Abstract
Barrett's oesophagus is premalignant. Oesophagectomy is traditionally regarded as the standard treatment option in the presence of high grade intraepithelial neoplasia or intramucosal cancer. However, oesophagectomy is associated with high rates of mortality and morbidity. Endoscopic ablative therapies are limited by the lack of tissue for histological assessment, and the ablation may be incomplete. Endoscopic mucosal resection is an alternative to surgery in the management of high grade intraepithelial neoplasia and intramucosal cancer. It is less invasive than surgery and, unlike ablative treatments, provides tissue for histological assessment. This review will cover the indications, techniques and results of endoscopic mucosal resection.
Barrett's oesophagus is a sequel of gastro‐oesophageal reflux disease (GORD) and may be present in 5–15% of GORD patients in the western population.1,2,3 Barrett's oesophagus is premalignant, with oesophageal adenocarcinoma occurring at an overall incidence rate of 0.4–0.5% per year.4 It progresses through stages of dysplasia to cancer. Patients without dysplasia and those with low grade intraepithelial neoplasia (LGIN) have low rates of disease progression. In the presence of high grade intraepithelial neoplasia (HGIN), disease may progress at rates >10% per year.5,6
Surveillance endoscopy for Barrett's oesophagus—with the aim of detecting HGIN or early cancer in order to facilitate earlier therapeutic interventions—has been advocated by the American College of Gastroenterology, with the time interval of endoscopy being dependent on the presence and severity of dysplasia. In the absence of dysplasia, follow up endoscopy is performed at 3 years. When there is LGIN, endoscopy is performed yearly. If focal HGIN is present, endoscopy is repeated at 3‐monthly intervals, but in the presence of multifocal HGIN or intramucosal cancer (IMC), intervention is required.7
Oesophagectomy is traditionally regarded as the standard treatment option in the presence of HGIN or cancer. It is a definitive treatment which removes all neoplastic epithelia. This is important because of the limitation of endoscopic biopsy, which may not detect other foci of HGIN or IMC. In a series of patients who underwent oesophagectomy for HGIN detected by endoscopy, surgery revealed invasive cancer in 30–40% of cases which was missed preoperatively.8 However, oesophagectomy is also associated with the highest rates of procedure related mortality and long term morbidity. Mortality rates ranging from 2.5–20.3% have been reported, and 30–50% of patients may develop serious postoperative complications such as pneumonia, anastomotic leaks and myocardial infarction.9 In addition, there have been reports of patients whose preoperative biopsy specimens showed IMC that was not seen in the surgical specimens.10 There is thus a need for a less invasive alternative treatment strategy.
Endoscopic ablative therapies such as argon plasma coagulation (APC) and photodynamic therapy (PDT) have been proposed as less invasive alternatives to oesophagectomy, but are clearly not optimal. These therapies are limited by the lack of tissue for histological assessment, which is crucial for determining treatment adequacy, and the possibility that the ablation may be incomplete, with remnant Barrett's mucosa post treatment; this persistent Barrett's oesophagus will remain at risk for progression to adenocarcinoma.9 In a multicentre randomised study which compared PDT using porfimer sodium, combined with omeprazole, versus omeprazole alone, it was shown that although PDT was superior to omeprazole alone, complete ablation of HGIN was achieved in only 77% of cases, while complete ablation of Barrett's oesophagus was achieved in only 52%. In addition, oesophageal adenocarcinoma still occurred in 13% of cases in the treated group; strictures also occurred in 36% of cases.11 Poor results were also obtained when 5‐aminolevulinic acid‐PDT was used to treat patients with residual HGIN and IMC after endoscopic mucosal resection, with failure of PDT in 25% of cases, and recurrence of HGIN in 27% of successfully treated cases on follow up.12 APC has been used to ablate Barrett's oesophagus with HGIN and IMC as well, but the failure rate was 20%.13 In a study of patients with Barrett's oesophagus (both without dysplasia as well as with LGIN) treated with APC and acid suppression, a relapse rate of 62% over a median period of 36 months was reported. In addition, 5% of patients progressed to adenocarcinoma during this period.14
Endoscopic mucosal resection (EMR) is increasingly being utilised as an alternative to surgery in the management of HGIN and IMC of the gastrointestinal tract. Performing EMR is similar to resecting the diseased mucosa surgically. It is less invasive than surgery and, unlike ablative therapies, it provides tissue for histological assessment. The role of EMR in the treatment of early oesophageal squamous cell carcinoma, early gastric cancer and early colonic cancer is established and had been previously reviewed.15 More data on the role of EMR in the context of Barrett's oesophagus with HGIN and IMC are now available. This review will cover the rationale and indication for EMR in the context of Barrett's oesophagus, the techniques of EMR, the limitations of EMR for Barrett's oesophagus, and results of key published data.
