Abstract
Tako‐tsubo syndrome (TTS) or stress‐related acute reversible ventricular apical dysfunction is an emerging but seemingly under‐recognised cardiomyopathy mimicking acute ST elevation myocardial infarction (STEMI) without concomitant epicardial coronary artery disease. Severe emotional stress is the most common trigger for this syndrome in the published series, but it can also be precipitated by severe intercurrent medical illness. Precise epidemiological data are not yet available, although TTS most commonly affects elderly women. The exact cause of this syndrome is undetermined, but proposed mechanisms include epicardial coronary artery vasospasm, impaired multivessel coronary microcirculation, calcium overload with direct myocyte damage and disrupted fatty acid metabolism with prolonged myocardial stunning. The time course of electrocardiographic changes is very similar to that of an acute STEMI due to an acute occlusion of the left anterior descending coronary artery. The left ventricular dysfunction typically displays an akinetic apical half of the left or both ventricles with hyperkinetic basal segments, although a variant with apical sparing has also been described recently. The ventricular dysfunction usually resolves within weeks and carries a generally favourable prognosis.
Transient acute left ventricular apical ballooning in the absence of significant coronary artery disease was first described by Hikaru Sato and colleagues in 1990.1 Sato termed this syndrome tako‐tsubo because of the similarity in appearance of the left ventricle to that of a narrow‐necked, wide‐based clay container used by Japanese fishermen to trap octopus (Japanese tako: octopus; tsubo: pot). Since then, only sporadic cases were published by Japanese authors, and just a few non‐Asian publications are currently available. Those studies suggest that tako‐tsubo syndrome (TTS) is actually more frequent than previously thought and may have simply gone unnoticed in the western world due to lack of awareness. The incidence in Japan is estimated to be as high as 1–2% of all admissions for suspected acute ST elevation myocardial infarctions (STEMIs).2 There is increasing evidence from studies with Caucasian patients from the United States, Europe and Australia indicating that TTS is unlikely to be a geographically isolated phenomenon.
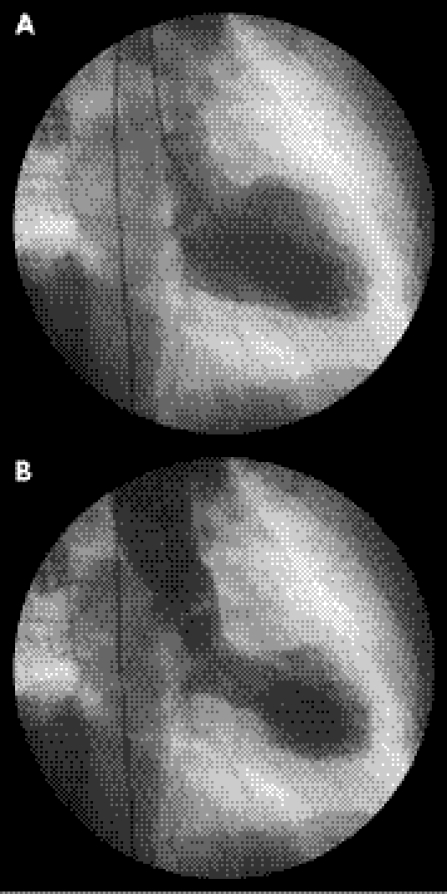
Bybee et al3 have proposed four main diagnostic criteria for TTS: (a) transient apical and midventricular a/dyskinesis that extend beyond the distribution of a single coronary artery (fig 1), (b) absence of significant coronary artery disease or acute plaque rupture on coronary angiography, (c) electrocardiographic evidence of new ST elevation or T wave inversion and (d) absence of recent head trauma or intracranial haemorrhage, phaeochromocytoma, myocarditis or evidence of hypertrophic cardiomyopathy.
Figure 1 Left ventriculography demonstrating apical ballooning in diastole (A) and systole (B).
