Abstract
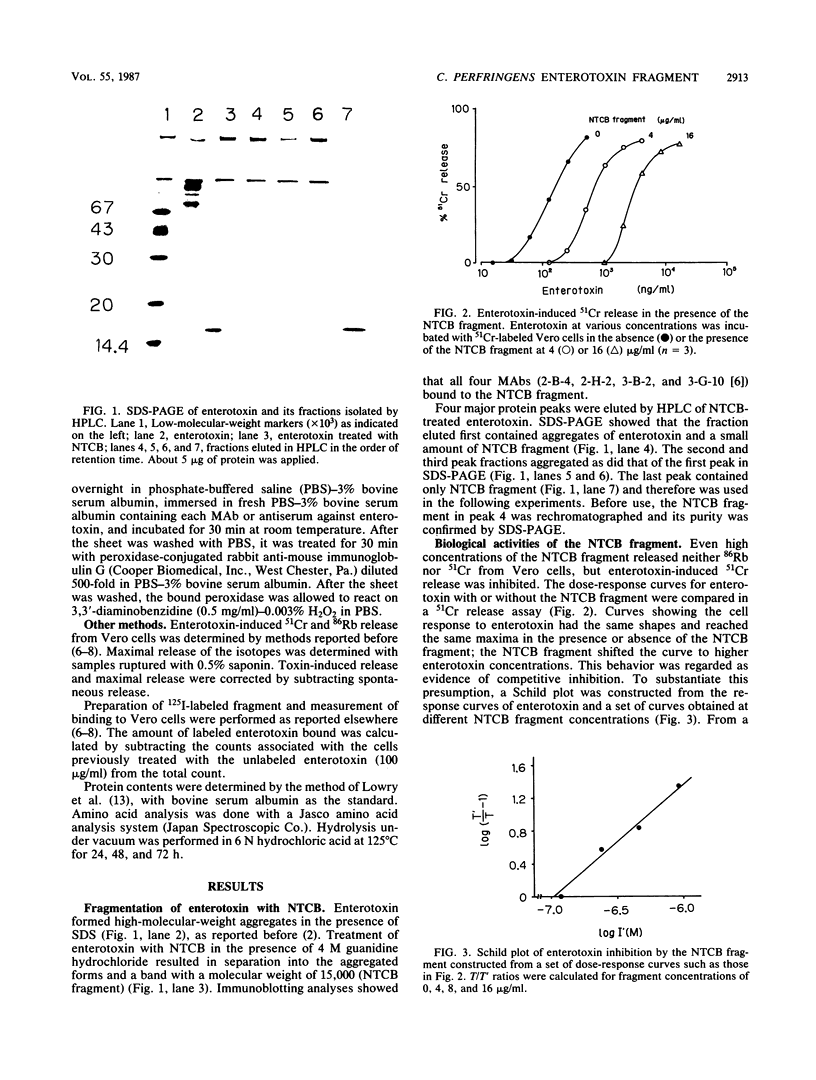
A fragment was obtained by treating Clostridium perfringens enterotoxin with 2-nitro-5-thiocyanobenzoic acid, a reagent which specifically cleaves the amino-terminal peptide bond of cysteine residues. The fragment (molecular weight, 15,000) was purified by high-performance liquid chromatography. The fragment had no cytotoxic effect on Vero cells but competitively inhibited enterotoxin-induced 51Cr release. Binding of 125I-labeled fragment to Vero cells was comparable to that of enterotoxin. Moreover, 125I-labeled fragment did not bind to FL cells, which lack receptor for enterotoxin. We conclude that the fragment contains the binding domain of enterotoxin. The amino acid composition of the fragment suggests that it is located on the carboxyl-terminal part of enterotoxin.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Degani Y., Patchornik A. Cyanylation of sulfhydryl groups by 2-nitro-5-thiocyanobenzoic acid. High-yield modification and cleavage of peptides at cysteine residues. Biochemistry. 1974 Jan 1;13(1):1–11. doi: 10.1021/bi00698a001. [DOI] [PubMed] [Google Scholar]
- Enders G. L., Jr, Duncan C. L. Anomalous aggregation of Clostridium perfringens enterotoxin under dissociating conditions. Can J Microbiol. 1976 Sep;22(9):1410–1414. doi: 10.1139/m76-209. [DOI] [PubMed] [Google Scholar]
- Granum P. E. Inhibition of protein synthesis by a tryptic polypeptide of Clostridium perfringens type A enterotoxin. Biochim Biophys Acta. 1982 Oct 20;708(1):6–11. doi: 10.1016/0167-4838(82)90196-0. [DOI] [PubMed] [Google Scholar]
- Hauschild A. H., Hilsheimer R. Purification and characteristics of the enterotoxin of Clostridium perfringens type A. Can J Microbiol. 1971 Nov;17(11):1425–1433. doi: 10.1139/m71-227. [DOI] [PubMed] [Google Scholar]
- Horiguchi Y., Uemura T., Kamata Y., Kozaki S., Sakaguchi G. Production and characterization of monoclonal antibodies to Clostridium perfringens enterotoxin. Infect Immun. 1986 Apr;52(1):31–35. doi: 10.1128/iai.52.1.31-35.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi Y., Uemura T., Kozaki S., Sakaguchi G. Effects of Ca2+ and other cations on the action of Clostridium perfringens enterotoxin. Biochim Biophys Acta. 1986 Oct 31;889(1):65–71. doi: 10.1016/0167-4889(86)90009-1. [DOI] [PubMed] [Google Scholar]
- Ittelson T. R., Gill D. M. Diphtheria toxin: specific competition for cell receptors. Nature. 1973 Mar 30;242(5396):330–332. doi: 10.1038/242330b0. [DOI] [PubMed] [Google Scholar]
- Jacobson G. R., Schaffer M. H., Stark G. R., Vanaman T. C. Specific chemical cleavage in high yield at the amino peptide bonds of cysteine and cystine residues. J Biol Chem. 1973 Oct 10;248(19):6583–6591. [PubMed] [Google Scholar]
- Jarmund T., Telle W. Binding of Clostridium perfringens enterotoxin to hepatocytes, small intestinal epithelial cells and Vero cells. Acta Pathol Microbiol Immunol Scand B. 1982 Oct;90(5):377–381. doi: 10.1111/j.1699-0463.1982.tb00134.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsuda M., Ozutsumi K., Iwahashi H., Sugimoto N. Primary action of Clostridium perfringens type A enterotoxin on HeLa and Vero cells in the absence of extracellular calcium: rapid and characteristic changes in membrane permeability. Biochem Biophys Res Commun. 1986 Dec 15;141(2):704–710. doi: 10.1016/s0006-291x(86)80229-7. [DOI] [PubMed] [Google Scholar]