INDICATIONS AND RATIONALE FOR EMR
Indications for EMR
EMR is indicated as an alternative treatment to oesophagectomy when HGIN or well to moderately differentiated IMC occurs in the context of Barrett's oesophagus. However, nodal metastases should first be excluded by endoscopic ultrasound (EUS) (table 1).
Table 1 Indications for endoscopic mucosal resection in Barrett's oesophagus.
| • High grade intraepithelial neoplasia |
| • Well or moderately differentiated T1 m intramucosal cancer |
| • Absence of suspicious surrounding lymph nodes by endoscopic ultrasound |
Pathological basis for EMR
The basis for the indications in performing EMR stems from the clinicopathologic data of patients who underwent curative surgery for early Barrett's cancer. Studies have shown that there is no or minimal risk of distant metastases in the context of IMC. In the absence of distant metastases, curative EMR is thus feasible. The risk for lymph node metastases is 0% for cancer limited to the mucosal layer. When there is submucosal involvement, nodal metastases may occur in 16–22.2%.16,17,18 In a study which analysed the histological characteristics of endoscopic resection specimens of Barrett's neoplasia, it was shown that the prevalence of lymphatic vessel involvement was only 0.3% in T1 m cancer, compared to 3% of cases with submucosal involvement. In all these patients, no invasion into a submucosal blood vessel was observed. In addition, well differentiated carcinomas were generally limited to the mucosa (92.7%), unlike the moderately (73.7%) and poorly differentiated (22.7%) carcinomas.19 EMR is limited to well differentiated and moderately differentiated cancer because of the concern of occult metastases with poorly differentiated cancer. This is mainly extrapolated from the Japanese experience with early gastric cancer.20
EUS is frequently performed before EMR in order to assess the depth of mucosal involvement, and the possibility of regional nodal metastases, especially in the case of IMC. However, EUS tends to over‐stage the depth of mucosal involvement, whether a conventional 12 MHz echoendoscope21 or a 20 MHz high frequency mini‐probe is used.22 This is due to underlying inflammation. Hence the depth of penetration of IMC on EUS cannot be used as the sole criterion for not performing EMR, especially if the lesion meets the generally accepted endoscopic criteria of size and appearance for EMR.15 The accuracy of high resolution endoscopy in staging superficial oesophageal cancer was compared with high resolution EUS using a 20 MHz mini‐probe and it was shown that results were comparable (83.4% vs 79.6%).23 A recent study suggested that EUS and EUS‐guided fine needle aspiration may be useful in excluding nodal metastases.24
TECHNIQUES AND STRATEGIES OF EMR FOR BARRETT'S OESOPHAGUS
EMR techniques
Various EMR techniques have been described.15 These may be broadly classified into techniques with and without suction. Examples of techniques without suction include the “inject and cut” technique25 and the “strip biopsy” technique.26 Techniques with suction include “the simple snare resection” technique using a 0.3 mm monofilament stainless steel wire snare,27 cap assisted endoscopic mucosal resection (EMRC),28 the use of the oesophageal endoscopic mucosal resection tube,29 and endoscopic mucosal resection with ligation (EMRL).30,31,32 In addition to these traditional EMR techniques, the technique of endoscopic submucosal dissection (ESD), which facilitates en‐bloc resection, has also been recently introduced.33,34,35 ESD has been used mainly for early gastric cancer, although its use has also been expanded to include oesophageal lesions as well.36,37
Based on published literature, the EMR techniques that have been used for Barrett's oesophagus include the following: the “inject and cut” technique,25 “the simple snare resection technique”,27 EMRC,28 and EMRL.30,31,32 The details of these techniques will be expanded upon further (table 2). Chromoendoscopy using dyes such as acetic acid, methylene blue, indigo carmine and crystal violet, either alone or in combination with magnification endoscopy, has been used to improve the detection of specialised intestinal metaplasia as well as dysplasia in columnar lined oesophagus.38 One could consider using chromoendoscopy to highlight the Barrett's mucosa in order to facilitate resection; however it is generally not needed or used.