Precise epidemiological data are not yet available. The largest case series to date was published in 2001 originating from Japan.4 A total of 88 patients, who fulfilled criteria similar to those aforementioned for diagnosing TTS, were analysed (12 men and 76 women, aged 67 (SD 13) years). In all, 43% had preceding medical problems such as epilepsy, exacerbation of bronchial asthma or acute stroke syndromes, and 27% presented with acute emotional and physical stress. Interestingly, 28% of all patients did not show a significant rise in troponin T levels. After treatment of acute pulmonary oedema (22%), cardiogenic shock (15%) and ventricular tachycardia/fibrillation (9%), 85 patients had class I New York Heart Association function on discharge. Left ventricular ejection fraction improved from an average of 41% to 64% at 24 (+/−11) days.
A European study retrospectively reviewed almost 17 000 cases with diagnostic coronary angiographies for intermittent left ventricular apical ballooning in conjunction with normal coronary arteries.5 They identified 32 patients fulfilling the criteria for TTS (incidence 0.2%). The majority were women (>90%) with a median age of >67 years, which is in keeping with all the other available published case reports and series to date. Almost half of this study's patients had a history of chronic obstructive pulmonary disease or bronchial asthma, raising the possibility of an increased risk between TTS and chronic pulmonary disease. Another study found the prevalence of arterial hypertension in patients with acute TTS to be as high as 76%.6
Park et al7 prospectively evaluated the incidence of stress‐induced cardiomyopathy in an intensive care setting in patients acutely admitted with a non‐cardiac diagnosis and with no history of cardiovascular disease. About a third of their patients had acute left ventricular apical ballooning with an average ejection fraction of 33% on initial echocardiography. The left ventricular function normalised in the majority of these patients within 7 days.
Although our own observations suggest that TTS can be triggered by exacerbation of pulmonary disease, urosepsis and pneumonia, emotional rather than physical stress seems to be the precipitant in the majority of cases reported. A study by Wittstein et al8 concluded that extreme emotional stress triggered severe yet reversible left ventricular dysfunction in 19 patients (median age 63 years, 95% women) without coronary artery disease. Those triggers included events such as car accidents, surprise reunions, death of a close relative or friend, armed robbery, fear of procedure, public speaking and court appearance. Three of these patients required intra‐aortic balloon pump counterpulsation for cardiogenic shock and one patient developed ventricular fibrillation. The median left ventricular ejection fraction was found to be only 20% during the acute phase. Plasma catecholamine levels were significantly higher than among a control group with Killip class III myocardial infarction due to acute coronary occlusion. This finding could indicate a possible catecholamine‐mediated process either due to epicardial or microvascular coronary artery spasm or due to direct myocyte damage from intracellular calcium overload. On the other hand, none of the 19 patients had angiographic evidence of epicardial spasm, and another study only confirmed a limited number (10/48, 21%) of coronary spasms in response to acetylcholine provocative testing.4
Another proposed mechanism for the development of TTS is impaired myocardial fatty acid metabolism. Using thallium‐201 and iodine‐123‐beta‐methyl‐p‐iodophenyl penta‐decanoic acid (201TI and 123I‐BMIPP) myocardial single‐photon emission computed tomography in 14 patients with TTS, Kurisu et al9 demonstrated a more severely impaired and prolonged defect in fatty acid metabolism (using 123I‐BMIPP) when directly compared with myocardial perfusion (using 201TI), especially in the early phase of TTS. They suggest that the left ventricular dysfunction may essentially be stunned myocardium. This finding was also suggested by another study using 123I‐metaiodobenzylguanidine, demonstrating significant sympathetic cardiac adrenergic dysfunction most likely caused by neurogenic myocardial stunning.10
One study also measured the Thrombolysis in Myocardial Infarction Trial frame count, an index of coronary blood flow velocity, representing the number of angiographic frames required for contrast to reach standardised distal landmarks.9 The authors found the Thrombolysis in Myocardial Infarction Trial frame count to be significantly higher in all coronary arteries in patients with TTS compared with control subjects, even after resolution of the left ventricular dysfunction. They speculate that impaired multivessel coronary artery microcirculation may be one causative mechanism.
