Abstract
Levodopa is the most effective drug for treating Parkinson's disease. However, long‐term use of levodopa is often complicated by significantly disabling fluctuations and dyskinesias negating its beneficial effects. Younger age of Parkinson's disease onset, disease severity, and high levodopa dose increase the risk of development of levodopa‐induced dyskinesias (LID). The underlying mechanisms for LID are unclear though recent studies indicate the importance of pulsatile stimulation of striatal postsynaptic receptors in their pathogenesis. The non‐human primates with MPTP‐induced parkinsonism serve as a useful model to study dyskinesia. Once established, LID are difficult to treat and therefore efforts should be made to prevent them. The therapeutic and preventative strategies for LID include using a lower dosage of levodopa, employing dopamine agonists as initial therapy in Parkinson's disease, amantadine, atypical neuroleptics, and neurosurgery. LID can adversely affect the quality of life and increase the cost of healthcare.
Levodopa‐induced dyskinesias (LID) were first reported by Cotzias et al, the group credited with the first successful use of levodopa in treating Parkinson's disease.1 Subsequent reports highlighted their high incidence, varied phenomenology, and treatment‐limiting effect. Initially thought to be associated only with the peak plasma levels of levodopa, later reports of diphasic dyskinesias and early morning dystonia emphasised a rather complex picture of LID.
EPIDEMIOLOGY AND RISK FACTORS
The reported incidence rates of LID show a wide range, from 9–80%.2,3 This is unsurprising as the risk of developing LID depends on age of onset and severity of Parkinson's disease, dose and duration of levodopa therapy, and possibly on some hitherto unknown factors. Moreover, methodological differences in the reporting of LID (for example, relying on patient's history alone versus detection of any dyskinesia by a detailed neurological examination) and lack of a universally agreed assessment scale may account for the differences among studies. The earlier reports of higher rates (81% after 12 months of levodopa therapy)2 as compared to the significantly lower rates (17% after 12 months) observed in later studies3 could be explained on the basis of introduction of levodopa at high dosage in relatively advanced stage of Parkinson's disease in the immediate post‐levodopa era.
Young‐onset Parkinson's disease is associated with a higher incidence of LID.4 The 5‐year risk of LID was 50% in patients with disease onset between 40–59 years of age as compared to 16% in those with disease onset after 70 years. The reason for this propensity is not entirely clear though an interesting suggestion is that there are age‐related differences in levodopa dynamics in Parkinson's disease.5 The presence and extent of nigral denervation (disease severity) are important risk factors. With the dose of levodopa used in clinical practice, LID occur almost exclusively in patients with idiopathic Parkinson's disease, whereas normal people and those with other neurological diseases do not develop LID.6,7 In an animal model of MPTP (1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine)‐induced Parkinson's disease, animals receiving saline plus levodopa did not develop dyskinesias in contrast to the MPTP‐treated group that developed significant dyskinesias, implying that nigral damage is required for the production of LID.8 Patients with post‐encephalitis parkinsonism and MPTP‐induced parkinsonism develop dyskinesias within weeks of starting levodopa, suggesting severity of the nigral lesion as an important risk factor.9,10 The observation that dyskinesias tend to develop first on the side worst affected by Parkinson's disease also lends support to this theory.
The dose of levodopa is important in the production of LID. In the DATATOP study,3 at the same follow‐up time, a mean (SD) daily levodopa dose of 338 (140) mg was not associated with LID, while LID developed at a mean daily dose of 387 (169) mg. Larger doses of levodopa are associated with prolonged dyskinesias.11 When used in very high doses, levodopa can produce dyskinesias in normal monkeys. LID do not appear early in the course of therapy. Most studies have reported an increase in the frequency of LID with increasing period of follow‐up.2,3 Recently, an association of LID with weight loss has been reported.12
A significant number of patients do not develop dyskinesias despite prolonged continued levodopa therapy. Genetic factors could play a role in determining the occurrence of peak‐dose dyskinesias. Certain types of genetic polymorphism of the dopamine receptor D2 gene have been associated with a reduced the risk of developing peak‐dose dyskinesias.13
PATHOPHYSIOLOGY OF LID
Despite significant advances, the pathogenesis of LID remains incompletely understood. It is known that dyskinesias appear only after dopaminergic therapy and there is a time lag between the start of treatment and the emergence of LID.