Table 2 Endoscopic mucosal resection techniques and strategies.
| Common EMR techniques for Barrett's oesophagus |
| “Inject and cut” technique |
| “Simple snare resection” technique |
| Cap assisted endoscopic mucosal resection |
| Endoscopic mucosal resection with ligation |
| EMR strategies used for Barrett's oesophagus |
| Localised EMR |
| Circumferential EMR |
EMR, endoscopic mucosal resection.
The “inject and cut” technique is also known as submucosal injection polypectomy. In this technique, the diseased mucosa is raised off the muscularis propria by the injection of saline with or without diluted epinephrine (adrenaline) to create a submucosal bleb which is strangulated by a braided snare and then resected using electrocautery. The problem with this approach is that flat lesions can be difficult to capture, and the injected solution may also dissipate rapidly.
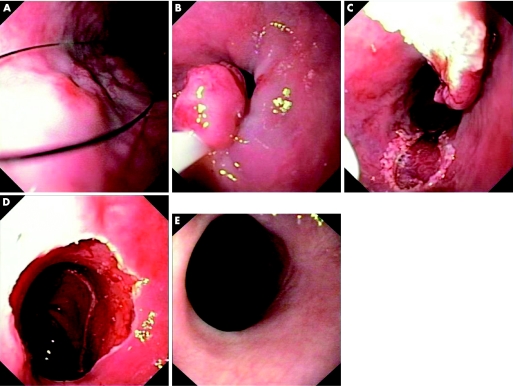
With the “the simple snare resection technique”,27 instead of using a braided snare, a stiff monofilament snare (Medwork, Germany) is utilised (fig 1). With this stiff snare, it is possible to press down on the diseased flat mucosa and ensnare it after simple aspiration, without the need for prior submucosal injection. This is not possible when the softer braided snare is used. The lesion is then resected by electrocautery. In our units we use the Erbotom ICC 200 electrosurgical device (Erbe Inc, Tϋbingen, Germany) with a pure coagulation current (output 60 W, effect 3).
Figure 1 (A) 3 cm long Barrett's oesophagus with a nodule proven as intramucosal cancer (IMC). The monofilament snare was pressed firmly around the Barrett's mucosa with the IMC. As the snare was closed, gentle aspiration was applied to draw the tumour bearing mucosa into the snare. (B) The Barrett's mucosa with IMC had been caught by the snare. (C) The lesion was resected by electrocautery. (D) Further resection of the Barrett's oesophagus was performed using the monofilament snare until the entire Barrett's epithelium had been completely resected. (E) Follow up endoscopy 12 months later. The Barrett's epithelium was completely replaced by new squamous cell epithelium.
With the EMRC technique,28 a specialised transparent plastic cap is fitted to the tip of a standard therapeutic endoscope. The margins of the lesion are first demarcated using a diathermy needle, followed by injection of saline or diluted epinephrine into the submucosa. A special soft crescent‐shaped snare (SD221L‐25 or SD‐7P‐1, Olympus, Tokyo, Japan) is then pre‐looped into the groove of the rim of the cap. This pre‐looping is performed by lightly pressing against and suctioning normal mucosa to seal the cap outlet. The snare is opened and rested along the inside groove of the cap to form the loop. The cap is used to suck the lesion into the cap, and the lesion is captured by closing the snare. Blended electrosurgical current is used to resect the lesion. The soft snare is easily deformed and usually suitable for single use only.
EMRL is a simple and easy method of performing EMR.30,31,32 It uses a variceal ligation device fitted onto the endoscope. The diseased mucosa is ligated, creating a pseudopolyp. The size of the threading channels of the standard band ligators does not permit the insertion of a polypectomy snare into the working channel of the endoscope. Therefore to resect the pseudopolyp, the endoscope must be withdrawn so that the cranking device of the band ligator can removed, in order to pass the snare through the working channel of the endoscope, or a second endoscope is required. The endoscope is then reinserted and the pseudopolyp resected using electrocautery. To circumvent the problem of repeat endoscope insertions when using the technique of EMRL for performing extensive EMR, the original Saeed Six Shooter Multi‐band Ligator (Wilson‐Cook Medical, Winston‐Salem, North Carolina, USA) has been modified, creating the Duette Multiband Mucosectomy Kit (Cook Ireland Ltd, Ireland).32 The modification consists of widening the threading channel of the cranking device from 2 mm to 3.2 mm, which facilitates the insertion of a 7 French polypectomy snare. The Duette Multiband Mucosectomy Kit consists of the modified multi‐band ligator and a hexagonal polypectomy snare sized 1.5×2.5 cm made of braided wire (AcuSnare SASMH‐1, Wilson‐Cook, USA). Up to six resections per device can be made. Band ligation can be performed with the snare still within the working channel. This modification enables sequential banding and snare resection of the oesophageal mucosa without a need to change the endoscope, thereby facilitating extensive EMR.
