion and significant benefits in health related QoL and physical function were noted.41
In an early trial of 108 patients with moderate to severe CD, 81% responded to a single dose of infliximab 5 mg/kg compared to 17% on placebo. Remarkably, clinical remission was achieved in almost half of those on active treatment.42 In the ACCENT I trial, patients who demonstrated an initial response were found to be more likely to sustain a clinical response with infliximab maintenance therapy than those on placebo.43 Clinical improvement was associated with endoscopic evidence of mucosal healing.44 The approval for infliximab maintenance therapy in fistulising CD was based on the results of the ACCENT II study.45 At the end of 1 year, maintenance therapy with infliximab resulted in a more sustained clinical response, a higher rate of complete fistula closures and improvement in QoL. Importantly, infliximab maintenance therapy also reduced the number of hospitalisations and need for surgery.46
Box 1 American College of Rheumatology response criteria for 20% improvement (ACR20)
20% reduction in the number of tender and swollen joints, plus 20% improvement in 3 of the following 5 parameters:
Physician global assessment of disease
Patient global assessment of disease
Patient assessment of physical function
Patient assessment of pain
Erythrocyte sedimentation rate or C‐reactive protein
Based on success in RCTs, infliximab was the first biologic approved for the treatment of UC and a licence has also been granted for use in AS. Additional case reports have demonstrated the therapeutic value of infliximab in many other IMIDs (table 1).
Etanercept
Etanercept (Enbrel, Wyeth‐Ayerst/Amgen) is a soluble TNF receptor fusion protein which comprises two TNFα receptors linked to human IgG and binds soluble TNFα. It is given as a 25 mg subcutaneous injection twice weekly or 50 mg weekly. Etanercept is licensed for use in RA, psoriasis, PsA, AS and juvenile idiopathic arthritis (JIA) (tables 2 and 3). Unlike infliximab, etanercept has not shown benefit in CD as mentioned previously.
The efficacy of etanercept in RA was initially evaluated as monotherapy. Significant improvements were found in all ACR responder indices and QoL measures in the etanercept groups compared with placebo.47 The TEMPO study, combining etanercept with MTX, however, indicated that the two drugs are significantly more effective in retarding disease progression than either agent alone.48
In the ERA trial, etanercept was directly compared with MTX in patients with RA diagnosed within the previous 3 years. Although response rates were initially more rapid with etanercept, responses converged at 12 months.49 After 2 years, however, significantly more etanercept‐treated patients met the ACR20 improvement criteria than those on MTX monotherapy, and the rate of erosive change was also retarded.50 In a 5 year open‐label extension study, sustained efficacy was observed with etanercept and erosive change was clearly retarded. Furthermore, many patients were able to discontinue MTX and corticosteroid therapy.51 These findings support prompt treatment of early aggressive RA.
Approval for the use of etanercept in psoriasis was based on data from two large phase III trials involving over 1200 patients. Similar results were obtained from both studies, with almost half of the patients on etanercept achieving a PASI 75 response compared to 4% on placebo. With extended etanercept therapy, clinical improvement was sustained and patient‐reported outcomes reflected a positive effect on QoL.52,53 For PsA, 59% of etanercept‐treated patients achieved an ACR20 response compared to 15% on placebo at 12 weeks. Improvement was sustained with maintenance therapy at 1 year and radiographic progression of disease was halted.54
Approval for etanercept has also been given for JIA and AS following positive results in RCTs. Pilot studies have also shown benefit in many other IMIDs (table 1).
Adalimumab
Adalimumab (Humira, Abbott), a fully human anti‐TNFα monoclonal antibody, is licensed for clinical use in RA, PsA and AS. NICE appraisals are in development for all these indications. The drug is administered as a 40 mg subcutaneous injection every other week.
Adalimumab has been assessed in over 2000 RA patients in RCTs. In the ARMADA trial, patients taking adalimumab plus MTX showed significant improvements in all ACR responder indices at 6 months, with effects seen as early as the first week.55 In a similar 12 month study, clinical benefit was accompanied by marked improvements in radiographic and functional outcomes.56 Evidence supporting the use of adalimumab as monotherapy has also been demonstrated.57
Approval has also been granted for adalimumab as first‐line therapy for early progressive RA based on data from the PREMIER study. Although clinical responses were comparable when either adalimumab and MTX were used alone, impressive results were seen when the two drugs were combined. Nearly 50% of patients achieved clinical remission, rates approximately double those found among those receiving monotherapy. Furthermore, three‐quarters of the patients on combination therapy showed no radiographic progression of disease for 2 years.58 Again, these data support the notion that early treatment of RA may prevent long‐term disability.
In a 6 month study of PsA, patients treated with adalimumab showed a dramatic improvement in all arthritis severity scores in addition to a reduction in joint damage progression and an improvement in QoL. Among the adalimumab‐treated patients with plaque psoriasis, 59% achieved a PASI 75 response, compared with 1% on placebo.59 Phase III trials for psoriasis are in progress.
For CD, limited published data indicate that adalimumab is safe and effective for inducing remission in up to 36% of patients.60 The efficacy of adalimumab has also been demonstrated in AS and phase III clinical trials are underway in UC and JIA (table 1).