Several possible mechanisms, both peripheral and central, have been proposed. They include reduced buffering capacity of the remaining intact neurons, dietary proteins, role of D3 receptors, and the role of glutamate receptors. While dietary proteins and gastric absorption have some relevance in producing fluctuations and “wearing off” associated with chronic levodopa therapy, central mechanisms are of greater importance for the genesis of LID.
It is suggested that pulsatile (as opposed to a continuous, physiological) stimulation of the postsynaptic receptors due to intermittent administration of levodopa leads to downstream changes in proteins and genes, causing alterations in striatal output in a way that promotes dyskinesias.14 Disinhibition of the primary and associated motor cortex secondary to increased outflow (pallidothalamocortical motor pathway) may account for LID.15
Recent observations indicate overactivity of glutamatergic systems (using N‐methyl‐D‐aspartate (NMDA) receptors) in the basal ganglia in patients with LID.16 Both dopamine and NMDA receptors are expressed along the dendritic spines of striatal medium sized γ‐aminobutyric acid (GABA)‐ergic neurones. Corticostriatal glutamatergic projections synapse primarily at the distal tips of these spines, whereas nigrostriatal dopaminergic fibres synapse slightly more proximally, allowing close functional alterations. Chronic intermittent stimulation of normally tonically active dopaminergic receptors brings about alterations in cell signals in striatal dopaminergic medium spiny neurones. This causes potentiation of the GABA‐ergic efferents, particularly, glutamate receptors of the NMDA‐subtype. In postmortem samples from levodopa treated parkinsonian patients, increased GABA (A) receptors content in the internal globus pallidus were found in dyskinetic patients compared with non‐dyskinetic patients.17 Further support for this theory comes from animal studies showing reduction of dyskinesias using NMDA antagonist dextrophan with levodopa in MPTP treated monkeys18 and from clinical observations of the efficacy of amantidine in treating LID.
Abnormalities in other non‐dopaminergic transmission—for example, α2 adrenergic, serotonergic (5HT), cannabinoid and opioid mechanisms—in both priming and expression of LID have also been reported.19
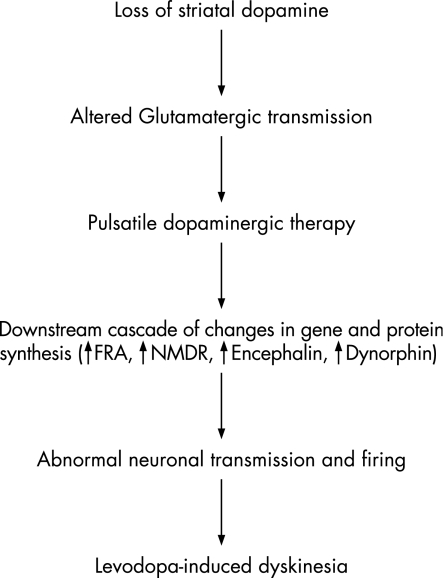
The details of changes in genes and proteins caused by abnormalities in the dopaminergic and non‐dopaminergic transmission eventually leading to LID are beginning to be clear. Fos‐ and Fos‐related proteins (FRA) are induced in the striatal neurons by excessive glutamatergic inputs (caused by loss of striatal dopamine).20 FRAs couple with a transcription factor, called Jun‐D, forming activator protein‐1AP‐1 complexes that can affect several proteins and genes, including encephalin, dynorphin and NMDA receptors (fig 1).
Figure 1 Schematic representation of sequence of events leading to levodopa‐induced dyskinesia (LID). FRA, Fos‐related proteins; NMDA, N‐methyl‐D‐aspartate.
CLINICAL FEATURES AND CLASSIFICATION OF LID
LID are clinically heterogeneous. They commonly present as chorea or choreoathetosis, though myoclonus, akathasia, ballism and other forms of abnormal movements have also been described. LID generally first appear on the side worst affected by Parkinson's disease and in legs before arms. This could be related to an early dopaminergic loss in the dorsolateral striatum, the region corresponding somatotopically to the foot area.
Chorea refers to involuntary, rapid, irregular, purposeless, and unsustained movements that seem to flow from one body part to another. The severity of these movements can vary from occasional abnormal movements that are absent at rest and provoked only during active movement—for example, walking or talking (the so‐called overflow chorea)—to violent large amplitude flinging and flailing arm movements—the ballism. Often, there are superimposed writhing athetoid movements—choreoathetosis. Dyskinesias may predominantly affect particular body parts—for example, torso, head and neck, limbs—or speech or respiratory muscles.
