Abstract
Acute rhinosinusitis is a common disease with worldwide prevalence. It is a significant burden on the health services. It is most commonly caused by viruses and is self‐limiting in nature. The diagnosis of acute rhinosinusitis is clinical and sinus radiography is not indicated routinely. Most cases of acute rhinosinusitis are treated symptomatically. However, symptoms may persist beyond 10 days when secondary bacterial infection prevails. Antibiotics are reserved for moderate or severe cases or when there is development of complications of acute rhinosinusitis. This paper provides an update on the current management of acute rhinosinusitis.
Rhinosinusitis is a significant health problem worldwide. It is an infection of the nasal passages and the paranasal sinuses. The term “sinusitis” typically carries different meaning for the patient and the primary care physician. Patients commonly attribute symptoms such as headache, facial pain, nasal congestion, or rhinorrhoea to “sinus trouble” when in fact it may be due to various other reasons. Primary care physicians often tend to think of sinusitis as an acute bacterial infection, hence antibiotics are prescribed in 92% of patients in the UK1 and 85–98% of sinusitis patients in the US.2 In 2003, the number of medical prescriptions for acute bacterial sinusitis was over 7.6 million in Germany.3 In France, around 7% of all antibiotics are prescribed to treat suspected acute bacterial sinusitis.4 The estimated annual cost of treatment in the UK is £10 million (€14.7 million, US$20 million).5 In 1996, the total cost of prescription and non‐prescription medications used for the treatment of sinusitis in the US was estimated at $3.39 billion (€2.5 billion, £1.7 billion).6 Rhinosinusitis has accounted for 12 to 17 million annual visits to physicians and for 12% of antibiotics prescribed to adults in the US, making it one of the 10 most common conditions to be treated in ambulatory practice.7
The enormous use of antibiotics has a financial impact on the health services. More importantly, it can contribute to the emergence and spread of antibiotic‐resistant bacteria.8 It is therefore important to appropriately identify and manage this common condition. This article provides an evidence based update on the current management of acute rhinosinusitis.
DEFINITION
“Rhinitis” is the inflammation of the nasal mucosa. It can be defined as symptoms of nasal irritation, sneezing, rhinorrhoea and nasal blockage lasting for at least 1 h a day on most days. The term “sinusitis” refers to inflammation of the mucosa of the paranasal sinuses, regardless of the cause. As the understanding of the pathophysiology of the nasal mucosa has evolved, the differentiation between rhinitis and sinusitis has become less apparent. Because sinusitis is invariably accompanied by rhinitis, the term rhinosinusitis instead of sinusitis was recommended by the 1997 Task Force of the Rhinology and Paranasal Sinus Committee.
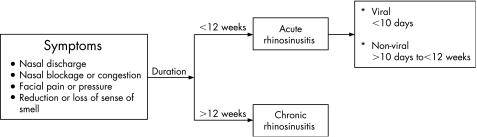
The term acute rhinosinusitis describes a sudden onset of two or more symptoms of nasal discharge, nasal blockage or congestion, facial pain or pressure and reduction or loss of sense of smell, which are less than 12 weeks in duration. If these symptoms are less than 10 days, it is considered to be of viral aetiology and hence called acute viral rhinosinusitis (common cold) (fig 1).
Figure 1 Symptoms and classification of rhinosinusitis.
CLINICAL FEATURES
Acute rhinosinusitis may be accompanied by low‐grade fever, malaise, headache and possibly a cough. Typical physical signs include bilateral nasal mucosal oedema, purulent nasal secretions and sinus tenderness, although this is not a sensitive or specific finding. Pain on palpation over the frontal sinuses can indicate inflammation. Maxillary sinus infection can cause toothache with tenderness over the molar region. Ethmoid sinusitis maybe associated with swelling, tenderness and pain around the eyes. Purulent drainage may be evident on examination as anterior rhinorrhoea or posterior pharyngeal drip with associated clinical symptoms of sore throat and cough. The nasal drainage is serous at first, changing to mucopurulent, with resolution within 10 days. However, if symptoms deteriorate after 5 days of onset or persist beyond 10 days, it is likely that there is secondary bacterial infection and it becomes known as acute non‐viral rhinosinusitis. Chronic rhinosinusitis includes all symptoms of acute rhinosinusitis but is >12 weeks in duration.