Rituximab
Rituximab (Mabthera, Roche; Rituxan, Genentech/Biogen) is a chimeric anti‐CD20 monoclonal antibody of human and mouse origin that was initially approved for treatment of non‐Hodgkin's B cell lymphoma. The drug has since been investigated in a variety of IMIDs in which B cells have been suggested to play a role.
Convincing evidence of efficacy comes from trials in RA with significant benefit being demonstrated when using rituximab either alone or in combination with MTX or cyclophosphamide.61 The subsequent DANCER trial, involving 645 patients, confirmed the benefit of rituximab plus MTX and the effect was independent of glucocorticoids.62 In another phase III trial, the REFLEX study, patients with an inadequate response to anti‐TNFα therapy also noted marked clinical improvement with rituximab therapy.63 The efficacy was similar to that of the TNFα inhibitors.
In 2006, the FDA and European Medicines Agency (EMEA) approved rituximab for the treatment of RA in patients who have failed anti‐TNFα therapy. The recommended dose is 1000 mg given intravenously 2 weeks apart with concomitant MTX. Although the safest and most effective timing for repeat treatment has yet to be determined, preliminary data suggest that the interval will likely be 6–12 months.
The use of rituximab has not been limited to RA. Published open‐label studies have shown efficacy in the treatment of more than 30 other IMIDs including SLE, dermatomyositis and Wegener's granulomatosis (table 1).
Abatacept
Abatacept (Orencia, Bristol Myers Squibb) is a recombinant fusion protein consisting of the extracellular domain of human cytotoxic T lymphocyte antigen‐4 (CTLA‐4) with the Fc domain of IgG. Classed as a co‐stimulation blocker, the drug is designed to inhibit T cell activation by blocking the interaction between antigen presenting cells and T cells. This interaction is mediated via the CD28‐CD80/86 pathway. In 2005, the FDA approved abatacept for the treatment of moderate to severe RA in patients who have had an inadequate response to one or more DMARDs or TNFα antagonists. The recommended dose is 10 mg/kg by intravenous infusion at 0, 2 and 4 weeks initially, and then monthly thereafter.
The observation that the CD28 and CD80/86 ligands are highly expressed on cells within the rheumatoid synovium suggests that co‐stimulation may play a direct role in the pathogenesis as well as progression of RA.64 Endogenous CTLA4 is also expressed on T cells in the rheumatoid joint and is thought to function as a regulator of T cell activation by interrupting the CD28 pathway, in addition to stimulating the release of immunoregulatory cytokines, such as TGFβ.65,66 CTLA4 can also mediate antigen‐specific apoptosis of T cells.67
In the clinical setting, two pivotal efficacy studies have now been published for RA. In the first, 339 subjects with an inadequate response to MTX were randomised to receive abatacept plus MTX or MTX alone. At 1 year, marked clinical improvements were observed with abatacept, with almost twice as many patients achieving an ACR20 response compared to placebo.68 Striking radiographic improvements have since been demonstrated in a subsequent phase III study.69 Abatacept has also been evaluated in patients with an inadequate response to anti‐TNFα therapy, and again, significant improvements in all ACR indices were seen with active treatment compared to placebo.70
Success with abatacept has been noted in psoriasis, with early studies showing a clear clinical and biochemical improvement in addition to a quantitative reduction of T cells infiltrating the skin.32 Overall, these findings support the view that T cell activation plays an essential role in the pathogenesis of IMIDs, and therefore it is likely that abatacept will have great utility in the treatment of T cell mediated diseases in the future.
Alefacept and efalizumab
Two additional agents have been approved for the treatment of plaque psoriasis. Efalizumab (Raptiva, Genentech/Xoma), a humanised monoclonal antibody, binds to the alpha subunit (CD11a) of leukocyte‐function‐associated antigen type‐1 (LFA‐1) and inhibits the activation of T cells. Alefacept (Amevive, Biogen/Idec), a fully human LFA‐3/IgG1 fusion protein, also inhibits T cell activation and selectively reduces memory T cells. These drugs have shown excellent responses in RCTs, with approximately a quarter of patients achieving 75% improvement in skin lesions within 3 months.71,72 Alefacept has also demonstrated efficacy in RA, in combination with MTX, and in PsA.73,74
Certolizumab pegol (CDP870)
A new anti‐TNFα agent, certolizumab (Cimzia, Nektar), previously known as CDP870, has recently been submitted to the FDA and EMEA for appraisal for the treatment of CD. Certolizumab consists of the Fab fragment of a humanised anti‐TNFα antibody, coupled to polyethylene glycol (PEG). This produces a drug that can remain in circulation longer and can be conveniently administered once a month via subcutaneous injection.
Data from a 12 week phase II clinical trial demonstrated that certolizumab produced a significant clinical benefit in CD over placebo from weeks 2 to 10, but not at week 12.23 In a more recent trial, however, certolizumab convincingly induced clinical remission for up to 6 months.75 Overall, the drug is well tolerated with a safety profile similar to other anti‐TNFα agents. Not surprisingly, certolizumab has also demonstrated efficacy in RA with outcomes comparable to that of etanercept and infliximab.76 Studies have also begun in psoriasis.
Anakinra
Anakinra (Kineret, Amgen), a recombinant IL1 receptor antagonist, was approved for use in RA in 2001. In the UK, however, NICE did not approve its use in RA as it was deemed to be cost ineffective. To date, RCTs involving nearly 3000 patients have demonstrated efficacy of anakinra in RA, either as monotherapy or in combination with MTX.77,78 Rapid improvements in functional status reflected radiographic evidence of reduced joint damage.