Dystonia is the second most common form of LID presenting as sustained muscle contractions. It occurs either in pure form or in combination with the chorea, in the latter case manifesting as twisting of the leg on walking or the arm being pulled behind the back. Dystonia accounts for greater disability than chorea. “Off” time dystonias are usually painful.
Uncommon forms of LID include akathasia (excessive motor restlessness), a high stepped overshooting gait, rapid alternating movements (RAM) of legs, blepharospasm, and mixed pattern of abnormal movements.21
Based on their relationship with levodopa dosing, LID are classified as peak‐dose, diphasic, off state, on state, and yo yo dyskinesias (box 1).
Box 1 Classification of levodopa‐induced dyskinesias
Peak dose dyskinesia
Diphasic dyskinesia
“Off” state dystonia
“On” state dystonia
Yo‐yoing
Peak‐dose dyskinesias—These are the most common forms of LID and are related to peak plasma (and possibly high striatal) levels of levodopa. They involve the head, trunk, and limbs, and sometimes respiratory muscles. Dose reduction can ameliorate them, frequently at the cost of deterioration of parkinsonism. Peak‐dose dyskinesias are usually choreiform, though in the later stages dystonia can superimpose.
Diphasic dyskinesias—These develop when plasma levodopa levels are rising or falling, but not with the peak levels. They are also called D‐I‐D (dyskinesia‐improvement‐dyskinesia). D‐I‐D are commonly dystonic in nature, though chorea or mixed pattern may occur. They do not respond to levodopa dose reduction and may rather improve with high dose of levodopa.
“Off” state dystonias—These occur when plasma levodopa levels are low (for example, in the morning). They are usually pure dystonia occurring as painful spasms in one foot. They respond to levodopa therapy. Rare forms of LID include “on” state dystonias (occurring during higher levels of levodopa) and yo‐yo dyskinesia (completely unpredictable pattern).
CONSEQUENCES OF LID
The emergence of dyskinesias often proves to be a turning point in the course of treated Parkinson's disease. In their mildest form, dyskinesias may not bother the patient and be noticed only by the spouse or carers. In fact, patients may prefer mobility associated with dyskinesia to immobility with no dyskinesia. With the worsening of dyskinesias, significant limitations ensue. LID lead to exhaustion and fatigue. The risk of injury to the patient (and carers) is constant. The weight loss caused by excessive movements may prompt extensive and often unnecessary investigations. The painful dystonias cause pronounced discomfort and physical limitation. The patients often limit their social life leading to isolation, frustration, anger, and depression. The burden of care is increased. Rare fatalities (possibly related to cardiac arrhythmias) have also been reported.22
From the view of health care services, dyskinesias have major consequences. LID tend to appear at a time when the disease is advancing and there is often a need to increase the dose of levodopa. Increasing the dose of levodopa is associated with a worsening of dyskinesias, while dose reductions lead to poor control of Parkinson's disease. LID are associated with poor quality of life and increased healthcare costs.23
ASSESSMENT OF LID
There is no universally agreed tool available for recording dyskinesias accurately and reliably. This may account for the variability of results in various studies of incidence and prevalence rates of dyskinesias. Given their intermittent nature, dyskinesias may not be present during a clinical encounter with the patient. The Unified Parkinson's Disease Rating Scale (UPDRS), the most commonly used scale to assess disability in Parkinson's disease, includes a section on dyskinesia wherein the patient's account of duration of dyskinesia and associated disability and pain are recorded.24 The patient may not be relied upon to give an accurate account, especially when dyskinesias are mild and intermittent. The assessment of dyskinesia, therefore, remains largely subjective and often inaccurate.
The indicators of severity of dyskinesias include amplitude, duration, the number of body parts affected, or by the disability caused. The Global Primate Dyskinesia Rating Scale (GPDRS) has been developed and validated for studying dyskinesias in primates.25 There is an urgent need to develop a similar scale in humans. Recently, the use of digitised spiral drawing tasks has been suggested as a method to quantify drug‐induced dyskinesias in upper limbs.26
PREVENTION OF LID
As pulsatile stimulation of denervated dopaminergic receptors with consequent downstream molecular changes is considered to be important in the genesis of LID, it is anticipated that therapeutic strategies aimed to counter them should prevent LID.
Use of controlled‐release preparations of levodopa
The standard preparation of levodopa with a short half‐life has the potential for pulsatile stimulation of the post‐synaptic receptors. It was hoped that a controlled‐release formulation (for example, Sinemet CR) with a longer half‐life would obviate this problem. However, a study comparing standard formulation of levodopa with a controlled‐release type in early Parkinson's disease showed no difference in the frequency of LID after 5 years of follow‐up.27 However, the controlled release formulation has variable absorption and it was administered in a twice‐daily regimen. This could have accounted for the failure to achieve continuous dopaminergic stimulation.