Ueyama11 found that acute left ventricular ballooning in rats induced by immobilisation stress can be abolished using combined α and β adrenoceptor blockade, concluding that the mechanism for the development of TTS is based on adrenoceptor hyperactivity. In a different experimental approach, they demonstrated that the degree of stress‐induced ventricular dysfunction and tachycardia in oestradiol‐supplemented ovariectomised female rats was significantly reduced compared with non‐oestradiol‐supplemented rats.12 From these results, they speculated that a reduction of oestrogen levels may underlie the high incidence of TTS in postmenopausal women.
Furthermore, Ibanez et al13 prospectively evaluated in five TTS patients the hypothesis that a ruptured coronary plaque with angiographically insignificant coronary atherosclerosis and normal coronary blood flow could be part of the underlying aetiology. They found in all patients a well‐developed left anterior descending artery (LAD) with a large diaphragmatic course and, utilising intravascular ultrasound, a solitary, ruptured, non‐occlusive atherosclerotic plaque in the middle portion of the LAD. These findings would lend gravitas to the theory that in some patients with TTS, the underlying cause could well be an acute coronary syndrome (ACS) with early reperfusion and wide left ventricular stunned myocardium in the presence of a large wrap‐around LAD. Indeed, clinical data seem to indicate that only a fraction of ACSs are actually caused by critical coronary artery stenoses.14 It is rather the plaque disruption due to fibrous cap fracture or superficial intimal erosion at sites of non‐critical narrowing of coronary arteries that frequently triggers an ACS and that might go unnoticed on angiography once the acute thrombus has been resolved.15
It is worth mentioning though that the ventricular asynergy in TTS may not be limited to the left ventricle alone, since our own echocardiographic studies of yet unpublished acute cases of TTS and other previously published data suggest a biventricular involvement.16 A more recently published Australian study has also demonstrated the phenomenon of apical sparing with the TTS.17
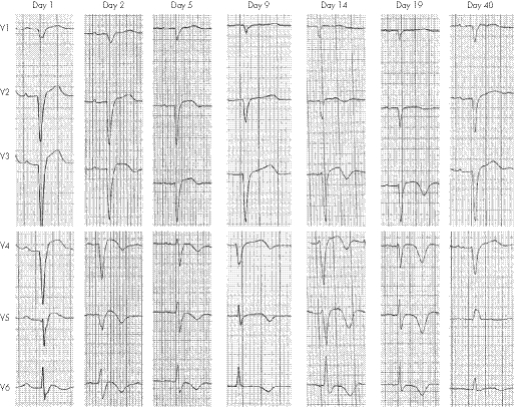
The time course of electrocardiographic changes in patients with TTS (fig 2) compared with that in patients with STEMI treated with early percutaneous coronary intervention (PCI) seems similar.18 Initial ECG changes are that of acute ST elevation, especially in leads V3–V6, followed by progressive T wave inversion to a first negative peak at 3 days. The T waves then shallow for several days, with a second negative peak at 2–3 weeks. The corrected QT interval becomes prolonged as the T waves deepen, and shortens as the T waves become shallower. Left ventricular dysfunction significantly resolved in both patient groups approximately 2 weeks after initial presentation.
Figure 2 Electrocardiographic changes in tako‐tsubo syndrome over a 40‐day period.
The fatality rate from TTS is much lower than that of acute myocardial infarction: 1 of 25 patients and 2 of 88 patients in two Japanese studies,2,4 1 of 10 patients in a French study19 and none of 19 patients in an American study8 died as a consequence of an acute TTS.
The optimal management of an acute TTS is unclear and is based only on outcomes of anecdotal case reports and small series and therefore, at the present time, no official treatment guidelines have been published. Given the underlying proposed mechanisms for the development of this syndrome, treatment should be supportive and, in our opinion, closely related to the treatment of an ACS. Patients presenting with chest pain and ST elevation should be managed as cases of an acute anterior infarct. Urgent coronary angiography and ventriculography, if available, is the management strategy of choice, as it will provide the diagnosis and avoid exposure to thrombolysis. It will also allow appropriate revascularisation in the event of an acute occlusion of the LAD. However, if urgent angiography is not available, thrombolysis should not be withheld as the vast majority of patients with chest pain and anterior ST elevation will have acute occlusion of the LAD and should not be denied reperfusion treatment given that there are no clinical, ECG or echocardiographic features that will reliably differentiate the two syndromes.