EMR strategies: localised EMR vs circumferential EMR for Barrett's oesophagus
In localised EMR, the area with HGIN and IMC is targeted and resected. In circumferential EMR, both the diseased area as well as all underlying Barrett's mucosa is completely resected (in reality the resection may not be really fully circumferential if the Barrett's oesophagus comprises isolated tongues that do not completely encircle the circumference of the oesophagus). This circumferential approach removes all at risk epithelium, and thus addresses both the problem of missing synchronous lesions at other sites as well as the development of metachronous lesions over the course of time. To perform localised EMR, all of the four techniques listed above have been utilised. To perform circumferential EMR, techniques that have been used include the “simple snare resection” technique,39 the “inject and cut” technique,40 and EMRL using the Duette Multiband Mucosectomy Kit.32 If a normal ligation device were to be used in EMRL, the endoscope would have to be withdrawn several times to allow extensive resection. EMRC is not ideal for repeat resection because the braided snare is easily deformed after single use.
Comparing the various techniques for circumferential EMR, the Duette Multiband Mucosectomy Kit is probably the easiest and least cumbersome method and hence will be expanded upon further. There is no need for submucosal saline injection (which may rapidly dissipate anyway), and flat lesions can be easily ensnared after creation of a pseudopolyp. In fact, a prospective randomised study of EMRL without submucosal saline injection against EMRC with prior submucosal injection of saline showed that both techniques were similar with respect to efficacy and safety for EMR of early oesophageal cancers.41 Multiple resections without prior submucosal injection can also be performed easily with the Duette Multiband Mucosectomy Kit. After the initial ligation and resection, the subsequent ligation is performed by sucking the adjacent mucosa with a bit of overlapping to ensure that no ridges of diseased mucosa remain. After EMR, resected specimens are collected using the Roth Net (US Endoscopy, Mentor, Ohio, USA), fixed onto a piece of cork before being immersed in 4% formalin solution and sent for histopathological assessment. At the end of EMR, if small remnant ridges of diseased mucosa persist, these can be easily removed with the stiff monofilament snare. If circumferential EMR is planned, resection can be performed in a single session or as a staged procedure with a hemi‐circumferential resection at the first setting depending on the length of the Barrett's oesophagus. Although circumferential EMR in one single setting is associated with stricture formation, conditions for a complete resection are most optimal in the first EMR session. In further sessions fibrosis with scar formation makes EMR more difficult. If EMR is performed in multiple sessions, repeat endoscopy and EMR is performed at 3‐ to 4‐week intervals until complete resection of all Barrett's mucosa. Patients are also treated with high dose proton pump inhibitors during this period.
RESULTS AND LIMITATIONS OF EMR FOR BARRETT'S OESOPHAGUS WITH HGIN OR IMC
Localised EMR
The first report of localised EMR for Barrett's oesophagus in a cohort of 64 patients showed that for low risk patients (defined as lesion diameter <20 mm; macroscopically type I, IIa lesions or IIC lesions ⩽10 mm; well or moderately differentiated adenocarcinoma or high grade dysplasia; limited to the mucosa) EMR could achieve complete remission in 97% of cases. For high risk patients (lesion diameter >20 mm and limited to the mucosa; and/or macroscopically type III; and/or poorly differentiated adenocarcinoma; and/or submucosal involvement) complete remission was achieved in only 59%. Apart from a case of bleeding needing endoscopic therapy, no severe complications occurred. Over a mean follow up period of 12 months, recurrent or metachronous carcinomas were found in 14% of cases.42 The same group subsequently published data for a longer period of follow up. Over a mean follow up period of 34 months, higher recurrence rates of 23–30% were noted.43,44 No major complications such as severe bleeding or perforation occurred. Similar results have been published by other groups.45,46
Local recurrence is thus a major limitation of localised EMR. This is especially so for long segment Barrett's oesophagus, even if histology shows complete removal of the malignant lesion by localised EMR. The high recurrence rate is due to the existence of multifocal premalignant and malignant areas in the Barrett's oesophagus overlooked by biopsy before EMR, as well as metachronous development of new foci of dysplasia. The issue of inaccurate pre‐EMR biopsy is not surprising, because studies addressing the question of 2 cm, four quadrant biopsies for surveillance have shown that cancers may be missed in 33–56% of cases,47,48 although this may be potentially halved with the use of a more intensive 1 cm, four quadrant biopsy protocol.49 Localised EMR has been combined with PDT and APC to address the issue of remnant Barrett's oesophagus and promising results have been obtained.43,44,50 However, similar to ablative therapy alone, there remains the serious concern over the lack of histological assessment and the distinct possibility of incomplete ablation.