Continuous delivery of levodopa
Continuous intraduodenal infusion of the levodopa/carbidopa enteral gel has been used successfully in treating patients with advanced Parkinson disease and shows no increase in dyskinesia as compared to oral polypharmacy.28 Subcutaneous or intramuscular injections of levodopa methyl ester and intravenous infusion of levodopa have also given similar results.29,30 However, limitations of such strategies in clinical practice are obvious.
Using catechol‐O‐methyl transferase (COMT) inhibitors
The inhibitors of the enzyme catechol‐O‐methyl transferase (COMT) extend the half‐life of levodopa. Entacapone and tolcapone are two such agents used in clinical practice. Tolcapone has been associated with significant hepatotoxicity, necessitating regular monitoring of liver function tests. In an animal study using rats, co‐administration of entacapone with levodopa attenuated all kinds of dyskinesia when compared to levodopa monotherapy.31 Stalevo (Orion), a commercially available formulation, combines levodopa, dopa‐decarboxylase inhibitor carbidopa and entacapone in a single tablet. It is hoped that early use of Stalevo might reduce the incidence of dyskinesia.
Using dopamine receptor agonists
Dopamine receptor agonists have a longer half‐life than levodopa. The indication of their potential to prevent dyskinesias was first reported in an animal study using bromocriptine.32 Since then there have been long‐term, prospective studies confirming the efficacy of dopamine agonists in protecting against dyskinesias. A prospective, randomised, double‐blind study, compared the safety and efficacy of the dopamine D2‐receptor agonist ropinirole with that of levodopa over a period of five years in patients with early Parkinson's disease and the primary outcome measure was the occurrence of dyskinesia.33 If symptoms were not adequately controlled by ropinirole, patients could receive supplementary levodopa, administered in an open‐label fashion. At 5 years, the cumulative incidence of dyskinesia regardless of levodopa supplementation was 20% in the ropinirole group and 45% in the levodopa group. Similar results have been reported with pramipexole.34
Neuroprotective agents
As the extent of striatal degeneration is a potent risk factor for LID, agents that can protect against this degeneration are expected to protect against LID. Though several such drugs have been studied, none has yet been proven to be safe and effective in Parkinson's disease.
Initial claims of selegeline being a neuroprotective agent were subsequently rejected. It has a proven symptomatic effect in Parkinson's disease, confounding any possible neuroprotective effect.
The suggestion that dopamine receptor agonists may have neuroprotective effects35 is not universally accepted. In a small study, Brooks et al reported attenuation of the decline in striatal fluorodopa uptake on positron emission tomography scans in patients on ropinirole when compared with those on levodopa.36
Riluzole, an anti‐NMDA receptor agent, delays disability in some patients with amyotrophic lateral sclerosis. However, it showed disappointing results in a small, double‐blind study in LID.37
A number of other putative neuroprotective agents have been tested. These include neurotrophic factors, immunomodulators, antioxidants and free radical scavengers, anti‐apoptotic agents, capsase inhibitors, etc. Some of these agents have also been tested in small clinical trials, but none can currently be recommended for routine clinical use. A detailed account of neuroprotection is beyond the scope of this article and readers are referred to a recent review on this subject.38
TREATMENT OF ESTABLISHED LID
Reduction of levodopa doses
The peak‐dose LID almost always respond to a dose reduction. However, this results in worsening of parkinsonism and increasing “off” periods. The strategy of temporary withdrawal of levodopa (“drug holiday”) is not used as it is often associated with significant worsening of Parkinson's disease and dyskinesias are only slightly reduced for a short period of time.39 The frequent and small doses often fail to achieve desired results. Patients prefer mobility associated with dyskinesias to immobility with no dyskinesias.
Using dopamine receptor agonists
As mentioned previously, initiating treatment with dopamine receptor agonists is associated with less dyskinesias. Monotherapy with dopamine receptor agonists has been shown to be effective in controlling symptoms of Parkinson's disease for up to 5 years.34 However, most patients eventually need additional levodopa to optimise control of Parkinson's disease. The ropinirole study showed lower incidence of LID even when levodopa was added to ropinirole. In clinical practice, dopamine receptor agonists are often added to keep doses of levodopa lower. Though an effective strategy in improving disease control, it is not known whether it reduces dyskinesias in patients already with levodopa priming.