PATHOPHYSIOLOGY
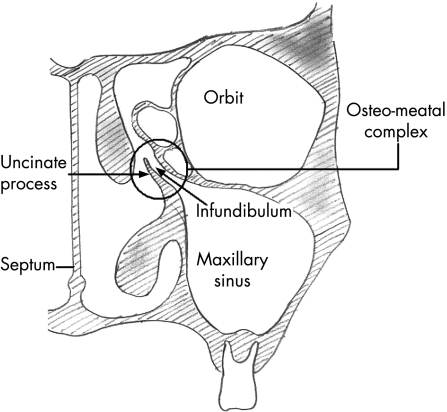
The paranasal sinuses are lined with pseudo‐stratified columnar epithelium, which is continuous with the lining of the nasal cavity. This epithelium contains a number of mucus‐producing goblet cells. Under physiologic conditions the sinuses are normally sterile. Their function depends on regular transport of the mucus layer from paranasal sinuses through their natural openings into a common area, known as the infundibulum, in the middle meatus of the nasal cavity. This area is the focal point of sinus drainage and is known as the osteomeatal complex (fig 2). It is situated in the lateral wall of the nose. From the nasal cavity the mucus then drains into the oropharynx.
Figure 2 The osteomeatal complex.
Acute rhinosinusitis starts as a viral infection of the nose resulting in inflammation and/or viral infection of the adjoining sinuses. There may be development of negative atmospheric pressure within the sinus cavities and a decrease in oxygen partial pressure. There is also excessive mucus production with or without transudation of plasma. This results in malfunction or complete cessation of movement of the cilia lining the sinuses leading to stasis of mucus and occlusion of the osteomeatal complex. This creates an environment within the sinuses that supports the growth of pathogenic organisms. Therefore, the development of rhinosinusitis is mainly attributed to blockage of the osteomeatal complex.
A wide range of factors predispose to obstruction and decreased ciliary function of the sinuses (box 1). These can be viral or non‐viral in origin. The most common cause of acute rhinosinusitis is a viral upper respiratory infection. Approximately 9 out of 10 patients who have viral upper respiratory tract infections have involvement of the adjacent sinuses. Up to 0.5% of upper respiratory infections in adults develop into documented sinusitis. However, only 5–10% of these patients have bacterial superinfection requiring antimicrobial treatment.6
Box 1 Predisposing factors for sinusitis
Upper respiratory infections
Anatomic variations
Allergic rhinitis
Nasal dryness
Dental infections and procedures, trauma
Barotrauma
Hormone factors
Immunodeficiency disease
Inhalation of irritants
Mechanical ventilation
Nasotracheal and nasogastric tubes
Although sinusitis is considered as rhinogenous in origin, dental infections are vital predisposing factors to be considered, as they can account for approximately 10–12% of cases of acute maxillary sinusitis.9 An odontogenic source should be considered in patients with symptoms of maxillary sinusitis who have a positive history for odontogenic infection or dentoalveolar surgery. Review of the literature suggests that many cases of recurrent acute sinusitis are due to secondary rhinogenous bacterial colonisation of antral mucosa that have been weakened and degenerated by chronic dental infection/inflammation.9
Box 2 Common causative organisms for acute rhinosinusitis
Viruses
Rhinovirus
Influenza virus
Parainfluenza virus
Bacteria
Streptococcus pneumoniae
Haemophilus influenzae
Moraxella catarrhalis
Anaerobic bacteria
Staphylococcus aureus
Streptococcus aureus
Gram‐negative bacteria
Acute non‐viral rhinosinusitis is mainly caused by bacteria (box 2). Haemophilus influenzae has been reported to produce toxins that interferes with ciliary function and damages the mucosal cells.10 The typical organisms in an odontogenic sinusitis include anaerobic streptococci (Streptococcus sanguis, Streptococcus salivarius, Streptococcus mutans), Bacteroides, Proteus, and coliform bacilli.11 Experimental studies in rabbits has shown that infection with Streptococcus pneumoniae or H influenzae is rapidly followed by destruction of the majority of the ciliated epithelial cells in the maxillary sinuses leading to accumulation of mucopus in the sinus cavities.12 Subsequently, this can either lead to resolution of symptoms or develop into chronic infection. It may