- Matsuda M., Sugimoto N. Calcium-independent and dependent steps in action of Clostridium perfringens enterotoxin on HeLa and Vero cells. Biochem Biophys Res Commun. 1979 Nov 28;91(2):629–636. doi: 10.1016/0006-291x(79)91568-7. [DOI] [PubMed] [Google Scholar]
- McClane B. A., McDonel J. L. Characterization of membrane permeability alterations induced in Vero cells by Clostridium perfringens enterotoxin. Biochim Biophys Acta. 1980 Aug 14;600(3):974–985. doi: 10.1016/0005-2736(80)90499-x. [DOI] [PubMed] [Google Scholar]
- McClane B. A., McDonel J. L. Protective effects of osmotic stabilizers on morphological and permeability alterations induced in Vero cells by Clostridium perfringens enterotoxin. Biochim Biophys Acta. 1981 Mar 6;641(2):401–409. doi: 10.1016/0005-2736(81)90496-x. [DOI] [PubMed] [Google Scholar]
- McClane B. A. Osmotic stabilizers differentially inhibit permeability alterations induced in Vero cells by Clostridium perfringens enterotoxin. Biochim Biophys Acta. 1984 Oct 17;777(1):99–106. doi: 10.1016/0005-2736(84)90501-7. [DOI] [PubMed] [Google Scholar]
- McDonel J. L. Binding of Clostridium perfringens [125I]enterotoxin to rabbit intestinal cells. Biochemistry. 1980 Oct 14;19(21):4801–4807. doi: 10.1021/bi00562a014. [DOI] [PubMed] [Google Scholar]
- Richardson M., Granum P. E. The amino acid sequence of the enterotoxin from Clostridium perfringens type A. FEBS Lett. 1985 Mar 25;182(2):479–484. doi: 10.1016/0014-5793(85)80358-6. [DOI] [PubMed] [Google Scholar]
- Sakaguchi G., Uemura T., Riemann H. P. Simplified method for purification of Clostridium perfringens type A enterotoxin. Appl Microbiol. 1973 Nov;26(5):762–767. doi: 10.1128/am.26.5.762-767.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinovich O., Mattice W. L., Blakeney E. W., Jr Effects of temperature, pH and detergents on the molecular conformation of the enterotoxin of Clostridium perfringens. Biochim Biophys Acta. 1982 Sep 22;707(1):147–153. doi: 10.1016/0167-4838(82)90408-3. [DOI] [PubMed] [Google Scholar]
- Skjelkvåle R., Tolleshaug H., Jarmund T. Binding of enterotoxin from Clostridium perfringens type A to liver cells in vivo and in vitro. The enterotoxin causes membrane leakage. Acta Pathol Microbiol Scand B. 1980 Apr;88(2):95–102. doi: 10.1111/j.1699-0463.1980.tb02612.x. [DOI] [PubMed] [Google Scholar]
- Stark R. L., Duncan C. L. Purification and biochemical properties of Clostridium perfringens type A enterotoxin. Infect Immun. 1972 Nov;6(5):662–673. doi: 10.1128/iai.6.5.662-673.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto N., Ozutsumi K., Matsuda M. Morphological alterations and changes in cellular cations induced by Clostridium perfringens type A enterotoxin in tissue culture cells. Eur J Epidemiol. 1985 Dec;1(4):264–273. doi: 10.1007/BF00237101. [DOI] [PubMed] [Google Scholar]
- Tolleshaug H., Skjelkvåle R., Berg T. Quantitation of binding and subcellular distribution of Clostridium perfringens enterotoxin in rat liver cells. Infect Immun. 1982 Aug;37(2):486–491. doi: 10.1128/iai.37.2.486-491.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wnek A. P., Strouse R. J., McClane B. A. Production and characterization of monoclonal antibodies against Clostridium perfringens type A enterotoxin. Infect Immun. 1985 Nov;50(2):442–448. doi: 10.1128/iai.50.2.442-448.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]



