Abstract
Background and aims
Data regarding the effect of peroxisome proliferator‐activated receptor γ (PPAR‐γ) ligands on the invasive ability of colon cancer cells are currently limited. This study was designed to examine the effects of PPAR‐γ agonists on the proliferation and invasion of two colon cancer cells to identify the role of PPAR‐γ in colon cancer growth and metastasis.
Methods
SW480 and LS174T cells were treated with PPAR‐γ ligands, pioglitazone and 15‐deoxy‐δ(12,14)‐prostaglandin J2 (15d‐PGJ2), as well as their combinations with the PPAR‐γ antagonist GW9662. MTT assay was used to determine the antiproliferative effects. Cell cycle analysis was conducted by flow cytometry. The mRNA and protein expression were detected by reverse transcriptase polymerase chain reaction (RT‐PCR) and western blot, respectively. The invasive ability of cells was determined by the BD BioCoat Matrige invasion chamber.
Results
Pioglitazone and 15d‐PGJ2 inhibited the proliferation of both colon cancer cell lines in a dose‐dependent manner. This growth inhibitory effect was reversed by GW9662. Results from flow cytometry demonstrated G1 arrest following treatment with pioglitazone and 15d‐PGJ2. The expression of matrix metalloproteinase‐7 (MMP‐7) was only detected in LS174T cells, while its tissue inhibitor‐1 (TIMP‐1) was expressed in both colon cancer cells. 15d‐PGJ2 and pioglitazone downregulated MMP‐7 expression and upregulated TIMP‐1 expression. PPAR‐γ agonists can only inhibit invasive activity of LS174T cells.
Conclusions
PPAR‐γ agonists have inhibitory effects on the proliferation of colon cancer cell lines associated with G1 cell cycle arrest and invasive activity. The latter effect is demonstrated in certain cell lines through the down‐regulation of MMP‐7 synthesis.
The conventional treatments of colorectal cancer have only limited effectiveness. Between 25–35% patients experience haematogenous metastasis with worse prognosis,1 resulting in a need for new therapeutic approaches for this highly prevalent disease. Current reports indicate that the incidence and mortality of colorectal cancer is generally greater among people with diabetes.2 This suggests that the application of some anti‐diabetic agents may be promising in the development of new strategies to inhibit the growth and metastasis of colorectal cancers. Recently a number of experimental models (such as colonic, gastric, pancreatic, breast and testicular) further supported the suggestion that modulation of the peroxisome proliferator‐activated receptor γ (PPAR‐γ), which affects the regulation of lipid and glucose metabolism,3 plays an important role in carcinogenesis.4,5,6,7,8 Therefore, PPAR‐γ ligands may be able to prevent and treat colorectal cancer. Although contradictory results from the adenomatous polyposis coli (APCmin/+) mice suggested that PPAR‐γ agonists promoted colorectal tumours,9 results from human cell lines and nude mice indicated that PPAR‐γ agonists might have therapeutic value for the treatment of established colorectal cancers.10 A recent study also showed that PPAR‐γ ligands inhibited the invasion and metastasis of human breast cancer cells.11 However, there is no definitive evidence to show the effect of PPAR‐γ agonists on the invasion of human colon cancer cells. In this study we investigated the growth inhibitory effect of PPAR‐γ agonists, 15‐deoxy‐δ(12,14)‐prostaglandin J2 (15d‐PGJ2) and pioglitazone, on SW480 and LS174T colon cancer cells, both of which were APC mutant. We further demonstrated the anti‐invasive activities of PPAR‐γ agonists on colon cancer cells, and explored the roles of matrix metalloproteinase‐7 (MMP‐7) and its tissue inhibitor‐1 (TIMP‐1) during the procedure.
MATERIALS AND METHODS
Materials and reagents
The human colon adenocarcinoma cell lines SW480 and LS174T were purchased from Wuhan University Cultures Center, Wuhan, China. PPAR‐γ agonist 15d‐PGJ2 was obtained from Oncogene Science (Cambridge, Massachusetts, USA), while pioglitazone was kindly donated by the Deyuan Medical Company (Lian‐Yun‐Gang, China). GW9662 was a product of Sigma‐Aldrich, Inc (St Louis, Missouri, USA). All PPAR‐γ ligands were dissolved in dimethyl sulfoxide (DMSO). Trizol reagent was obtained from Omega (Parsippany, New Jersey, USA). Oligo (dT) and reverse transcriptional enzyme (M‐MLV) were products of Promega Corp (Madison, Wisconsin, USA). Primers were synthesised by Sangong Biological Company (Shanghai, China). Rabbit anti‐human PPAR‐γ polyclonal antibody, rabbit anti‐human TIMP‐1 polyclonal antibody, mouse anti‐human MMP‐7 monoclonal antibody and β‐actin were all products of Santa Cruz Biotechnology, Inc (Santa Cruz, California, USA). They were diluted to working concentrations of 1:500. Goat anti‐rabbit and anti‐mouse IgG‐HRP were obtained from Huamei Biological Company (Wuhan, China), with a working concentration of 1:1000. BioCoat Matrige invasion chamber was purchased from BD Biosciences, Inc (Rockville, Maryland, USA).