Apomorphine, a parenteral dopamine receptor agonist, can reduce “off” states and reduce dyskinesia. A meta‐analysis of several long‐term, open‐label, uncontrolled studies involving a total of 233 patients reported efficacy of continuous subcutaneous apomorphine infusions in advanced Parkinson's disease.40 The use of subcutaneous apomorphine infusion was successful in aborting “off” periods, reducing dyskinesias and improving Parkinson's disease motor scores, with the added benefit of a substantial levodopa‐sparing effect.
Drugs acting on NMDA receptors
Based on the importance of overexpression of NMDA receptors in LID, NMDA antagonists have been tried as potential treatment for LID. In monkeys with MPTP‐induced lesions, Papa et al reported useful antidyskinetic effects using an experimental selective NMDA antagonist.41 In humans, amantadine can reduce dyskinesias without worsening parkinsonian symptoms. The antidyskinetic effect of amantidine is mediated via the inhibition of NMDA receptors. In a randomised, double‐blind, placebo‐controlled study of 18 consecutive Parkinson's disease patients, amantidine reduced the duration of LID by 60%.42 A recent evidence‐based review supports the use of amantidine in LID.43 Tachyphylaxis can be a limiting factor in its use.
Drugs acting on serotonergic systems
The basal ganglia have dense serotonergic (5‐HT) innervation. It is suggested that serotonergic transmission has an inhibitory effect on dopaminergic transmission. There are reports of successful use of 5‐HT agents in treating LIDs.44,45 However, these studies included very small numbers and were mostly uncontrolled.
Miscellaneous agents
Several agents of different classes have been tried in treating LID. They include drugs acting on opiate receptors, adenosine A2A receptors, cannabinoid receptors, and noradrenergic systems.46 There have been no large scale randomised trials with these drugs. Currently, none can be recommended for routine use. Clozapine, an atypical neuroleptic, has been reported to have antidyskinetic effects in double‐blind studies.47 However, quetiapine, another agent of this class, failed to show an antidyskinetic effect when used in a controlled study.48
Role of neurosurgery
Deep brain stimulation targeting the internal globus pallidum (GPi) or subthalamic nucleus (STN) has been shown to be an effective treatment for advanced Parkinson's disease. The improvement in motor symptoms is associated with a reduction in dyskinesias. A randomised, blinded pilot study comparing the safety and efficacy of STN and GPi stimulation in patients with advanced Parkinson's disease showed a reported reduction of dyskinesia by stimulation of both the GPi and STN (89% vs 62%).49 The reduction in dyskinesias may not all be attributable to the diminished requirement for levodopa, as levodopa dose was more reduced in the STN group while dyskinesias were more reduced in the GPi group.
Surgical lesions (by radiofrequency or gamma knife) targeting the thalamus, STN or GPi have been used to treat advanced Parkinson's disease. Pallidotomy is probably the most effective surgical treatment for LID. It is effective in young as well as older patients50 and benefit may persist for up to 5 years.51 Bilateral pallidotomy is associated with significant complications.
Experimental neurosurgical approaches—for example, strategies of “regeneration” or “re‐innervation”—include adrenal medullary implantation, gene therapy, and use of trophic factors.
Main points
Levodopa‐induced dyskinesias (LID) are common and serious complications of long‐term treatment with levodopa
The pathogenesis of LID is not fully known, though pulsatile stimulation of post‐synaptic striatal receptors appears to be important in their genesis
The risk factors of LID include younger age of disease onset, extent of striatal damage, and duration and doses of levodopa
Attempts should be made to prevent LID as treatment of established LID is usually very difficult
Practical approach
In routine clinical practice, attempts should be made to prevent LID. This is possible, particularly in younger and biologically fit older patients by using dopamine receptor agonists as initial monotherapy to control symptoms of Parkinson's disease. This strategy may work for a long time in some patients. However, a proportion of patients do not tolerate dopamine agonists and the majority of patients eventually need levodopa for symptom control. Levodopa should not be withheld in patients with late‐onset Parkinson's disease with significant symptoms, as the risk of LID is substantially low in these patients. Once established, LID are difficult to treat. Peak‐dose LID may respond to dose reductions. However, this leads to worsening of motor symptoms. Dose reduction plus adjunctive dopamine receptor agonist may reduce dyskinesias in some patients. When this fails, amantidine can be tried to control LID. Next line antidyskinetic therapy includes low dose clozapine with close haematological monitoring in view of its potential to cause serious blood dyscrasias. Continuous subcutaneous infusion of apomorphine can be an alternative strategy to treat difficult LIDS. When all this fails, neurosurgical approaches should be considered in appropriate patients.