Treatment recommendations would also include appropriate antiplatelet treatment with aspirin and clopidogrel depending on the clinical context. Having said that, TTS has been associated with the development of an acute left ventricular mural thrombus,20,21 which in turn can be further complicated by acute renal infarction22 and acute ischaemic stroke.23 In view of this, short‐term therapeutic anticoagulation treatment with heparin or warfarin (rather than antiplatelet treatment) might help prevent ventricular mural thrombus formation until such time when the left ventricular dysfunction improves or resolves.
Furthermore, in our view, β blockers should be given in the acute and also in the chronic phase unless contraindicated, and probably indefinitely, given the fact that TTS can reoccur. Special attention should be paid by the attending physicians to the initial acute phase of QT interval prolongation, as commonly used drugs such as amiodarone or sotalol might cause complications such as torsade‐de‐pointes by further prolonging the repolarisation phase. Given the evidence of severe left ventricular dysfunction, use of ACE inhibitors or, alternatively, angiotensin receptor blockers seems logical.
Learning points
In patients presenting with suspected ST elevation myocardial infarction (STEMI) who are treated with primary non‐facilitated percutaneous coronary intervention (PCI), angiography will suggest the diagnosis of tako‐tsubo syndrome (TTS) by showing no significant coronary artery disease.
A suspicion of TTS in patients presenting with STEMI is not sufficient reason to withhold primary PCI or thrombolytic therapy, as the vast majority of STEMI presentations will be due to underlying coronary artery disease.
Management is usually supportive with most patients being treated with appropriate antiplatelet treatment, ACE inhibitors or angiotensin‐receptor blockers. β‐Blockers should be given in the acute and also in the chronic phase unless contraindicated. Short‐term prophylactic or therapeutic anticoagulation treatment with heparin or warfarin might help prevent left ventricular thrombus formation. Drugs that potentially prolong the QT interval should be avoided, especially in the acute phase of the TTS.
Given the probable sympathetic overactivity, avoiding β‐agonists or inotropic substances would seem appropriate, although there is no evidence from randomised controlled trials to support this assumption. Special care should of course be taken in patients with acute cardiogenic shock, and the use of intra‐aortic balloon counterpulsation has been associated with a positive outcome.
More information about the underlying pathophysiology and optimal supportive treatment is needed, as these may not necessarily be the same for all patients.
Abbreviations
ACS - acute coronary syndrome
LAD - left anterior descending artery
PCI - percutaneous coronary intervention
STEMI - ST elevation myocardial infarction
TTS - tako‐tsubo syndrome
Footnotes
Competing interests: None.
References
- 1.Sato H, Tateishi H, Uchida T.et al Takotsubo type cardiomyopathy due to multivessel spasm. In: Kodama K, Haze K, Hon M, eds. Clinical aspect of myocardial injury: from ischaemia to heart failure Tokyo: Kagakuhyouronsya, 1990, 56–64 (in Japanese)
- 2.Kawai S. Ampulla‐shaped ventricular dysfunction or ampulla cardiomyopathy? Respir Circ 2000481237–48 in Japanese. [Google Scholar]
- 3.Bybee K A, Kara T, Prasad A.et al Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST‐segment elevation myocardial infarction. Ann Intern Med 2004141858. [DOI] [PubMed] [Google Scholar]
- 4.Tsuchihashi K, Ueshima K, Uchida T.et al Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. J Am Coll Cardiol 20013811–18. [DOI] [PubMed] [Google Scholar]