Methods
Cell culture and grouping
SW480 and LS174T colon cancer cell lines were cultured in RPMI (Roswell Park Memorial Institute) 1640 supplemented with 10% fetal calf serum, 100 units/ml of penicillin, and 100 μg/ml of streptomycin, in a humidified 5% carbon dioxide atmosphere at 37°C for 48 h. For MTT (1‐(4, 5‐Dimethylthiazol‐2‐yl)‐3, 5‐diphenylformazan) assay, colon cancer cells were grouped into: (1) control group (received an equivalent volume of DMSO, the final concentration ⩽0.1%); (2) 15d‐PGJ2 group (5, 10, 20, 40 μmol/l); (3) pioglitazone group (20, 30, 40, 50 μmol/l); (4) 15d‐PGJ2 (10 μmol/l) + GW9662 (1 μmol/l) group; and (5) pioglitazone (30 μmol/l) + GW9662 (1 μmol/l) group. For invasion assay, groups were as follows: (1) control group, (2) 15d‐PGJ2 (5 μmol/l) group, and (3) pioglitazone (20 μmol/l) group.
Cell growth assay
Cells were plated (3×104 cells/well) in triplicate onto a 96‐well cultured plate, and treated with each chemical agent for 48 h (grouped as above). Each experiment was repeated three times. The antiproliferative effects were determined using the MTT dye uptake method. Cell viability was expressed as optical density (OD), which was detected in the enzyme‐linked immunosorbent assay (ELISA) reader (American Research Company, USA) at 570 nm wavelength. The following formula was used: cell proliferation inhibited (%) = [1–(OD of the experimental samples/OD of the control)] ×100%.
Cell cycle analysis
Cells were treated with either vehicle or 10 μmol/l 15d‐PGJ2 and 30 μmol/l pioglitazone for 48 h, collected after brief trypsinisation, washed with phosphate buffered saline, and fixed in cold 70% ethanol. The samples were then treated with RNase, stained with 50 μg/ml propidium iodide (PI) and analysed by FACScan (Becton Dickinson, Franklin Lakes, New Jersey, USA).
Reverse transcription‐PCR to determine mRNA level of PPAR‐γ, MMP‐7, and TIMP‐1
The total RNA was extracted using Trizol reagent according to the product manual; 2 μg of total RNA was reverse‐transcribed to cDNA. The primers used for amplifying cDNA were as follows:
PPAR‐γ (474bp)
Sense primer: 5′‐TCTCTCCGTAATGGAA GACC‐3′
Antisense primer: 5′‐GCATTATGAGACATCCCCAC‐3′
MMP‐7 (365bp)
Sense primer: 5′‐AGATGTGGAGTGCCAGA TGT‐3′
Antisense primer: 5′‐TAGACTGCTACCATCCGTCC‐3′
TIMP‐1 (326bp)
Sense primer: 5′‐GCAACTCCGACCTTGTC ATC‐3′
Antisense primer: 5′‐AGCGTA GGTCTTGGTGAAGC‐3′
As an internal control, G3PDH mRNA was also amplified (598bp) using the following primers:
Sense primer: 5′‐CCACCCATGGCAAATTCCATGGCA‐3′
Antisense primer: 5′‐TCTAGAGGGCAGGTCAGGTCCACC‐3′
After denaturation at 94°C for 2 min, polymerase chain reaction (PCR) was carried out in a DNA thermal cycler (GeneAmp 2400, Hayward, California, USA) for 30 cycles. Each cycle includes denaturation at 94°C for 40 s, annealing at 54°C (PPAR‐γ)/60°C (MMP‐7)/58°C (TIMP‐1) for 40 s, and extension at 72°C for 60 s, followed by a final extension at 72°C for 5 min. The PCR products were run on 2% agarose gel in TAE buffer (40 mmol/l Tris acetate, 1 mmol/l ethylenediaminetetraacetic acid), and visualised by ethidium bromide staining. Autoradiography was performed using Bio‐1D Image (VL Company, (Vilber Lourmat), Marne‐la‐Vallée, France).