also lead to the development of one of the complications of bacterial rhinosinusitis. These complications can be orbital or intracranial (box 3). They arise either as a direct erosion of the thin walls of the sinuses adjoining the orbit and the cranium or via haematogenous spread. Early recognition of these complications is vital. Symptoms and signs to look for include inflammatory oedema of the eyelids with or without oedema of the orbital contents, displaced globe, ophthalmoplegia, diplopia, reduced visual acuity, frontal swelling, severe frontal headache, signs of meningitis or focal neurological signs.
Box 3 Complications of acute rhinosinusitis
Orbital
Preseptal cellulitis
Orbital cellulitis
Orbital abscess
Osteomyelitis
Subperiosteal orbital abscess
Intracranial
Subdural empyema
Epidural empyema
Meningitis
Brain abscess
Cortical thrombophlebitis
Cavernous/sagittal sinus thrombosis
INVESTIGATIONS
Acute rhinosinusitis is mainly a clinical diagnosis. More than 50% of patients with sinus symptoms who visit primary care physicians are unlikely to have bacterial sinusitis. The clinical diagnosis of acute bacterial sinusitis is most appropriately made on the basis of the medical history, symptoms, and clinical examination.13
Nasal cytology
Sinus puncture (maxillary or frontal sinus) remains the gold standard for obtaining sinus culture material, with many studies showing little correlation between nasal swab and sinus culture.14,15 Nasal cytology (Hansel, Wright of Gram stain) could be performed in cases of acute rhinosinusitis. Presence of neutrophils and bacteria suggests bacterial rhinosinusitis.
Radiology
Radiology has traditionally been used as an investigative tool to diagnose acute rhinosinusitis. This includes plain sinus radiographs and computed tomography (CT) scans of the paranasal sinuses.
X ray
Plain sinus radiographs are commonly used as a first‐line investigation for sinusitis. They are indicated in cases of acute rhinosinusitis only if symptoms persist despite adequate treatment. Sinus radiographs are not performed in children <3 years of age due to undeveloped sinuses and high false positive opacification rate. Waters view (occipitomental view) is usually sufficient for maxillary sinusitis. Caldwell‐Luc (frontal) view is used to demonstrate involvement of the frontal sinuses. The hallmark of acute sinusitis is an air–fluid level and standard radiography may be accurate in showing air and fluid levels in the frontal, maxillary, and sphenoid sinuses, but it significantly underestimates the degree of inflammatory disease present, particularly in the ethmoid sinuses. Sinus x rays apart from air‐fluid levels can also demonstrate sinus opacification and non‐specific mucosal thickening. Interobserver variability and inability to distinguish infection from polyp or tumour disease limits the use of plain radiography. Sinus radiographs have been shown to have a high number of false positive and negative results.13,16
CT scan
CT scanning provides a detailed view of the paranasal sinuses. Although considered to be the radiologic investigation of choice in chronic rhinosinusitis, it is not normally used as an assessment tool for uncomplicated acute sinusitis. Its limitations include a high frequency of abnormal scans in asymptomatic persons and the fact that CT cannot be used to distinguish viral from non‐viral sinusitis. It has a high sensitivity but low specificity for demonstrating acute sinusitis.17 Forty per cent of asymptomatic patients and 87% of patients with community‐acquired colds have sinus abnormalities on sinus CT scan.18 CT scans are only indicated in cases where patients have failed to respond to medical treatment or if they have a suspected complication of acute sinusitis. They should therefore be reserved for patients who fail to respond to medical treatment and for those who present with complications of sinusitis as periorbital or facial swelling with or without erythema, diplopia or neurologic symptoms.