Safety
Few serious adverse events were documented in the clinical trials of anti‐TNFα agents, however post‐marketing surveillance revealed several important complications. Principle among these was reactivation of latent mycobacterium tuberculosis (TB) infection as TNFα plays a key role in the integrity of granumolata.79 Serious bacterial infections have also been described, however a recent report from the British Society of Rheumatology Biologics Register found that the overall risk of serious infections in RA patients was not increased by anti‐TNFα therapy when compared to those on standard DMARD therapy. There were however more opportunistic infections, such as histoplasmosis, and more skin and soft tissue infections in the cohort on biologic therapy.80
Additional complications of TNFα blockade include possible exacerbation of congestive cardiac failure, demyelinating disease and lymphoproliferative disorders.81 For RA patients, the occurrence of lymphoma secondary to biologic therapy is controversial as there is a two‐ to threefold increased incidence of this disease with RA per se. The possible increased risk for the development of solid malignancy is far from clear. A further potential side effect of anti‐TNFα therapy is the development of antinuclear antibodies (ANA), although an SLE‐like illness is rare and ANA development alone does not necessitate stopping therapy.
The tolerability of rituximab and abatacept in clinical trials has been very favourable with integrated safety data indicating that adverse events, such as malignancy and serious infections, are rare. In relation to rituximab, the experience in lymphoma patients has been vast (>750 000 patients treated) and no increased risk of TB or opportunistic infection has been noted. Infusion reactions are common with rituximab, although these events may be minimised by the use of peri‐infusional corticosteroids.62
The commonest side effect of anakinra is injection‐site reaction. Although neutropaenia has been noted in clinical trials with the IL1 blocker, the rate of serious infection is comparable to control patients. In an RA study comparing the combined efficacy of anakinra and etanercept, however, no added benefit was seen and there was an unacceptably high incidence of serious infections.82 Thus treatment with concurrent biologic agents is not advised and a warning has been issued by the regulatory health bodies.
The precise relationship between several of these complications and biologic therapy is unknown, partly due to underreporting but also due to lack of comparison cohorts. It may be that several of these comorbidities are associated with the disease itself, or perhaps due to extended exposure to standard DMARD therapy. Robust safety data will emerge from the numerous biologic registries established across Europe and the US.83
Cost
One of the drawbacks of biologic therapy is the expense; the TNFα antagonists cost approximately US$15–18 000 per patient per annum in the US and around €10 000 in Europe.84,85 The cost of B cell targeted therapy may be less, however further work on the appropriate dosing regimen is needed. Due to the infrastructure required to manufacture these agents, the prices are unlikely to plummet following patent expiry. This cost, however, must be balanced against the significant economic burden IMIDs can pose to the individual patient and to society as a whole. Direct annual costs for IMIDs vary widely from US$791 for psoriasis86 to US$5822 for RA87 and US$12 417 for CD.88 The indirect costs however, such loss of productivity and income, may be several times greater as many IMIDs affect young people subjecting them to chronic disability.1 In 1992, the total annual cost of RA to UK society was over £1 billion, approximately half of which was attributed to loss of productivity.89 Clearly, those with severe, debilitating disease consume greater resources than those less afflicted. In CD for example, it has been estimated that 2% of the patient population accounts for 34% of the medical costs.90
Summary box
Biologic agents are highly effective disease‐modifying medications.
Currently licensed biologics target TNFα, interleukin‐1, T cell activation and B cells.
Biologic agents improve the signs and symptoms and quality of life of patients with rheumatoid arthritis, Crohn's disease, psoriasis and many orphan inflammatory conditions.
Physicians should be vigilant for the potential side‐effects of biologic agents.
Treatment should commence early for optimal outcome.
IMIDs represent a breakthrough in the way pathology is classified.
Conclusions
Biologics have revolutionised the treatment of autoimmune diseases due to their efficacy, speed of onset and tolerability. The fact that anti‐cytokine therapies, such as infliximab, have been effective in treating multiple orphan inflammatory conditions confirms that the IMID concept is valid. For the medical profession, this represents an important breakthrough in the way we classify pathology. Traditionally, IMIDs are treated by doctors who specialise in the organ most affected, however given that CD shares similar cytokine dysregulation to both RA and psoriasis, these systemic disorders may in the future be grouped as one disorder.
Although biologics provide a very useful addition to our therapeutic armamentarium, evidence suggests that they should not be considered a panacea. Serious adverse events have been reported and long‐term safety data are lacking. Furthermore, a substantial number of patients demonstrate a poor response to these agents, confirming that our understanding of IMID immunopathogenesis is far from complete.
The current challenge is to identify exactly when to introduce biologics into the therapeutic algorithm. Traditionally they have been used in those least likely to respond, that is, those who have failed multiple DMARDs and still have active disease. Certainly in rheumatology, the prevailing philosophy is to treat as early as possible in order to avoid the potential sequelae of joint destruction and functional loss.