Western blotting to detect protein expression of PPAR‐γ, MMP‐7, and TIMP‐1
The cell lines were collected by centrifugation and then lysed in solubilisation buffer. Total cellular protein (50 μg) was separated by using 10% sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE) and transferred onto a polyvinylidene difluoride (PVDF) membrane. The membrane was then blocked with 5% calf serum albumin. Afterwards the membrane was incubated for 2 h with primary antibodies as follows: rabbit anti‐human PPAR‐γ polyclonal antibody, rabbit anti‐human TIMP‐1 polyclonal antibody, and mouse anti‐human MMP‐7 monoclonal antibody. As an internal control, β‐actin was detected with mouse monoclonal antibody. The membrane was then probed for 1 h at room temperature with horseradish peroxidase‐labelled secondary antibody. The signal was revealed using a DAB system. Quantification of the western blots was determined by scanning the blots with Adobe Photoshop and performing densitometry with Bio‐1D Image (VL Company, France).
In vitro invasion assay
Cell invasion assay was carried out with the BioCoat Matrige invasion chamber kit following the manufacturer's instructions. Briefly, 500 μl suspension of NIH3T3 cells was added into the lower compartments of the chambers. Cells were seeded at a concentration of 2×104/ml into the upper compartment. After 48 h incubation, the cells on the upper surface of the filters were completely removed by wiping with cotton swabs. Then the filters were fixed in methanol and stained with haematoxylin and eosin. Each in vitro invasion assay was performed in triplicate. For quantification purpose, cells which migrated to the lower surface were counted under a microscope in five random fields at a magnification of ×200. Cells were tested for their relative invasion ability as percentages of untreated controls.
Statistical analysis
All results are presented as mean (SD). The statistical significance of differences was determined by one‐way analysis of variance (ANOVA) and subsequently by Fisher's least significant difference (LSD) test using SPSS 11.0.
RESULTS
PPAR‐γ mRNA and protein expression in colon cancer cells
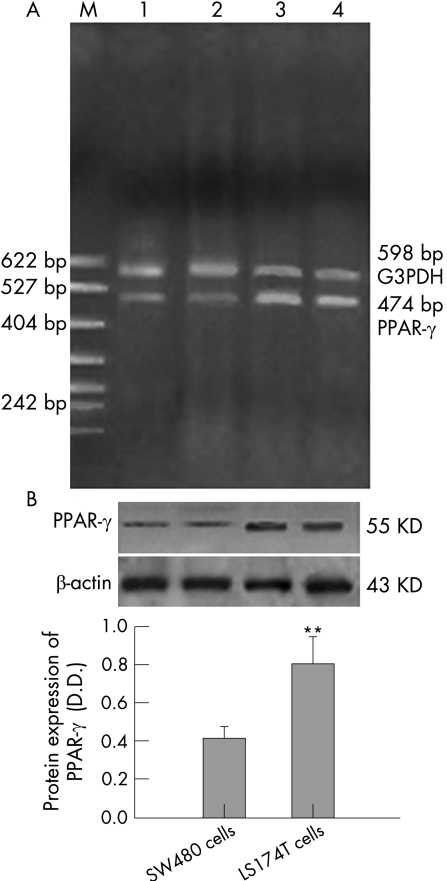
RT‐PCR and western blot showed that PPAR‐γ mRNA and protein were expressed in both cell lines. The fragments of PPAR‐γ and G3PDH cDNA were 474bp and 598bp in length, respectively. A specific band corresponding to PPAR‐γ protein at a molecular size of about 55 kDa was detected. As shown in fig 1, LS174T cells expressed more PPAR‐γ mRNA (fig 1A) and protein (fig 1B) than SW480 cells.
Figure 1 Expression of peroxisome proliferator‐activated receptor γ (PPAR‐γ) in two colon cancer cells. The result was a representative of three independently performed experiments. (A) Expression of PPAR‐γ mRNA in two colon cancer cell lines. G3PDH served as an internal control. M: PBR322 DNA/MSP1 markers; line 1, 2: SW480 cells; line 3, 4: LS174T cells. (B) Expression of PPAR‐γ protein in two colon cancer cell lines. β‐actin served as an internal control. n = 3; mean (SD). **p<0.01, compared with SW480 cells.