Abbreviations
COMT - catechol‐O‐methyl transferase
D‐I‐D - dyskinesia‐improvement‐dyskinesia
FRA - Fos‐related proteins
GABA - γ‐aminobutyric acid
GPDRS - Global Primate Dyskinesia Rating Scale
GPi - internal globus pallidum
5‐HT - 5‐hydroxytryptamine
LID - levodopa‐induced dyskinesias
MPTP - 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine
NMDA - N‐methyl‐D‐aspartate
RAM - rapid alternating movements
STN - subthalamic nucleus
UPDRS - Unified Parkinson's Disease Rating Scale
Footnotes
Conflict of interest: None declared
References
- 1.Cotzias G C, Papavasiliou P S, Gellene R. Modification of Parkinsonism – chronic treatment with L‐dopa. N Engl J Med 1969280337–345. [DOI] [PubMed] [Google Scholar]
- 2.Duvoisin R C. Hyperkinetic responses with L‐DOPA. In: Yahr MD, ed. Current concepts in the treatment of parkinsonism. New York: Raven Press, 1974203–210.
- 3.Parkinson's Study Group Impact of deprenyl and tocopherol treatment on Parkinson's disease in DATATOP patients requiring levodopa. Parkinson Study Group. Ann Neurol 19963937–45. [DOI] [PubMed] [Google Scholar]
- 4.Kumar N, Van Gerpen J A, Bower J H.et al Levodopa‐dyskinesia incidence by age of Parkinson's disease onset. Mov Disord 200520342–344. [DOI] [PubMed] [Google Scholar]
- 5.Sossi V, de la Fuente‐Fernandez R, Schulzer M.et al Age‐related differences in levodopa dynamics in Parkinson's: implications for motor complications. Brain 2006129(Pt 4)1050–1058. [DOI] [PubMed] [Google Scholar]
- 6.Markham C H. The choreoathetoid movement disorder induced by levodopa. Clin Pharmacol Ther 197112340–343. [DOI] [PubMed] [Google Scholar]
- 7.Chase T N, Holden E M, Brody J A. Levodopa‐induced dyskinesias. Comparison in Parkinsonism‐dementia and amyotrophic lateral sclerosis. Arch Neurol 197329328–333. [DOI] [PubMed] [Google Scholar]
- 8.Di Monte D A, McCormack A, Petzinger G.et al Relationship among nigrostriatal denervation, parkinsonism, and dyskinesias in the MPTP primate model. Mov Disord 200015459–466. [DOI] [PubMed] [Google Scholar]
- 9.Sacks O W, Kohl M, Schwartz W.et al Side‐effects of L‐dopa in postencephalic parkinsonism. Lancet 1970i1006. [DOI] [PubMed] [Google Scholar]
- 10.Ballard P A, Tetrud J W, Langston J W. Permanent human parkinsonism due to 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine (MPTP): seven cases. Neurology 198535949–956. [DOI] [PubMed] [Google Scholar]
- 11.Nutt J G, Woodward W R, Carter J H.et al Effect of long‐term therapy on the pharmacodynamics of levodopa. Relation to on‐off phenomenon. Arch Neurol 1992491123–1130. [DOI] [PubMed] [Google Scholar]
- 12.Sharma J C, Macnamara L, Hasoon M.et al Cascade of levodopa dose and weight‐related dyskinesia in Parkinson's disease (LD‐WD‐PD cascade). Parkinsonism Relat Disord. 2006 Aug 24; [Epub ahead of print] [DOI] [PubMed]
- 13.Oliveri R L, Annesi G, Zappia M.et al Dopamine D2 receptor gene polymorphism and the risk of levodopa‐induced dyskinesias in PD. Neurology 1999531425. [DOI] [PubMed] [Google Scholar]
- 14.Bibbiani F, Costantini L C, Patel R.et al Continuous dopaminergic stimulation reduces risk of motor complications in parkinsonian primates. Exp Neurol 200519273–78. [DOI] [PubMed] [Google Scholar]
- 15.Rascol O, Sabatini U, Brefel C.et al Cortical motor overactivation in parkinsonian patients with L‐dopa‐induced peak‐dose dyskinesia. Brain 1998121(Pt 3)527–533. [DOI] [PubMed] [Google Scholar]
- 16.Chase T N, Bibbiani F, Oh J D. Striatal glutamatergic mechanisms and extrapyramidal movement disorders. Neurotox Res 20035(1–2)139–146. [DOI] [PubMed] [Google Scholar]