While RCTs have demonstrated efficacy in early RA with the combination of MTX and TNFα inhibitors, it may be argued that evidence is lacking to promote this as initial therapy for all patients. In up to one third of patients with RA, progressive joint destruction does not develop and anti‐TNFα therapy may be not only costly but unnecessary.91 Furthermore, combination conventional DMARD therapy has shown to be very effective in reducing disease activity in RA.92,93,94 There is however evidence from early RA trials that infliximab, etanercept and adalimumab, when used in combination with MTX, are more effective in rapidly suppressing disease activity than MTX alone in patients with aggressive disease diagnosed within the preceding 3 years.35,49,58 Clearly further studies are required to determine exactly who and when to treat. If we could identify the patients most likely to respond, it may be possible to save time and expense and to avert potential toxic reactions. The field of pharmacogenetics will address this.
Overall, biologic therapies represent an exciting advance in the treatment of autoimmune diseases. For millions of patients, treatment success may translate to rapid suppression of inflammation, prevention of disability, improved quality of life, and the goal of complete disease remission – something unthinkable a decade ago.
Key references
33 Maini R, St Clair EW, Breedveld F, et al. Infliximab (chimeric anti‐tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet 1999;354:1932–9.
49 Bathon JM, Martin RW, Fleischmann RM, et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med 2000;343:1586–93.
58 Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: a multicenter, randomized, double‐blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum 2006;54:26–37.
62 Emery P, Fleischmann R, Filipowicz‐Sosnowska A, et al. DANCER Study Group. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double‐blind, placebo‐controlled, dose‐ranging trial. Arthritis Rheum 2006;54:1390–400.
94 Goekoop‐Ruiterman YP, de Vries‐Bouwstra JK, Allaart CF, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum 2005;52:3381–90.
Multiple choice questions (true (T)/false (F), answers after the references)
Choose the best of the five options for each question.
-
Which of the following is true regarding anti‐TNFα therapy?
Anti‐TNFα therapy can be administered both subcutaneously and orally
Infliximab and etanercept are of comparable efficacy in treating Crohn's disease
Etanercept can be used as first line therapy in psoriasis
D. Adalimumab is administered subcutaneously every other week
E. Only one anti‐TNFα agent is licensed in the UK for the treatment of rheumatoid arthritis
-
Which of the following is true regarding rituximab?
It decreases the number of circulating B cells
It is licensed for the treatment of rheumatoid arthritis and juvenile idiopathic arthritis
It is licensed in Europe as a second line agent for patients who have failed methotrexate
The efficacy of rituximab is far greater than infliximab in the treatment of rheumatoid arthritis
The recommended dose is 1000 mg given intravenously every month.
-
Which of the following are IMIDs?
Systemic lupus erythematosus
Type 1 diabetes
Osteoarthritis
Multiple sclerosis
Ankylosing spondylitis
-
In clinical trials which of the following have been shown to be beneficial?
Abatacept for rheumatoid arthritis
Etanercept for Crohn's disease
Alefacept for psoriasis
Anakinra for rheumatoid arthritis
Infliximab for ankylosing spondylitis
-
Which of the following is true regarding the safety of biologics?
Infliximab is safer than adalimumab
Anti‐TNFα treatment is associated with an increased risk of lung cancer
Patients must be screened for tuberculosis before commencing anti‐TNFα therapy
Patients commonly have infusion reactions with rituximab
The development of antinuclear antibodies necessitates stopping therapy
Abbreviations
ACR - American College of Rheumatology
ANA - antinuclear antibodies
AS - ankylosing spondylitis
CD - Crohn's disease
CTLA4 - cytotoxic T lymphocyte antigen‐4
DMARDs - disease modifying anti‐rheumatic drugs
EMEA - European Medicines Agency
FDA - US Food and Drug Administration
IL1 - interleukin 1
IL6 - interleukin 6
IL10 - interleukin 10
IMID - immune‐mediated inflammatory disease
JIA - juvenile idiopathic arthritis
MTX - methotrexate
NICE - National Institute for Health and Clinical Excellence
PEG - polyethylene glycol
PsA - psoriatic arthritis
QoL - quality of life
RA - rheumatoid arthritis
RCT - randomised controlled trial
TB - tuberculosis
TNFα - tumour necrosis factor‐α
UC - ulcerative colitis
Answers
(A) F, (B) F, (C) F, (D) T, (E) F.
(A) T, (B) F, (C) F, (D) F, (E) F.
(A) T, (B) T, (C) F, (D) T, (E) T.
(A) T, (B) F, (C) T, (D) T, (E) T.
(A) F, (B) F, (C) T, (D) T, (E) F.
Footnotes
Financial support was provided by CARE (Cambridge Arthritis Research Endeavour) Charity.
Competing interests: Dr Annabel Kuek declares no conflicts of interest. Dr Andrew Östör and Dr Brian Hazleman receive sponsorship from Schering‐Plough, Wyeth, Abbott and Roche.