Effects of PPAR‐γ agonists on the proliferation of colon cancer cells by MTT
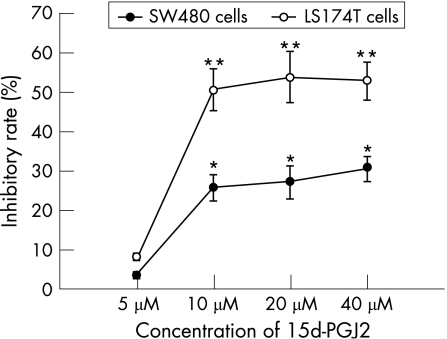
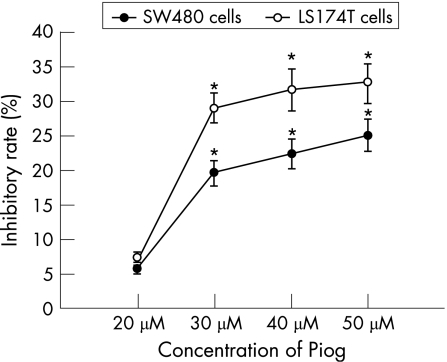
After 48 h incubation, 15d‐PGJ2 and pioglitazone inhibited the proliferation of both SW480 and LS174T cells in a dose‐dependent manner (figs 2 and 3). The OD values of 15d‐PGJ2 (10 μmol/l, 20 μmol/l, 40 μmol/l) and pioglitazone‐treated (30 μmol/l, 40 μmol/l, 50 μmol/l) groups were decreased significantly compared with the untreated groups (p<0.05 or p<0.01). But 5 μmol/l 15d‐PGJ2 and 20 μmol/l pioglitazone did not show significant inhibitory effects on both cell lines (p>0.05). Furthermore, the inhibitory effect of two PPAR‐γ agonists on the proliferation of LS174T cells was significantly higher than that on SW480 cells (p<0.05 or p<0.01). When LS174T cells were cultured with the combinations of 10 μmol/l 15d‐PGJ2 (or 30 μmol/l pioglitazone) and the PPAR‐γ antagonist GW9662 (1 μmol/l) for 48 h, the inhibitory rate on the growth of LS174T cells was decreased significantly compared with those without GW9662 (12.1 (2.1)% vs 50.3 (13.0)%; 9.8 (1.7)% vs 29.0 (6.7)%, both p<0.01).
Figure 2 The dose‐dependent response of growth inhibition in two colon cancer cell lines treated with 15‐deoxy‐δ(12,14)‐prostaglandin J2 (15d‐PGJ2) for 48 h. Growth inhibition was determined using an MTT assay and shown as inhibitory rate. Each value presented as mean (SD) of triplicate experiments.*p<0.05, **p<0.01, compared with untreated group.
Figure 3 The dose‐dependent response of growth inhibition in two colon cancer cell lines treated with pioglitazone (Pion) for 48 h. Growth inhibition was determined using an MTT assay and shown as inhibitory rate. Each value presented as mean (SD) of triplicate experiments. *p<0.05, compared with untreated group.
Changes of cell cycle profile by treatment with PPAR‐γ agonists
After 48 h, 10 μmol/l 15d‐PGJ2‐treated SW480 and LS174T cells exhibited a significant increase in G1 phase associated with a decrease in S phase (p<0.05 or p<0.01). Pioglitazone (30 μmol/l) had similar effects on both cells (table 1).
Table 1 Cell cycle profile in SW480 and LS174T cells treated with PPAR‐γ agonists for 48 h.
| LS174T | SW480 | |||||
|---|---|---|---|---|---|---|
| G1 | S | G2 | G1 | S | G2 | |
| Control | 59.7 (1.1) | 35.2 (1.1) | 5.1 (0.8) | 56.8 (1.2) | 32.8 (1.0) | 10.4 (1.1) |
| 15d‐PGJ2 (10 μmol/l) | 67.2 (1.2)** | 29.1 (0.9)* | 3.8 (0.6) | 65.2 (0.8)** | 26.5 (0.9)** | 8.3 (0.6) |
| Pioglitazone (30 μmol/l) | 63.2 (0.7)** | 31.9 (1.4)** | 4.9 (0.5) | 59.0 (1.1)* | 29.3 (0.9)* | 11.7 (1.0) |
Date presented as mean (SD).
The values represent the number of cells in a phase of the cell cycle as a percentage of total cells. Assessment of cell cycle was performed in triplicate and repeated three times.
*p<0.05, **p<0.01, compared with control group.