Circumferential EMR
To address these limitations, the concept of circumferential EMR to remove all underlying Barrett's mucosa upon detection of HGIN or IMC was mooted.38 Thus far only three full papers on this topic has been published, two from the centre that first conceptualised it,32,39 and another corroborative study.40 Although no recurrent or metachronous lesions were reported in two of the studies,32,39 in the third study a recurrence rate of 11% was reported over a mean follow up period of 18 months. This may be due to incomplete resection of the Barrett's mucosa due to repeated resection in multiple sessions. The issue of remnant ridges of diseased mucosa is very pertinent as none of the available techniques can achieve en bloc resection. Piecemeal resection is unavoidable. However, the risk can be potentially minimised by overlapping the margins of resection, as well as by using adjunctive measures such as additional simple snare resection or APC to remove remnant tissue ridges. It is probably better to remove all remnants in the first EMR session because fibrosis may make subsequent EMR more difficult. A problem with circumferential EMR in one session is the occurrence of strictures.32,39 If the Barrett's segment is short these strictures can usually be handled by bougienage without any difficulty.
Key references
Sharma P, McQuaid K, Dent J, et al. A critical review of the diagnosis and management of Barrett's esophagus: the AGA Chicago workshop. Gastroenterology 2004;127:310–30.
Vieth M, Ell C, Gossner L, et al. Histological analysis of endoscopic resection specimens from 326 patients with Barrett's esophagus and early neoplasia. Endoscopy 2004;36:776–81.
Soetikno RM, Gotoda T, Nakanishi Y, et al. Endoscopic mucosal resection. Gastrointest Endosc 2003;57:567–79.
Soehendra N, Seewald S, Groth S, et al. Use of modified multiband ligator facilitates circumferential endoscopic mucosal resection in Barrett's esophagus (+ video). Gastrointest Endosc 2006;63:847–52.
Ell C, May A, Gossner L, et al. Endoscopic mucosal resection of early cancer and high grade dysplasia in Barrett's esophagus. Gastroenterology 2000;118:670–7.
CONCLUSION
The early and intermediate term results of EMR for Barrett's oesophagus with HGIN and IMC are promising, with high treatment success rates and no occurrence of major complications. In particular, circumferential EMR to remove all the underlying Barrett's oesophagus, once HGIN or IMC is detected, is conceptually sound and attractive. However, more long‐term data for a larger number of treated patients are required in order to establish the role of EMR as a standard alternative treatment to surgery in Barrett's oesophagus. In addition, these high success and low complication rates have been mainly achieved by highly experienced therapeutic endoscopists in referral centres. More data are needed to determine whether these excellent clinical outcomes may be duplicated outside of these referral centres.
MULTIPLE CHOICE QUESTIONS (TRUE (T)/FALSE (F); ANSWERS AFTER THE REFERENCES)
1. Barrett's oesophagus is a premalignant condition. Adenocarcinoma may occur at a rate of:
0.5% per year
0.3% per year
1.5% per year
1.0% per year
2. Surveillance endoscopy using a 2 cm, four quadrant biopsy protocol:
Detects all cases of high grade dysplasia and cancer
Misses 70% of coexistent cancer
Misses 33–56% of coexistent cancers
Misses 10% of coexistent cancers
3. Which of the following statements about endoscopic mucosal resection (EMR) is true?
The risk of lymph node metastases for intramucosal cancer is 0.3%
EMR is indicated in the presence of both low grade and high grade dysplasia
In the absence of lymph node on endoscopic ultrasound, EMR of poorly differentiated intramucosal cancer can be safely performed