References
- 1.Williams J P, Meyers J A. Immune‐mediated inflammatory disorders (I.M.I.D. s): the economic and clinical costs, Am J Manag Care 200221(Suppl)S664–S681. [PubMed] [Google Scholar]
- 2.Davidson A, Diamond B. Autoimmune diseases. N Engl J Med 2001345340–350. [DOI] [PubMed] [Google Scholar]
- 3.Brophy S, Pavy S, Lewis P.et al Inflammatory eye, skin, and bowel disease in spondyloarthritis: genetic, phenotypic and environmental factors. J Rheumatol 2001282667–2673. [PubMed] [Google Scholar]
- 4.O'Shea J J, Ma A, Lipsky P. Cytokines and autoimmunity. Nat Immunol 2002237–45. [DOI] [PubMed] [Google Scholar]
- 5.Niessner M, Volk B A. Altered Th1/Th2 cytokine profiles in the intestinal mucosa of patients with inflammatory bowel disease as assessed by quantitative reversed transcribed polymerase chain reaction (RT‐PCR). Clin Exp Immunol 1995101428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lucey D R, Cerici M, Shearer G M. Type 1 and type 2 cytokine dysregulation in human infections, neoplastic, and inflammatory diseases. Clin Microbiol Rev 19969532–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Youinou P, Hillion S, Jamin C.et al B lymphocytes on the front line of autoimmunity. Autoimmun Rev 20065215–221. [DOI] [PubMed] [Google Scholar]
- 8.Edwards J C, Szczepanski L, Szechinski J.et al Efficacy of B‐cell‐targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med 20043502572–2581. [DOI] [PubMed] [Google Scholar]
- 9.Alamanos Y, Drosos A A. Epidemiology of adult rheumatoid arthritis. Autoimmun Rev 20054130–136. [DOI] [PubMed] [Google Scholar]
- 10.Scott D L, Symmons D P, Coulton B L.et al Long‐term outcome of treating rheumatoid arthritis: results after 20 years. Lancet 198711108–1111. [DOI] [PubMed] [Google Scholar]
- 11.Gregersen P K, Silver J, Winchester R J. The shared epitope hypothesis: an approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum 1987301205–1213. [DOI] [PubMed] [Google Scholar]
- 12.Choy E H, Panayi G S. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med 2001344907–916. [DOI] [PubMed] [Google Scholar]
- 13.Sugiyama E, Kuroda A, Taki H.et al Interleukin 10 cooperates with interleukin 4 to suppress inflammatory cytokine production by freshly prepared adherent rheumatoid synovial cells. J Rheumatol 1995222020–2026. [PubMed] [Google Scholar]
- 14.van Roon J A, van Roy J L, Duits A.et al Proinflammatory cytokine production and cartilage damage due to rheumatoid synovial T helper‐1 activation is inhibited by interleukin‐4. Ann Rheum Dis 199554836–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards J C W, Cambridge G, Abrahams V M. Do self‐perpetuating B lymphocytes drive human autoimmune disease? Immunology 199997188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takemura S, Klimiuk P A, Braun A.et al T cell activation in rheumatoid synovium is B cell dependent. J Immunol 20011674710–4718. [DOI] [PubMed] [Google Scholar]
- 17.Shanahan F. Crohn's disease. Lancet 200235962–69. [DOI] [PubMed] [Google Scholar]
- 18.Hanauer S B. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis 200612(Suppl 1)S3–S9. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz D A, Pemberton J H, Sandborn W J. Diagnosis and treatment of perianal fistulas in Crohn's disease. Ann Intern Med 2001135906–918. [DOI] [PubMed] [Google Scholar]
- 20.Loftus E V, Jr, Schoenfeld P, Sandborn W J. The epidemiology and natural history of Crohn's disease in population–based patient cohorts from North America: a systematic review. Ailment Pharmacol Ther 20021651–60. [DOI] [PubMed] [Google Scholar]
- 21.Reinecker H C, Steffen M, Witthoeft T.et al Enhanced secretion of tumour necrosis factor‐alpha, IL‐6, and IL‐1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin Exp Immunol 199394174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fedorak R N, Ganl A, Elson C O.et al Recombinant human interleukin‐10 in the treatment of patients with mild to moderate active Crohn's disease. Gastroenterology 20001191473–1482. [DOI] [PubMed] [Google Scholar]
- 23.Schreiber S, Rutgeerts P, Fedorak R N.et al CDP870 Crohn's Disease Study Group. A randomized, placebo‐controlled trial of certolizumab pegol (CDP870) for treatment of Crohn's disease. Gastroenterology 2005129807–818. [DOI] [PubMed] [Google Scholar]
- 24.Sandborn W J, Hanauer S B, Katz S.et al Etanercept for active Crohn's disease: a randomized, double‐blind, placebo‐controlled trial. Gastroenterology 20011211088–1094. [DOI] [PubMed] [Google Scholar]
- 25.Van den Brande J M, Bratt H, van den Brink G R.et al Infliximab but not etanercept induces apoptosis in lamina propria T‐lymphocytes from patients with Crohn's disease. Gastroenterology 20031261774–1785. [DOI] [PubMed] [Google Scholar]
- 26.Gelfand J M, Weinstein R, Porter S B.et al Prevalence and treatment of psoriasis in the United Kingdom: a population‐based study. Arch Dermatol 20051411537–1541. [DOI] [PubMed] [Google Scholar]