- 5.Hertting K, Krause K, Harle T.et al Transient left ventricular apical ballooning in a community hospital in Germany. Int J Cardiol 2006112282–288. [DOI] [PubMed] [Google Scholar]
- 6.Stollberger C, Finsterer J, Schneider B. Tako‐tsubo‐like left ventricular dysfunction: clinical presentation, instrumental findings, additional cardiac and non‐cardiac diseases and potential pathomechanisms. Minerva Cardioangiol 200553139–145. [PubMed] [Google Scholar]
- 7.Park J H, Kang S J, Song J K.et al Left ventricular apical ballooning due to severe physical stress in patients admitted to the medical ICU. Chest 2005128296–302. [DOI] [PubMed] [Google Scholar]
- 8.Wittstein I S, Thiemann D R, Lima J A C.et al Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 2005352539–548. [DOI] [PubMed] [Google Scholar]
- 9.Kurisu S, Inoue I, Kawagoe T.et al Myocardial perfusion and fatty acid metabolism in patients with tako‐tsubo‐like left ventricular dysfunction. J Am Coll Cardiol 200341743–748. [DOI] [PubMed] [Google Scholar]
- 10.Akashi Y J, Nakazawa K, Sakakibara M.et al123I‐MIBG myocardial scintigraphy in patients with “takotsubo” cardiomyopathy. J Nucl Med 2004451121–1127. [PubMed] [Google Scholar]
- 11.Ueyama T. Emotional stress‐induced Tako‐tsubo cardiomyopathy: animal model and molecular mechanism. Ann N Y Acad Sci 20041018437–444. [DOI] [PubMed] [Google Scholar]
- 12.Ueyama T, Hano T, Kasamatsu K.et al Estrogen attenuates the emotional stress‐induced cardiac responses in the animal model of tako‐tsubo (Ampulla) cardiomyopathy. J Cardiovasc Pharmacol 200342(Suppl 1)117–119. [DOI] [PubMed] [Google Scholar]
- 13.Ibanez B, Navarro F, Cordoba M.et al Tako‐tsubo transient left ventricular apical ballooning: is intravascular ultrasound the key to resolve the enigma? Heart 200591102–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Libby P. Act local, act global: inflammation and the multiplicity of “vulnerable” coronary plaques. J Am Coll Cardiol 2005451600–1602. [DOI] [PubMed] [Google Scholar]
- 15.Nissen S E. Pathobiology, not angiography, should guide management in acute coronary syndrome/non‐ST‐segment elevation myocardial infarction. J Am Coll Cardiol 200341103S–12S. [DOI] [PubMed] [Google Scholar]
- 16.Nyui N, Yamanaka O, Nakayama R.et al ‘Tako‐tsubo' transient ventricular dysfunction: a case report. Jpn Circ J 200064715–719. [DOI] [PubMed] [Google Scholar]
- 17.Abdulla I, Kay S, Mussap C.et al Apical sparing in tako‐tsubo cardiomyopathy. Int Med J 200636414–418. [DOI] [PubMed] [Google Scholar]
- 18.Kurisu S, Inoue I, Kawagoe T.et al Time course of electrocardiographic changes in patients with tako‐tsubo syndrome: comparison with acute myocardial infarction with minimal enzymatic release. Circ J 20046877–81. [DOI] [PubMed] [Google Scholar]
- 19.Lipiecki J, Durel N, Decalf V.et al Transient left ventricular apical ballooning or the tako‐tsubo syndrome. Arch Mal Coeru Vaiss 200598275–280. [PubMed] [Google Scholar]
- 20.Tibrewala A V, Moss B N, Cooper H A. A rare case of tako‐tsubo cardiomyopathy complicated by a left ventricular thrombus. South Med J 2006992–3. [DOI] [PubMed] [Google Scholar]
- 21.Yasuga Y, Inoue M, Takeda Y.et al Tako‐tsubo‐like transient left ventricular dysfunction with apical thrombus formation: a case report. J Cardiol 20044375–80. [PubMed] [Google Scholar]
- 22.Sasaki N, Kinugawa T, Yamawaki M.et al Transient left ventricular apical ballooning in a patient with bicuspid aortic valve created a left ventricular thrombus leading to acute renal infaction. Circ J 2004681081–1083. [DOI] [PubMed] [Google Scholar]
- 23.Kurisu S, Inoue I, Kawagoe T.et al Left ventricular apical thrombus formation in a patient with suspected tako‐tsubo‐like left ventricular dysfunction. Circ J 200367556–558. [DOI] [PubMed] [Google Scholar]