- 17.Calon F, Morissette M, Rajput A H.et al Changes of GABA receptors and dopamine turnover in the postmortem brains of parkinsonians with levodopa‐induced motor complications. Mov Disord 200318241–253. [DOI] [PubMed] [Google Scholar]
- 18.Blanchet P J, Metman L V, Mouradian M M.et al Acute pharmacologic blockade of dyskinesias in Parkinson's disease. Mov Disord 199611580–581. [DOI] [PubMed] [Google Scholar]
- 19.Brotchie J M. Nondopaminergic mechanisms in levodopa‐induced dyskinesia. Mov Disord 200520919–931. [DOI] [PubMed] [Google Scholar]
- 20.Calon F, Grondin R, Morissette M.et al Molecular basis of levodopa‐induced dyskinesias. Ann Neurol 200047(4 Suppl 1)S70–S78. [PubMed] [Google Scholar]
- 21.Fahn S. The spectrum of levodopa‐induced dyskinesias. Ann Neurol. 2000;47: S2–9; discussion S9–11, (4 Suppl 1) [PubMed]
- 22.Marsden C D, Parkes J D, Quinn N. Fluctuations of disability in Parkinson's disease: clinical aspects. In: Marsden CD, Fahn S, eds. Movement disorders. London: Butterworth, 198196–122.
- 23.Pechevis M, Clarke C E, Vieregge P, Trial Study Group et al Effects of dyskinesias in Parkinson's disease on quality of life and health‐related costs: a prospective European study. Eur J Neurol 200512956–963. [DOI] [PubMed] [Google Scholar]
- 24.Fahn S, Elton R L. UPDRS Development Committee. Unified Parkinson's disease Rating Scale. In: Fahn Marsden CD, Clane DB, Goldstein M, eds, Recent developments in Parkinson's disease. Florham Park, New Jersey: Macmillan 1987153–163.
- 25.Langston J W, Quik M, Petzinger G.et al Investigating levodopa‐induced dyskinesias in the parkinsonian primate. Ann Neurol 200047(4 Suppl 1)S79–S89. [PubMed] [Google Scholar]
- 26.Liu X, Carroll C B, Wang S Y.et al Quantifying drug‐induced dyskinesias in the arms using digitised spiral‐drawing tasks. J Neurosci Methods 200514447–52. [DOI] [PubMed] [Google Scholar]
- 27.Block G, Liss C, Reines S.et al Comparison of immediate‐release and controlled release carbidopa/levodopa in Parkinson's disease. A multicenter 5‐year study. The CR First Study Group. Eur Neurol 19973723–27. [DOI] [PubMed] [Google Scholar]
- 28.Nyholm D, Nilsson Remahl A I, Dizdar N.et al Duodenal levodopa infusion monotherapy vs oral polypharmacy in advanced Parkinson disease. Neurology 200564216–223. [DOI] [PubMed] [Google Scholar]
- 29.Djaldetti R, Melamed E. Levodopa ethylester: a novel rescue therapy for response fluctuations in Parkinson's disease. Ann Neurol 199639400–404. [DOI] [PubMed] [Google Scholar]
- 30.Schuh L A, Bennett J P., Jr Suppression of dyskinesias in advanced Parkinson's disease. I. Continuous intravenous levodopa shifts dose response for production of dyskinesias but not for relief of parkinsonism in patients with advanced Parkinson's disease. Neurology 1993431545–1550. [DOI] [PubMed] [Google Scholar]
- 31.Marin C, Aguilar E, Obeso J A. Coadministration of entacapone with levodopa attenuates the severity of dyskinesias in hemiparkinsonian rats. Mov Disord 200621646–653. [DOI] [PubMed] [Google Scholar]
- 32.Bedard P J, Di Paolo T, Falardeau P.et al Chronic treatment with L‐DOPA, but not bromocriptine induces dyskinesia in MPTP‐parkinsonian monkeys. Correlation with [3H] spiperone binding. Brain Res 1986379294–299. [DOI] [PubMed] [Google Scholar]
- 33.Rascol O, Brooks D J, Korczyn A D.et al A five‐year study of the incidence of dyskinesia in patients with early Parkinson's disease who were treated with ropinirole or levodopa. 056 Study Group. N Engl J Med 20003421484–1491. [DOI] [PubMed] [Google Scholar]