- 27.Zachariae H. Prevalence of joint disease in patients with psoriasis: implications for therapy. Am J Clin Dermatol 20034441–447. [DOI] [PubMed] [Google Scholar]
- 28.Krueger G, Ellis C N. Psoriasis – recent advances in understanding its pathogenesis and treatment. J Am Acad Dermatol 200553(Suppl 1)S94–100. [DOI] [PubMed] [Google Scholar]
- 29.Gudjonsson J E, Johnson A, Sigmundsdottir H.et al Immunopathogenic mechanisms in psoriasis. Clin Exp Immunol 20041351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veale D J, Ritchlin C, Fitzgerald O. Immunopathology of psoriasis and psoriatic arthritis. Ann Rheum Dis 200564(Suppl 2)ii26–ii29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naldi L, Griffiths C E. Traditional therapies in the management of moderate to severe chronic plaque psoriasis: an assessment of the benefits and risks. Br J Dermatol 2005152597–615. [DOI] [PubMed] [Google Scholar]
- 32.Abrams J R, Lebwohl M G, Guzzo C A.et al CTLA4‐Ig‐mediated blockade of T‐cell costimulation in patients with psoriasis vulgaris. J Clin Invest 19991031243–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maini R, St Clair E W, Breedveld F.et al Infliximab (chimeric anti‐tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet 19993541932–1939. [DOI] [PubMed] [Google Scholar]
- 34.Maini R N, Breedveld F C, Kalden J R.et al ATTRACT Study Group. Sustained improvement over two years in physical function, structural damage, and signs and symptoms among patients with rheumatoid arthritis treated with infliximab and methotrexate. Arthritis Rheum 2004501051–1065. [DOI] [PubMed] [Google Scholar]
- 35.St Clair E W, van der Heijde D M, Smolen J S.et al Active‐Controlled Study of Patients Receiving Infliximab for the Treatment of Rheumatoid Arthritis of Early Onset Study Group. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial, Arthritis Rheum 2004503432–3443. [DOI] [PubMed] [Google Scholar]
- 36.Smolen J S, van der Heijde D M, St Clair E W.et al ASPIRE Study Group. Predictors of joint damage in patients with early rheumatoid arthritis treated with high‐dose methotrexate with or without concomitant infliximab: results from the ASPIRE trial, Arthritis Rheum 200654702–710. [DOI] [PubMed] [Google Scholar]
- 37.Gottlieb A B, Evans R, Li S.et al Infliximab induction therapy for patients with severe plaque‐type psoriasis ‐ a randomized, double‐blind, placebo‐controlled trial. J Am Acad Dermatol 200451534–542. [DOI] [PubMed] [Google Scholar]
- 38.Reich K, Nestle F O, Papp K.et al EXPRESS study investigators. Infliximab induction and maintenance therapy for moderate‐to‐severe psoriasis: a phase III, multicentre, double‐blind trial. Lancet 20053661367–1374. [DOI] [PubMed] [Google Scholar]
- 39.Antoni C E, Kavanaugh A, Kirkham B.et al Sustained benefits of infliximab therapy for dermatologic and articular manifestations of psoriatic arthritis: results from the infliximab multinational psoriatic arthritis controlled trial (IMPACT). Arthritis Rheum 2005521227–1236. [DOI] [PubMed] [Google Scholar]
- 40.Antoni C, Krueger G G, de Vlam K.et al IMPACT 2 Trial Investigators. Infliximab improves signs and symptoms of psoriatic arthritis: results of the IMPACT 2 trial. Ann Rheum Dis2005641150–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kavanaugh A, Antoni C, Krueger G G.et al Infliximab improves health related quality of life and physical function in patients with psoriatic arthritis. Ann Rheum Dis 200664471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Targan S R, Hanauer S B, van Deventer S J.et al A short‐term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med 19973371029–1035. [DOI] [PubMed] [Google Scholar]
- 43.Hanauer S B, Feagan B G, Lichtenstein G R.et al ACCENT I Study Group. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet 20023591541–1549. [DOI] [PubMed] [Google Scholar]
- 44.Goboes K, Rutgeerts P, Opdenakker G.et al Endoscopic and histologic evidence of persistent mucosal healing and correlation with clinical improvement following sustained infliximab treatment for Crohn's disease. Curr Med Res Opin 2005211741–1754. [DOI] [PubMed] [Google Scholar]
- 45.Sands B E, Anderson F H, Bernstein C N.et al Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med 2004350876–885. [DOI] [PubMed] [Google Scholar]
- 46.Lichtenstein G R, Yan S, Bala M.et al Infliximab maintenance treatment reduces hospitalizations, surgeries, and procedures in fistulizing Crohn's disease. A randomized, controlled trial. Gastroenterology 2005128862–869. [DOI] [PubMed] [Google Scholar]
- 47.Moreland L W, Schiff M H, Baumgartner S W.et al Etanercept therapy in rheumatoid arthritis. A randomized, controlled trial. Ann Intern Med 1999130478–486. [DOI] [PubMed] [Google Scholar]
- 48.Klaresog L, van der Heijde D, de Jager J P.et al TEMPO (Trial of Etanercept and Methotrexate with Radiographic Patient Outcomes) study investigators. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double‐blind randomised controlled trial. Lancet 2004363675–681. [DOI] [PubMed] [Google Scholar]