Effect of PPAR‐γ agonists on MMP‐7 and TIMP‐1 expression
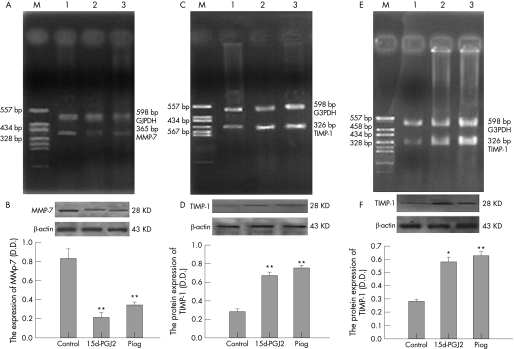
MMP‐7 was only expressed in LS174T cells, while TIMP‐1 was expressed in both cell lines. As shown in RT‐PCR and western blot analysis (fig 4), when LS174T cells were cultured with 5 μmol/l 15d‐PGJ2 or 20 μmol/l pioglitazone, the MMP‐7 mRNA (fig 4A) and protein levels (fig 4B) were significantly decreased compared with the control. Meanwhile, PPAR‐γ agonists upregulated the expression of TIMP‐1 at mRNA and protein levels in both LS174T (fig 4C, 4D) and SW480 cells (fig 4E, 4F).
Figure 4 Effect of PPAR‐γ agonists on matrix metalloproteinase‐7 (MMP‐7) and tissue inhibitor‐1 (TIMP‐1) expression. The result was a representative of three independently performed experiments. (A) Effect of PPAR‐γ agonists (15d‐PGJ2 and pioglitazone) on the mRNA expression of MMP‐7 in LS174T cells. M: PGEM‐7zf (+)/Hae III markers; line 1: control group; line 2: 15d‐PGJ2 group; line 3: pioglitazone group. (B) Effect of PPAR‐γ agonists (15d‐PGJ2 and pioglitazone) on the protein expression of MMP‐7 in LS174T cells. n = 3; mean (SD). **p<0.01 compared with control group. (C) Effect of PPAR‐γ agonists (15d‐PGJ2 and pioglitazone) on the mRNA expression of TIMP‐1 in LS174T cells. M: PGEM‐7zf (+)/Hae III markers; line 1: control group; line 2: 15d‐PGJ2 group; line 3: pioglitazone group. (D) Effect of PPAR‐γ agonists (15d‐PGJ2 and pioglitazone) on the protein expression of TIMP‐1 in LS174T cells. n = 3; mean (SD). **p<0.01 compared with control group. (E) Effect of PPAR‐γ agonists (15d‐PGJ2 and pioglitazone) on the mRNA expression of TIMP‐1 in SW480 cells. M: PGEM‐7zf (+)/Hae III markers; line 1: control group; line 2: 15d‐PGJ2 group; line 3: pioglitazone group. (F) Effect of PPAR‐γ agonists (15d‐PGJ2 and pioglitazone) on the protein expression of TIMP‐1 in SW480 cells. n = 3; mean (SD). *p<0.05 compared with control group.
Effect of PPAR‐γ agonists on the invasive ability of two colon cancer cells in vitro
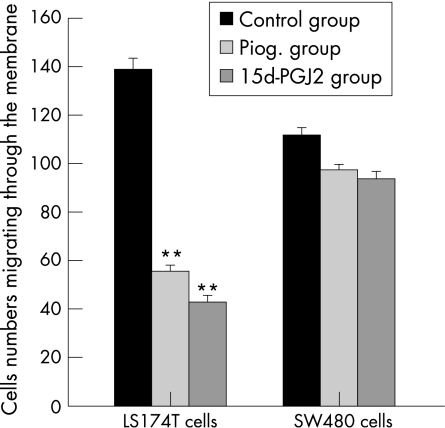
Both 15d‐PGJ2 (5 μmol/l) and pioglitazone (20 μmol/l) significantly reduced LS174T cell numbers through Matrigel gel after 48 h incubation (figs 5 and 6). The relative invasion rates (% of control) were only 30.4 (4.8)% and 39.9 (5.6)% when LS174T cells were treated with 15d‐PGJ2 and pioglitazone, respectively. 15d‐PGJ2 was more potent in inhibiting the invasion than pioglitazone (p<0.05). However, when incubating with 15d‐PGJ2 or pioglitazone, the invasion rate of SW480 cells did not change significantly (figs 5 and 7).
Figure 5 In vitro invasion assay, effect of PPAR‐γ agonists (15d‐PGJ2 and pioglitazone) on the invasive cell numbers of two colon cancer cell lines. n = 3; **p<0.01 compared with control group.