TREATMENT
The main aims of treatment of acute rhinosinusitis are to eradicate infection, prevent development of chronic disease, decrease the duration of illness, and prevent complications. Acute rhinosinusitis is a condition that is mainly managed medically. Primarily it includes the use of ancillary treatment and may include antimicrobial treatment.
Ancillary treatment
Ancillary treatment is designed to promote ciliary function and decrease oedema to improve drainage through the sinus ostia. This in effect provides symptomatic relief. Ancillary treatment includes topical and oral decongestants, mucolytic agents, antihistamines, intranasal corticosteroids, steam inhalation and saline nasal rinses. Unfortunately, the evidence supporting the use of ancillary treatment for acute rhinosinusitis is relatively weak.19,20,21 No treatment has shown to reduce the duration of illness (box 4).
Box 4 Ancillary treatment for acute rhinosinusitis
Likely to be effective:
Oral decongestants
Topical decongestants
Possibly effective:
Topical anticholinergics
Antihistamines
Mucolytic agents
Nasal corticosteroids
Hypertonic and normal saline nasal irrigation
No proven benefit:
Saline spray
Zinc salt lozenges
Vitamin C
Less sedating antihistamines
Pseudoephedrine
Xylometazoline
Oxymetazoline
Phenylephrine
Ipratropium bromide
Chlorpheniramine
Diphenhydramine
Guaiphensin
Mometasone furoate
Decongestants
Topical decongestants
Decongestants may provide temporary relief in nasal congestion. The commonly available topical decongestants are phenylephrine, oxymetazoline and xylometazoline. In the form of spray or drops, they act by constricting the sinusoids in the nasal mucosa. These sinusoids are controlled by adrenoreceptors types α1 and α2.22 α1 agonists, such as phenylephrine, are preferred because the nasal mucosal blood flow is not significantly altered by the α1 agonists as compared to a selective α2 adrenoreceptor agonist (for example, oxymetazoline) which interferes with the healing of sinusitis by decreasing nasal mucosal blood flow.23 Following intranasal administration, local vasoconstriction occurs within 10 min irrespective of the drug used. The effect lasts longer for oxymetazoline (8–12 h) and xylometazoline. This may be explained by its slow mucosal clearance due to decreased mucosal blood flow. Side‐effects of the use of topical nasal decongestants include stinging, dryness or ulceration of the nasal mucosa. Prolonged use (>10 days) of intranasal vasoconstrictors may lead to tachyphylaxis and a rebound swelling of the nasal mucosa (rhinitis medicamentosa). The use of topical decongestants should therefore be restricted to <10 days.
Oral decongestants
Oral decongestants (for example, pseudoephedrine, ephedrine, phenylephrine) are commonly used. They are prescribed usually on a short‐term basis to provide fast acting relief. Oral decongestants have a weaker effect in relieving the nasal obstruction when compared to topical intranasal decongestants. However, they do not cause rebound phenomenon so they could be prescribed for long‐term. Following oral administration, the effect of nasal decongestion occurs within 30 min and persists for up to 6 h. Phenylepherine has a high first pass metabolism and is therefore least likely to be effective. Oral decongestants have certain adverse effects including agitation and nervousness, drowsiness and arrhythmias. Oral decongestants should be avoided in combinations with alcohol or certain drugs, including monoamine oxidase inhibitors and sedatives. There is usually no significant increase in blood pressure in patients with stable hypertension.24 However, precautionary use is advised in patients with ischaemic heart disease, glaucoma or prostatic hypertrophy.