- 49.Bathon J M, Martin R W, Fleischmann R M.et al A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med 20003431586–1593. [DOI] [PubMed] [Google Scholar]
- 50.Genovese M C, Bathon J M, Martin R W.et al Etanercept versus methotrexate in patients with early rheumatoid arthritis: two‐year radiographic and clinical outcomes. Arthritis Rheum 2002461143–1150. [DOI] [PubMed] [Google Scholar]
- 51.Genovese M C, Bathon J M, Fleischmann R M.et al Longterm safety, efficacy, and radiographic outcome with etanercept treatment in patients with early rheumatoid arthritis. J Rheumatol 2005321232–1242. [PubMed] [Google Scholar]
- 52.Leonardi C L, Powers J L, Matheson R T.et al Etanercept Psoriasis Study Group. Etanercept as monotherapy in patients with psoriasis. N Engl J Med 20033492014–2022. [DOI] [PubMed] [Google Scholar]
- 53.Papp K A, Tyring S, Lahfa M.et al Etanercept Psoriasis Study Group. A global phase III randomized controlled trial of etanercept in psoriasis: safety, efficacy, and effect of dose reduction. Br J Dermatol 20051521304–1312. [DOI] [PubMed] [Google Scholar]
- 54.Mease P J, Kivitz A J, Burch F X.et al Etanercept treatment of psoriatic arthritis: safety, efficacy, and effect on disease progression. Arthritis Rheum 2004502264–2272. [DOI] [PubMed] [Google Scholar]
- 55.Weinblatt M E, Keystone E C, Furst D E.et al Adalimumab, a fully human anti‐tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum 20034835–45. [DOI] [PubMed] [Google Scholar]
- 56.Keystone E D, Kavanaugh A F, Sharp J T.et al Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti‐tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo‐controlled, 52‐week trial. Arthritis Rheum 2004501400–1411. [DOI] [PubMed] [Google Scholar]
- 57.van der Putte L B, Atkins C, Malaise M.et al Efficacy and safety of adalimumab as monotherapy in patients with rheumatoid arthritis for whom previous disease modifying antirheumatic drug treatment has failed. Ann Rheum Dis 200463508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Breedveld F C, Weisman M H, Kavanaugh A F.et al The PREMIER study: a multicenter, randomized, double‐blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum 20065426–37. [DOI] [PubMed] [Google Scholar]
- 59.Mease P J, Gladman D D, Ritchlin C T.et al Adalimumab effectiveness in Psoriatic Arthritis Trial Study Group. Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double‐blind, randomized, placebo‐controlled trial. Arthritis Rheum 2005523279–3289. [DOI] [PubMed] [Google Scholar]
- 60.Hanauer S B, Sandborn Q J, Rutgeerts P.et al Human anti‐tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC‐I trial. Gastroenterology 2006130323–333. [DOI] [PubMed] [Google Scholar]
- 61.Edwards J C, Szcepanski L, Szechinski J.et al Efficacy of B‐cell‐targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med 20043502572–2581. [DOI] [PubMed] [Google Scholar]
- 62.Emery P, Fleischmann R, Filipowicz‐Sosnowska A.et al DANCER Study Group. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double‐blind, placebo‐controlled, dose‐ranging trial. Arthritis Rheum 2006541390–1400. [DOI] [PubMed] [Google Scholar]
- 63.Cohen S B, Greenwald M, Dougados M R.et al Efficacy and safety of rituximab in active RA patients who experienced an inadequate response to one or more anti‐TNFα therapies (REFLEX Study). Arthritis Rheum 200552(Suppl)S677 [Google Scholar]
- 64.Liu M F, Kohsaka H, Sakurai H.et al The presence of costimulatory molecules CD86 and CD28 in rheumatoid arthritis synovium. Arthritis Rheum 199639110–114. [DOI] [PubMed] [Google Scholar]
- 65.Verwilghen J, Lovis R, De Boer M.et al Expression of functional B7 and CTLA4 on rheumatoid synovial T cells. J Immunol 19941531378–1385. [PubMed] [Google Scholar]
- 66.Salomon B, Bluestone J A. Complexities of CD28/B7: CTLA‐4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol 200119225–252. [DOI] [PubMed] [Google Scholar]
- 67.Gribben J G, Freeman G J, Boussiotis V A.et al CTLA4 mediates antigen‐specific apoptosis of human T cells. Proc Natl Acad Sci U S A 199592811–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kremer J M, Dougados M, Emery P.et al Treatment of rheumatoid arthritis with the selective costimulation modulator abatacept: twelve‐month results of a phase IIb, double‐blind, randomized, placebo‐controlled trial. Arthritis Rheum 2005522263–2271. [DOI] [PubMed] [Google Scholar]
- 69.Kremer J M, Genant H K, Moreland L W.et al Effects of abatacept in patients with methotrexate‐resistant active rheumatoid arthritis: a randomized trial. Ann Intern Med 2006144865–876. [DOI] [PubMed] [Google Scholar]
- 70.Genovese M C, Becker J C, Schiff M.et al Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med 20053531114–1123. [DOI] [PubMed] [Google Scholar]
- 71.Gordon K B, Papp K A, Hamilton T K.et al Efalizumab Study Group. Efalizumab for patients with moderate to severe plaque psoriasis: a randomized controlled trial, JAMA 20032903073–3080. [DOI] [PubMed] [Google Scholar]