Figure 6 In vitro invasion assay of LS174T cells; invasive cells were tested after 48 h incubation with or without PPAR‐γ agonists. Panel A, control group; panel B, 15d‐PGJ2 group; panel C, pioglitazone group (haematoxylin and eosin, ×200).
Figure 7 In vitro invasion assay of SW480 cells; invasive cells were tested after 48 h incubation with or without PPAR‐γ agonists. Panel A, control group; panel B, 15d‐PGJ2 group; panel C, pioglitazone group (haematoxylin and eosin, ×200).
DISCUSSION
PPAR‐γ plays a central role in adipocyte differentiation and lipid metabolism, and regulates genes involved in glucose utilisation.12 Recently it has been shown that ligand activation of endogenous or eotopically expressed PPAR‐γ is sufficient to induce growth arrest and apoptosis in different cancer cell lines.13 15d‐PGJ2 is metabolised from prostaglandin D2, and is an endogenous, high affinity natural ligand of PPAR‐γ.14 Thiazolidinedione (TZD) agents such as pioglitazone, which are newly developed anti‐diabetic agents, are the specific synthetic ligands and agonists of PPAR‐γ.15
PPAR‐γ is important in the normal and pathological function of the human colon.16 Our study revealed that 15d‐PGJ2 and pioglitazone inhibited the growth of SW480 and LS174T colon cancer cell lines in a dose‐dependent manner. The result was similar to the studies of Sarraf et al10 and Shimada et al,16 which demonstrated that PPAR‐γ agonists inhibited the growth and induced differentiation of human colon cancer cells, both in culture and in nude mice. However, Lefebvre et al9 presented evidence that APCmin/+ mice showed increased numbers of polyps when subjected to oral TZD dosing. In contrast, Osawa et al17 recently showed that continuous feeding of pioglitazone reduced the aberrant crypt foci formation and notably suppressed colon tumours. These conflicting results have been partially explained by the finding that in mice with a mutated APC gene, PPAR‐γ loses its ability to regulate colon tumorigenesis, whereas in wild‐type APC mice, PPAR‐γ functions as a tumour suppressor gene.4 However, APC gene mutation appears to be a crucial early event during colorectal tumorigenesis.18 In our study, we have observed that the inhibitory effect on proliferation was rather obvious with 15d‐PGJ2 and pioglitazone at concentrations of 10 μmol/l and 30 μmol/l, respectively. The inhibitory rate on LS174T cells was significantly higher than that on SW480 cells. The possible explanation was that the mRNA and protein levels of PPAR‐γ were significantly higher in LS174T cells than those in SW480 cells. In addition, the inhibitory effects of 15d‐PGJ2 and pioglitazone can be counteracted by PPAR‐γ antagonist GW9662. Our study suggested that 15d‐PGJ2 and pioglitazone inhibited the growth of APC mutant colon cancer cells, probably through a PPAR‐γ‐dependent route, within a certain range of concentrations. Although we demonstrated the inhibitory effect on APC mutant cells through PPAR‐γ activation, our study was performed in cultured human colon cancer cells rather than one of the animal models. At high concentration, PPAR‐γ ligands can have PPAR‐γ‐independent effects,19 and our study did not exclude the possibility of such effects.
Next we analysed the cell cycle profile to identify the mechanism by which PPAR‐γ agonists inhibit the growth of colon cancer cells. According to our data, 15d‐PGJ2 and pioglitazone increased the population of cells in G1 phase and reduced the cells in S phrase in both SW480 and LS174T cells. It suggested that the inhibitory effects of PPAR‐γ agonists may be associated with G1 cell cycle arrest. As the cell proliferation was not changed at a concentration of <10 μmol/l 15d‐PGJ2 and of <30 μmol/l pioglitazone, we chose 5 μmol/l 15d‐PGJ2 and 20 μmol/l pioglitazone to perform the invasion assay in order to avoid cytotoxicity.
MMP‐7 is thought to be one of the key MMPs in the invasion step of colorectal cancer.20,21 In our study, we found that the expression of MMP‐7 could only be detected in LS174T cells, but not in SW480 cells. This result has also been demonstrated by Witty.22 However, the expression of TIMP‐1, which is the natural tissue inhibitor of MMPs,23 was detectable in both cells. Our study further showed that when treated with 15d‐PGJ2 and pioglitazone, the expression of MMP‐7 in LS174T cells was significantly decreased. In the meantime, the expression of TIMP‐1 was dramatically upregulated in both cell lines. All this evidence indicated that PPAR‐γ agonists may reduce the invasive ability of colon cancer cells. Similar results were also reported in HT‐29 colon cancer cells.24 However, to our knowledge, there were no in vitro data to identify the inhibitory ability of PPAR‐γ agonists on human colon cancer cells. In our subsequent study, the invasive ability of two colon cancer cell lines was detected. It is clearly demonstrated that PPAR‐γ agonists had a significant inhibitory effect on LS174T cells, but the invasive ability of SW480 cells was not significantly affected. The results indicated that the invasive ability of certain colon cancer cell lines in vitro was inhibited by PPAR‐γ agonists at least partially through the downregulation of MMP‐7 expression.