- 34.Holloway R G, Shoulson I, Fahn S.et al Parkinson Study Group. Pramipexole vs levodopa as initial treatment for Parkinson disease: a 4‐year randomized controlled trial [Erratum, Arch Neurol. 2005;62: 430]. Arch Neurol2004611044–1053. [DOI] [PubMed] [Google Scholar]
- 35.Olanow C W, Jenner P, Brooks D. Dopamine agonists and neuroprotection in Parkinson's disease. Ann Neurol 199844(3 Suppl 1)S167–S174. [DOI] [PubMed] [Google Scholar]
- 36.Whone A L, Watts R L, Stoessl A J, REAL‐PET Study Group et al Slower progression of Parkinson's disease with ropinirole versus levodopa: The REAL‐PET study. Ann Neurol 20035493–101. [DOI] [PubMed] [Google Scholar]
- 37.Braz C A, Borges V, Ferraz H B. Effect of riluzole on dyskinesia and duration of the on state in Parkinson disease patients: a double‐blind, placebo‐controlled pilot study. Clin Neuropharmacol 20042725–29. [DOI] [PubMed] [Google Scholar]
- 38.Schapira A H. Present and future drug treatment for Parkinson's disease. J Neurol Neurosurg Psychiatry 2005761472–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goetz C G, Tanner C M, Klawans H L. Drug holiday in the management of Parkinson disease. Clin Neuropharmacol 19825351–364. [DOI] [PubMed] [Google Scholar]
- 40.Deleu D, Hanssens Y, Northway M G. Subcutaneous apomorphine: an evidence‐based review of its use in Parkinson's disease. Drugs Aging 200421687–709. [DOI] [PubMed] [Google Scholar]
- 41.Papa S M, Chase T N. Levodopa‐induced dyskinesias improved by a glutamate antagonist in Parkinsonian monkeys. Ann Neurol 199639574–578. [DOI] [PubMed] [Google Scholar]
- 42.da Silva‐Junior F P, Braga‐Neto P, Sueli Monte F.et al Amantadine reduces the duration of levodopa‐induced dyskinesia: a randomized, double‐blind, placebo‐controlled study. Parkinsonism Relat Disord 200511449–452. [DOI] [PubMed] [Google Scholar]
- 43.Pahwa R, Factor S A, Lyons K E.et al Practice parameter: treatment of Parkinson disease with motor fluctuations and dyskinesia (an evidence‐based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 200666983–995. [DOI] [PubMed] [Google Scholar]
- 44.Bonifati V, Fabrizio E, Cipriani R.et al Buspirone in levodopa‐induced dyskinesias. Clin Neuropharmacol 19941773–82. [DOI] [PubMed] [Google Scholar]
- 45.Durif F, Vidailhet M, Bonnet A M.et al Levodopa‐induced dyskinesias are improved by fluoxetine. Neurology 1995451855–1858. [DOI] [PubMed] [Google Scholar]
- 46.Fox S H, Lang A E, Brotchie J M. Translation of nondopaminergic treatments for levodopa‐induced dyskinesia from MPTP‐lesioned nonhuman primates to phase IIa clinical studies: keys to success and roads to failure. Mov Disord. 2006 Jul 27; [Epub ahead of print] [DOI] [PubMed]
- 47.Durif F, Debilly B, Galitzky M.et al Clozapine improves dyskinesias in Parkinson disease: a double‐blind, placebo‐controlled study. Neurology 200462381–388. [DOI] [PubMed] [Google Scholar]
- 48.Katzenschlager R, Manson A J, Evans A.et al Low dose quetiapine for drug induced dyskinesias in Parkinson's disease: a double blind cross over study. J Neurol Neurosurg Psychiatry 200475295–297. [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson V C, Burchiel K J, Hogarth P.et al Pallidal vs subthalamic nucleus deep brain stimulation in Parkinson disease. Arch Neurol 200562554–560. [DOI] [PubMed] [Google Scholar]
- 50.Uitti R J, Wharen R E, Jr, Turk M F.et al Unilateral pallidotomy for Parkinson's disease: comparison of outcome in younger versus elderly patients. Neurology 1997491072–1077. [DOI] [PubMed] [Google Scholar]
- 51.Fine J, Duff J, Chen R.et al Long‐term follow‐up of unilateral pallidotomy in advanced Parkinson's disease. N Engl J Med 20003421708–1714. [DOI] [PubMed] [Google Scholar]