Localised EMR has a recurrence rate of up to 30%
4. Submucosal injection of saline is not required in which of the following techniques of EMR?
“Simple snare resection” technique
EMR with cap technique
EMR with ligation technique
“Inject and cut” technique
5. Which of the following statements concerning circumferential EMR is true?
Circumferential EMR of Barrett's oesophagus is the standard of care
Stricture formation is unavoidable in circumferential EMR
Circumferential EMR has the advantage of removing all at risk mucosa
The issue of disease recurrence is not applicable to circumferential EMR
Abbreviations
APC - argon plasma coagulation
EMR - endoscopic mucosal resection
EMRC - cap assistant endoscopic mucosal resection
EMRL - endoscopic mucosal resection with ligation
EST - endoscopic submucosal dissection
EUS - endoscopic ultrasound
GORD - gastro‐oesophageal reflux disease
HGIN - high grade intraepithelial neoplasia
IMC - intramucosal cancer
LGIN - low grade intraepithelial neoplasia
PDT - photodynamic therapy
ANSWERS
(A) T (B) F (C) F (D) F;
(A) F (B) F (C) T (D) F;
(A) F (B) F (C) F (D) T;
(A) T (B) F (C) T (D) F;
(A) F (B) F (C) T (D) F.
Footnotes
Conflict of interest: none stated
References
- 1.Corder A P, Jones R H, Sadler G H.et al Heartburn, esophagitis and Barrett's esophagus in self‐medicating patients in general practice. Br J Clin Pract 199650245–248. [PubMed] [Google Scholar]
- 2.Winters C, Jr, Spurling T J, Chobanian S J.et al Barrett's esophagus. A prevalent, occult complication of gastroesophageal reflux disease. Gastroenterology 198792118–124. [PubMed] [Google Scholar]
- 3.Csendes A, Smok G, Burdiles P.et al Prevalence of Barrett's esophagus by endoscopy and histologic studies: a prospective evaluation of 306 control subjects and 376 patients with symptoms of gastroesophageal reflux. Dis Esophagus 2000135–11. [DOI] [PubMed] [Google Scholar]
- 4.Sharma P, McQuaid K, Dent J.et al A critical review of the diagnosis and management of Barrett's esophagus: the AGA Chicago workshop. Gastroenterology 2004127310–330. [DOI] [PubMed] [Google Scholar]
- 5.Miros M, Kerlin P, Walker N. Only patients with dysplasia progress to adenocarcinoma in Barrett's esophagus. Gut 1991321441–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reid B J, Levine D S, Longton G.et al Predictors of progression to cancer in Barrett's esophagus: baseline histology and flow cytometry identify low‐ and high‐risk patient subsets. Am J Gastroenterol 200 951669–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sampliner R E. Updated guidelines for the diagnosis, surveillance, and therapy of Barrett's esophagus. Am J Gastroenterol 2002971888–1895. [DOI] [PubMed] [Google Scholar]
- 8.Collard J M. High grade dysplasia in Barrett's esophagus. The case for esophagectomy. Chest Surg Clin North Am 20021277–92. [DOI] [PubMed] [Google Scholar]
- 9.Spechler S J. Dysplasia in Barrett's esophagus: limitations of current management strategies. Am J Gastroenterol 2005100927–935. [DOI] [PubMed] [Google Scholar]
- 10.Levine D S, Haggitt R C, Blount P L.et al An endoscopic biopsy protocol can differentiate high grade dysplasia from early adenocarcinoma in Barrett's esophagus. Gastroenterology 199310540–50. [DOI] [PubMed] [Google Scholar]
- 11.Overholt B F, Lightdale C J, Wang K K.et al Photodynamic therapy with porfimer sodium for ablation of high‐grade dysplasia in Barrett's esophagus: international, partially blinded, randomized phase III trial. Gastrointest Endosc 200562488–498. [DOI] [PubMed] [Google Scholar]
- 12.Peters F, Kara M, Rosmolen W.et al Poor results of 5‐aminolevulinic acid‐photodynamic therapy for residual high‐grade dysplasia and early cancer in Barrett's esophagus after endoscopic resection. Endoscopy 200537418–424. [DOI] [PubMed] [Google Scholar]
- 13.Van Laethem J L, Jagodzinski R, Peny M O.et al Argon plasma coagulation in the treatment of Barrett's high‐grade dysplasia and in situ adenocarcinoma. Endoscopy 200133257–261. [DOI] [PubMed] [Google Scholar]