Topical anticholinergics
Parasympathetic fibres are distributed widely in the nasal glands and blood vessels. Parasympathetic stimulation causes a watery secretion mediated by acetylcholine and vasodilatation of blood vessels serving the glands. Anticholinergic drugs can block the muscarinic receptors of the sero‐mucinous glands. Topical anticholinergics, such as ipratropium bromide nasal spray, are primarily used to control symptom of rhinorrhoea.21 It does not have a significant effect on nasal congestion, itching and sneezing.25 Nasal dryness, irritation and burning are the most prominent side‐effects followed by a stuffy nose, dry mouth and headache. Long term use has no effect on olfaction and ciliary beat frequency. The clinical appearance of the nasal mucosa remains unaltered.
Antihistamines
No clinical studies support the use of antihistamines for treatment of acute rhinosinusitis. They may probably be beneficial due to their anti‐inflammatory effect.25 On the other hand, anticholinergic effects of first generation antihistamines could impair clearance by thickening mucus.26 Second generation antihistamines are not recommended for acute rhinosinusitis as they do not have anticholinergic effect.
Mucolytic agents
Guaiphensin is a commonly used mucolytic agent. It is usually used in combination with a decongestant preparation. Although it is prescribed to thin the mucous secretions and improve drainage, studies comparing the effects of guaiphensin and placebo on nasal mucociliary clearance and ciliary beat frequency have failed to show any measurable effect.22
Nasal saline spray/saline irrigation
Saline sprays have been shown to reduce symptoms of rhinitis. Daily hypertonic saline nasal irrigation has been shown to result in improved sinus‐related quality of life, decreased symptoms and decreased medication use in patients with frequent sinusitis.27 There has been no reported serious adverse effect with saline irrigation.28
Topical corticosteroids
Most studies of intranasal steroid use in acute rhinosinusitis have not shown an effect on clinical outcome. The use of intranasal beclomethasone in the treatment of the common cold neither reduced symptoms caused by inflammation, nor shortened the recovery time.29 However, mometasone furoate nasal spray, used as an adjunctive treatment with an oral antibiotic, has been shown to be significantly more effective in reducing the symptoms of acute rhinosinusitis than antibiotic treatment alone.30,31 Fluticasone propionate treatment tends to prevent paranasal sinusitis, especially in rhinovirus‐positive subjects,32 but does not have any notable effects on the symptoms or recovery time of the common cold.33
Vitamin C, zinc salt lozenges
There is insufficient evidence to recommend the use of vitamin C or zinc salt lozenges in patients with acute bacterial rhinosinusitis. Using the outcome of cold symptoms after 7 days, a meta‐ analysis of eight clinical trials of zinc salt lozenge for the treatment of common cold did not find a significant benefit.34 In contrast, clinical trials showed that zinc effectively and significantly shortened the duration of the common cold when it was administered within 24 h of the onset of symptoms.35 Vitamin C may have a small role in preventing the common cold, especially in persons exposed to brief periods of severe physical activity or cold environments,36,37 but has no apparent effect on the duration or severity of symptoms.38
Antimicrobial treatment
The diagnosis between bacterial and viral infection at the onset of symptoms is difficult, as the symptoms of the two infections are often indistinguishable. Therefore, the use of an antibiotic as well as choosing the right time to start treatment is a very challenging task. As most of the episodes of acute rhinosinusitis start as a viral infection, about two‐thirds of patients improve without antibiotic treatment and most patients with viral upper respiratory infection improve within seven days.39 The emerging consensus is that symptom duration and severity are the most useful indicators of acute bacterial sinusitis. Therefore, antimicrobial treatment should be reserved for patients with persistent symptoms (present for at least 10 days) or those who develop a complication. When selecting antibiotic treatment for acute bacterial rhinosinusitis, the clinician should consider the severity of the disease, the rate of progression of the disease and recent antibiotic exposure.