- 72.Krueger G G, Papp K A, Stough D B.et al Alefacept Clinical Study Group. A randomized, double‐blind, placebo‐controlled phase III study evaluating efficacy and tolerability of 2 courses of alefacept in patients with chronic plaque psoriasis. J Am Acad Dermatol 200247821–833. [DOI] [PubMed] [Google Scholar]
- 73.Schneider M, Stahl H, Scaramucci J.et al Alefacept in subjects with active rheumatoid arthritis (abstract 1709). Presented at the American College of Rheumatology Annual Scientific Meeting, Orlando, FL, October 23–28 2003
- 74.Mease P J, Gladman D D, Keystone E C. Alefacept in combination with methotrexate for the treatment of psoriatic arthritis: results of a randomized, double‐blind, placebo‐controlled study. Arthritis Rheum 2006541638–1645. [DOI] [PubMed] [Google Scholar]
- 75.Schreiber S. Certolizumab pegol, a humanised anti‐TNF pegylated Fab' fragment, is safe and effective in the maintenance of response and remission following induction in active Crohn's disease: a phase 3 study (PRECISE) (abstract OP‐G‐355). Presented at the 13th United European Gastroenterology Week, Copenhagen, October 15–19 2005
- 76.Choy E H, Hazleman B, Smith M.et al Efficacy of a novel PEGylated humanized anti‐TNF fragment (CDP870) in patients with rheumatoid arthritis: a phase II double‐blinded, randomized, dose‐escalating trial. Rheumatology 2002411133–1137. [DOI] [PubMed] [Google Scholar]
- 77.Bresnihan B, Alvaro‐Gracia J M, Cobby M.et al Treatment of rheumatoid arthritis with recombinant human interleukin‐1 receptor antagonist. Arthritis Rheum 1998412196–2204. [DOI] [PubMed] [Google Scholar]
- 78.Fleischmann R M, Schechtman J, Bennett R.et al Anakinra, a recombinant human interleukin‐1 receptor antagonist (r‐metHuIl‐1ra), in patients with rheumatoid arthritis: a large, international, multicenter, placebo‐controlled trial. Arthritis Rheum 200348927–934. [DOI] [PubMed] [Google Scholar]
- 79.Gardam M A, Keystone E C, Menzies R.et al Anti‐tumour necrosis factor agents and tuberculosis risk: mechanisms of action and clinical management. Lancet Infect Dis 20033148–155. [DOI] [PubMed] [Google Scholar]
- 80.Dixon W G, Watson K, Lunt M.et al Rates of serious infection, including site‐specific and bacterial intracellular infection, in rheumatoid arthritis patients receiving anti‐tumor necrosis factor therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum 2006542368–2376. [DOI] [PubMed] [Google Scholar]
- 81.Hyrich K L, Silman A J, Watson K D.et al Anti‐tumour necrosis factor alpha therapy in rheumatoid arthritis: an update on safety. Ann Rheum Dis 2004631538–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Genovese M C, Cohen S, Moreland L.et al 20000223 Study Group. Combination therapy with etanercept and anakinra in the treatment of patients with rheumatoid arthritis who have been treated unsuccessfully with methotrexate. Arthritis Rheum 2004501412–1419. [DOI] [PubMed] [Google Scholar]
- 83.Hyrich K L. Assessing the safety of biologic therapies in rheumatoid arthritis: the challenges of study design. J Rheumatol Suppl 20057248–50. [PubMed] [Google Scholar]
- 84.Kavanaugh A. Health economics: implications for novel antirheumatic therapies. Ann Rheum Dis 200564(Suppl 4)iv65–iv69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kobelt G, Jonsson L, Young A.et al The cost‐effectiveness of infliximab (Remicade) in the treatment of rheumatoid arthritis in Sweden and the United Kingdom based on the ATTRACT study. Rheumatology 200342326–335. [DOI] [PubMed] [Google Scholar]
- 86.Javitz H S, Ward M M, Farber E.et al The direct cost of care for psoriasis and psoriatic arthritis in the United States. J Am Acad Dermatol 200246850–860. [DOI] [PubMed] [Google Scholar]
- 87.Cooper N J. Economic burden of rheumatoid arthritis: a systematic review. Rheumatology 20003928–33. [DOI] [PubMed] [Google Scholar]
- 88.Bodger K. Cost of illness of Crohn's disease. Pharmacoeconomics 200220639–652. [DOI] [PubMed] [Google Scholar]
- 89.McIntosh E. The cost of rheumatoid arthritis. Br J Rheumatol 199635781–790. [DOI] [PubMed] [Google Scholar]
- 90.Hay J W, Hay A R. Inflammatory bowel disease: costs‐of‐illness. J Clin Gastroenterol 199214309–317. [DOI] [PubMed] [Google Scholar]
- 91.Brook A, Corbett M. Radiographic changes in early rheumatoid disease. Ann Rheum Dis 19773671–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Boers M, Verhoeven A C, Markusse H M.et al Randomised comparison of combined step‐down prednisolone, methotrexate and sulphasalazine with sulphasalazine alone in early rheumatoid arthritis. Lancet 1997350309–318. [DOI] [PubMed] [Google Scholar]
- 93.Mottonen T, Hannonen P, Leirisalo‐Repo M.et al Comparison of combination therapy with single‐drug therapy in early rheumatoid arthritis: a randomised trial. FIN‐RACo trial group. Lancet 19993531568–1573. [DOI] [PubMed] [Google Scholar]
- 94.Goekoop‐Ruiterman Y P, de Vries‐Bouwstra J K, Allaart C F.et al Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum 2005523381–3390. [DOI] [PubMed] [Google Scholar]