- 14.Kahaleh M, Van Laethem J L, Nagy N.et al Long‐term follow‐up and factors predictive of recurrence in Barrett's esophagus treated by argon plasma coagulation and acid suppression. Endoscopy 200234950–955. [DOI] [PubMed] [Google Scholar]
- 15.Soetikno R M, Gotoda T, Nakanishi Y.et al Endoscopic mucosal resection. Gastrointest Endosc 200357567–579. [DOI] [PubMed] [Google Scholar]
- 16.Holscher A H, Bollschweiler E, Schneider P M.et al Early adenocarcinoma in Barrett's oesophagus. Br J Surg 1997841470–1473. [PubMed] [Google Scholar]
- 17.Stein H J, Feith M, Mueller J.et al Limited resection for early adenocarcinoma in Barrett's esophagus. Ann Surg 2000232733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feith M, Stein H J, Siewert J R. Pattern of lymphatic spread of Barrett's cancer. World J Surg 2003271052–1057. [DOI] [PubMed] [Google Scholar]
- 19.Vieth M, Ell C, Gossner L.et al Histological analysis of endoscopic resection specimens from 326 patients with Barrett's esophagus and early neoplasia. Endoscopy 200436776–781. [DOI] [PubMed] [Google Scholar]
- 20.Gotoda T, Yanagisawa A, Sasako M.et al Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric cancer 20003219–225. [DOI] [PubMed] [Google Scholar]
- 21.Falk G W, Catalano M F, Sivak M V., Jret al Endosonography in the evaluation of patients with Barrett's esophagus and high grade dysplasia. Gastrointest Endosc 199440207–212. [DOI] [PubMed] [Google Scholar]
- 22.Waxman I, Raju G S, Critchlow J.et al High frequency probe ultrasonography has limited accuracy for detecting invasive adenocarcinoma in patients with Barrett's esophagus and high grade dysplasia or intramucosal carcinoma: A case series. Am J Gastroenterol . 2006;;1011773–1779. [DOI] [PubMed]
- 23.May A, Günter E, Roth F.et al Accuracy of staging in early esophageal cancer using high resolution endoscopy and high resolution endosonography: a comparative, prospective, and blinded trial. Gut 200453634–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shami V M, Villaverde A, Stearns L.et al Clinical impact of conventional endosonography and endoscopic ultrasound guided fine needle aspiration in the assessment of patients with Barrett's esophagus and high grade dysplasia or intramucosal carcinoma who have been referred for endoscopic ablation therapy. Endoscopy 200638157–161. [DOI] [PubMed] [Google Scholar]
- 25.Deyhle P, Largiader F, Jenny S. A method for endoscopic electroresection of sessile colonic polyps. Endoscopy 1973538–40. [Google Scholar]
- 26.Tada M, Shimada M, Murukami F.et al Development of the strip‐off biopsy [in Japanese with English abstract]. Gastroenterol Endosc 198426833–839. [Google Scholar]
- 27.Soehendra N, Binmoeller K F, Bohnacker S.et al Endoscopic snare mucosectomy in the esophagus without any additional equipment: a simple technique for resection of flat early cancer. Endoscopy 199729380–383. [DOI] [PubMed] [Google Scholar]
- 28.Inoue H, Takeshita K, Hori H.et al Endoscopic mucosal resection with a cap‐fitted panendoscope for esophagus, stomach and colon mucosal lesions. Gastrointest Endosc 19933958–62. [DOI] [PubMed] [Google Scholar]
- 29.Makuuchi H, Machimura T, Soh Y. Endoscopic mucosectomy for mucosal carcinomas in the esophagus. J Jpn Gastroenterol Surg 1991242599–2603. [Google Scholar]
- 30.Chaves D M, Sakai P, Mester M.et al A new endoscopic technique for the resection of flat polypoid lesions. Gastrointest Endosc 199440224–226. [DOI] [PubMed] [Google Scholar]
- 31.Fleischer D E, Wang G Q, Dawsey S.et al Tissue band ligation followed by snare resection (band and snare): a new technique for tissue acquisition in the esophagus. Gastrointest Endosc 19964468–72. [DOI] [PubMed] [Google Scholar]
- 32.Soehendra N, Seewald S, Groth S.et al Use of modified multiband ligator facilitates circumferential endoscopic mucosal resection in Barrett's esophagus (+ video). Gastrointest Endosc 200663847–852. [DOI] [PubMed] [Google Scholar]