Over the past decade, newer chemotherapeutic agents such as biological treatments have been used for the management of colorectal cancer, with resulting improvements in response and survival rates.25,26 More recently there have been a few reports on the association between diabetes mellitus and cancer. Inoue et al27 demonstrated that patients with diabetes mellitus may be at an increased risk of colon cancer. A 10‐year prospective cohort study by Jee et al28 showed that elevated fasting serum glucose levels increased colon cancer incidence and mortality. The TZDs, a newly developed group of antidiabetic agents, have been recently demonstrated to inhibit colon cancer cell growth as well as induce cell differentiation. In this study we demonstrated that pioglitazone inhibited the growth and invasion of certain colon cancer cell lines through PPAR‐γ activation. We anticipate that TZDs may represent a new group of biological agents for the management of colon cancer, especially in the patients with diabetes mellitus.
In summary, our study identified that, in addition to the inhibition of colon cancer cell growth associated with G1 cell cycle arrest, PPAR‐γ agonists such as 15d‐PGJ2 and pioglitazone had an inhibitory effect on the invasive ability of certain colon cancer cell lines in vitro. Our study suggested that PPAR‐γ agonists may be promising novel agents for the treatment of human colon cancer.
ACKNOWLEDGEMENTS
We thank Xiqiu Yu for the suggestions made during the preparation of the manuscript. The excellent technical assistances of Shiquan Liu, Sufang Tian, and Yan Wang are gratefully acknowledged. We also thank Hui Shen and Xueyan Chen for help with the translation.
Abbreviations
15d‐PGJ2 - 15‐deoxy‐δ(12,14)‐prostaglandin J2
DMSO - dimethyl sulfoxide
ELISA - enzyme‐linked immunosorbent assay
MMP‐7 - matrix metalloproteinase‐7
OD - optical density
PI - propidium iodide
PPAR‐γ - peroxisome proliferator‐activated receptor γ
PVDF - polyvinylidene difluoride
RT‐PCR - reverse transcriptase polymerase chain reaction
SDS‐PAGE - sodium dodecyl sulfate‐polyacrylamide gel electrophoresis
TIMP‐1 - tissue inhibitor‐1
TZD - thiazolidinedione
Footnotes
Competing interests: There is no competing interest in the manuscript.
References
- 1.Aznavoorian S, Murphy A N, Stetler‐Stevenson W G. Molecular aspects of tumor cell invasion and metastasis. Cancer 1993711368–1383. [DOI] [PubMed] [Google Scholar]
- 2.Mayor S. Raised glucose concentrations and diabetes are associated with cancer risk. BMJ 2005330111 [Google Scholar]
- 3.Rosen E D, Spiegeiman B M. PPAR‐gamma: a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem 200127637731–37734. [DOI] [PubMed] [Google Scholar]
- 4.Bull A W. The role of peroxisome proliferator‐activated receptor γ in colon cancer and inflammatory bowel disease. Arch Pathol Lab Med 20031271121–1123. [DOI] [PubMed] [Google Scholar]
- 5.Sato H, Ishihara S, Kawashima K.et al Expression of peroxisome proliferator activated receptor (PPAR) gamma in gastric cancer and inhibitory effects of PPAR gamma agonist. Br J Cancer 2000831394–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eibl G, Wente M N, Reber H A.et al Peroxisome proliferator activated receptor gamma ligands induces pancreatic cancer cell apoptosis. Biochem Biophys Res Commun 2001287522–529. [DOI] [PubMed] [Google Scholar]
- 7.Chang T H, Szabo E. Ligands for peroxisome proliferator activated receptor gamma and retinoic acid receptor inhibit growth and induce apotosis of human breast cancer cells in vitro and in BNX mice. Cancer Res 2000601129–1138. [PubMed] [Google Scholar]
- 8.Hase T, Yoshimura R, Mitsuhashi M.et al Expression of peroxisome proliferator activated receptors in human testicular cancer and growth inhibition by its agonists. Urology 200260542–547. [DOI] [PubMed] [Google Scholar]