- 33.Hosokawa K, Yoshida S. Recent advances in endoscopic mucosal resection for early gastric cancer [in Japanese with English abstract]. Jpn J Cancer Chemother 199825476–483. [PubMed] [Google Scholar]
- 34.Ohkuwa M, Hosokawa K, Boku N.et al New endoscopic treatment for intramucosal gastric tumors using an insulated‐tip diathermic knife. Endoscopy 200133221–226. [DOI] [PubMed] [Google Scholar]
- 35.Ono H, Kondo H, Gotoda T.et al Endoscopic mucosal resection for treatment of early gastric cancer. Gut 200148225–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oyama T, Tomori A, Hotta K.et al Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol 20053(suppl 1)S67–S70. [DOI] [PubMed] [Google Scholar]
- 37.Rösch T, Sarbia M, Schumacher B.et al Attempted endoscopic en bloc resection of mucosal and submucosal tumors using insulated tip knives: a pilot series (including videos). Endoscopy 200436788–801. [DOI] [PubMed] [Google Scholar]
- 38.Canto M I. Chromoendoscopy and magnifying endoscopy for Barrett's esophagus. Clin Gastroenterol Hepatol 20053(Suppl 1)S12–S15. [DOI] [PubMed] [Google Scholar]
- 39.Seewald S, Akaraviputh T, Seitz U.et al Circumferential EMR and complete removal of Barrett's epithelium: a new approach to management of Barrett's esophagus containing high grade intraepithelial neoplasia and intramucosal carcinoma. Gastrointest Endosc 200357854–859. [DOI] [PubMed] [Google Scholar]
- 40.Giovannini M, Bories E, Pesenti C.et al Circumferential endoscopic mucosal resection in Barrett's esophagus with high grade intraepithelial neoplasia or mucosal cancer. Preliminary results in 21 patients. Endoscopy 200436782–787. [DOI] [PubMed] [Google Scholar]
- 41.May A, Gossner L, Behrens A.et al A prospective randomized trial of two different endoscopic resection techniques for early stage cancer of the esophagus. Gastrointest Endosc 200358167–175. [DOI] [PubMed] [Google Scholar]
- 42.Ell C, May A, Gossner L.et al Endoscopic mucosal resection of early cancer and high grade dysplasia in Barrett's esophagus. Gastroenterology 2000118670–677. [DOI] [PubMed] [Google Scholar]
- 43.May A, Gossner L, Pech O.et al Local endoscopic therapy for intraepithelial high grade neoplasia and early adenocarcinoma in Barrett's esophagus: acute phase and intermediate results of a new treatment approach. Eur J Gastroenterol Hepatol 2002141085–1091. [DOI] [PubMed] [Google Scholar]
- 44.May A, Gossner L, Pech O.et al Intraepithelial high grade neoplasia and early adenocarcinoma in short segment Barrett's esophagus (SSBE): curative treatment using local endoscopic treatment techniques. Endoscopy 200234604–610. [DOI] [PubMed] [Google Scholar]
- 45.Peters F P, Mohammed A K, Rosmolen W D.et al Endoscopic treatment of high grade dysplasia and early stage cancer in Barrett's esophagus. Gastrointest Endosc 200561506–514. [DOI] [PubMed] [Google Scholar]
- 46.Conio M, Repici A, Cestari R.et al Endoscopic mucosal resection for high grade dysplasia and intramucosal carcinoma in Barrett's esophagus: an Italian experience. World J Gastroenterol 2005116650–6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Falk G W, Rice T W, Goldblum J R.et al Jumbo biopsy forceps protocol still misses unsuspected cancer in Barrett's esophagus with high grade dysplasia. Gastrointest Endosc 199949170–176. [DOI] [PubMed] [Google Scholar]
- 48.Peters J H, Clark G W, Ireland A P.et al Outcome of adenocarcinoma arising in Barrett's esophagus in endoscopically surveyed and nonsurveyed patients. J Thorac Cardiovasc Surg 1994108813–821. [PubMed] [Google Scholar]
- 49.Reid B J, Blount P L, Feng Z.et al Optimizing endoscopic biopsy detection of early cancers in Barrett's high grade dysplasia. Am J Gastroenterol 2000953089–3096. [DOI] [PubMed] [Google Scholar]
- 50.Pacifico R J, Wang K K, Wongkeesong L M.et al Combined endoscopic mucosal resection and photodynamic therapy versus esophagectomy for management of early adenocarcinoma in Barrett's esophagus. Clin Gastroenetrol Hepatol 20031252–257. [PubMed] [Google Scholar]