- 9.Lefebvre A M, Chen I, Desreumaux P.et al Activation of the peroxisome proliferator activated receptor gamma promotes the development of conlon tumors in C57BL/6J‐APCMin/+mice. Nat Med 199841053–1057. [DOI] [PubMed] [Google Scholar]
- 10.Sarraf P, Mueller E, Jones D.et al Differentiation and reversal of malignant changes in colon cancer through PPARγ. Nat Med 199841046–1052. [DOI] [PubMed] [Google Scholar]
- 11.Liu H, Zang C, Fenner M H.et al PPARγ ligands and ATRA inhibit the invasion of human breast cancer cells in vitro. Breast Cancer Res Treat 20037963–74. [DOI] [PubMed] [Google Scholar]
- 12.Kersten S, Desvergne B, Wahli W. Role of PPARs in health and disease. Nature 2000405421–424. [DOI] [PubMed] [Google Scholar]
- 13.Grommes C, Landreth G E, Heneka M T. Antineoplastic effects of peroxisome proliferator activated receptor γ agonists. Lancet Oncology 20045419–429. [DOI] [PubMed] [Google Scholar]
- 14.Yang W L, Frucht H. Activation of the PPAR pathway induces apoptosis and COX‐2 inhibition in HT‐29 human colon cancer cells. Carcinogenesis 2001221379–1383. [DOI] [PubMed] [Google Scholar]
- 15.Kato M, Kusumi T, Tsuchida S.et al Induction of differentiation and peroxisome proliferator activated receptor γ expression in colon cancer cells by troglitazone. J Cancer Res Clin Oncol 200413073–80. [DOI] [PubMed] [Google Scholar]
- 16.Shimada T, Kojima K, Yoshiura K.et al Characteristics of the peroxisome proliferator‐ activated receptor γ (PPAR‐gamma) ligand induced apoptosis in colon cancer cells. Gut 200250658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osawa E, Nakajima A, Wada K.et al Peroxisome proliferator activated receptor gamma ligands suppress colon carcinogenesis induced by azoxymethane in mice. Gestroenterology 2003124564–567. [DOI] [PubMed] [Google Scholar]
- 18.Rao C V, Yang Y M, Swamy M V.et al Colonic tumorigenesis in BubR1+/‐ApcMin/+ compound mutant mice is linked to premature separation of sister chromatids and enhanced genomic instability. Proc Natl Acad Sci USA 20051024365–4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han S, Roman J. Rosiglitazone suppresses human lung carcinoma cell growth through PPARgamma‐dependent and PPARgamma‐independent signal pathways. Mol Cancer Ther 20065430–437. [DOI] [PubMed] [Google Scholar]
- 20.Baker E A, Bergin F G, Leaper D J. Matrix metalloproteinases, their tissue inhibitors and colorectal cancer staging. Br J Surg 2000871215–1221. [DOI] [PubMed] [Google Scholar]
- 21.Garbett E A, Reed M W R, Brown N J. Proteolysis in colorectal cancer. Mol Pathol 199952140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Witty J P. Modulation of matrilysin levels in colon carcinoma cell lines affects tumorigenicity in vivo. Cancer Res 1994544805–4812. [PubMed] [Google Scholar]
- 23.Kohn E C, Liotta L A. Molecular insight into cancer invasion: strategies for prevention and intervention. Cancer Res 1995551856–1862. [PubMed] [Google Scholar]
- 24.Sunami E, Tsuno N H, Kitayama J.et al Decreased synthesis of matrix metalloproteinase‐7 and adhesion to the extracellular matrix protein of human colon cancer cells treated with troglitazone. Surg Today 200232343–350. [DOI] [PubMed] [Google Scholar]
- 25.Goyle S, Maraveyas A. Chemotherapy for colorectal cancer. Dig Surg 200522401–414. [DOI] [PubMed] [Google Scholar]
- 26.Mazar D, Stebbing J, Heller W. Recent advances in the systemic management of colorectal cancer. Future Oncol 20062643–650. [DOI] [PubMed] [Google Scholar]
- 27.Inoue M, Iwasaki M, Otani T.et al Diabetes mellitus and the risk of cancer: results from a large‐scale population‐based cohort study in Japan. Arch Intern Med 20061661871–1877. [DOI] [PubMed] [Google Scholar]
- 28.Jee S H, Ohrr H, Sull J W.et al Fasting serum glucose level and cancer risk in Korean men and women. JAMA 2005293194–202. [DOI] [PubMed] [Google Scholar]