Prescription of appropriate antibiotics can be determined in two ways; either clinically or by aspirate cultures. Clinical monitoring as a proof of eradication is inaccurate. Aspirate cultures should ideally be done before treatment is started to establish the presence of bacterial infection, and after completion of treatment to confirm eradication of infection. However, this is not practical in every case. There have been no randomised controlled trials of antibiotic treatment using sinus aspirate cultures before and after treatment, although non‐randomised trials have demonstrated bacteriologic cures.
Five randomised controlled trials and two meta‐analyses have compared antibiotics, usually amoxicillin and trimethoprim–sulfamethoxazole, versus placebo, with clinical improvement as the main outcome.40,41 Overall, antibiotics have been found to be more effective than placebo, reducing the risk of clinical failure by about 25–30% within 7–14 days after initiation of treatment. However, improvement or resolution of symptoms has been seen in 65% of patients without any antibiotic treatment at all.42 It may be possible that a significant proportion of patients in this study may have had a viral rather than a bacterial infection.
Choice of first line antimicrobial agent varies among practices, as the decision to initiate antibiotic treatment is typically made empirically. However, a narrow spectrum agent that is active against the likely pathogen should be considered first. As the most common pathogens associated with bacterial acute rhinosinusitis are S pneumoniae or H influenzae, the use of amoxicillin (with or without clavulanate) is generally recommended for initial treatment in adult patients with mild disease who have not received an antibiotic in the previous 4 weeks. 43,44 Amoxicillin, which is a extended‐spectrum penicillin, has been broadly used worldwide as first line treatment of acute rhinosinusitis, with mild clinical features.43 Interestingly, H influenzae isolates can be highly resistant to ampicillin and amoxicillin.45 Recent studies suggested that the fluoroquinolones gatifloxacin, levofloxacin and moxifloxacin are efficacious for the treatment acute bacterial rhinosinusitis, with 90–92% predicted clinical efficacy.44 Fluoroquinolones remain highly active against both S pneumoniae and H influenzae, with <2% resistance of all isolates.46 Although the role of the fluoroquinolones is evolving, these agents are often recommended as second line treatment, or as first line in patients with mild disease who have had recent antimicrobial therapy, or for patients with moderate to severe disease. 47,48 An alternative option in these cases would be high dose amoxicillin/clavulanate (4 g/250 mg per day).43,44,47 Other efficacious antimicrobials that could be used for treatment of acute rhinosinusitis include cephalosporins.44 Third generation cephalosporins, such as ceftriaxone or cefdinir, have good efficacy against H influenzae but much lower activity against S pneumoniae.49 On the other hand, macrolides (erythromycin, clarithromycin) exert a bacteriostatic effect on Gram‐positive and some Gram‐negative bacteria. Although rates of macrolide resistance to S pneumoniae and H influenzae are increasing worldwide, they are still used as first line antibiotic in patients with β‐lactam allergies.44 Less commonly used treatments include tetracyclines and trimethoprim.
Amoxicillin, cephalosporins and macrolides have been studied extensively.46,49 All have demonstrated similar clinical success rates (>85%). Amoxicillin–clavulanate compared to antibiotics in the cephalosporin class was found to be 41% more effective in reducing clinical failure within 10–25 days after treatment initiation. In absolute terms, this means treating 100 patients with antibiotics in the cephalosporin class would lead to 3.5 more failures as compared to amoxicillin–clavulanate.46 There was no consistent trend observed when comparing amoxicillin–clavulanate, cephalosporins and quinolones to the group encompassing macrolides, azalides and ketolides.
There have been eight studies that report data on comparison of treatment duration with outcome efficacy. One study showed that 10 days vs 5 days of amoxicillin–clavulanate 500 mg three times a day showed a non‐significant 28% reduction in clinical failure rate.46 Two studies on 10 days vs 5 days of telithromycin showed that the clinical failure rate between the two treatment durations was comparable.50,51 The studies on gemifloxacin (5 days vs 7 days),52 azithromycin (3 days vs 6 days),53 and gatifloxacin (5 days vs 10 days)54 showed therapeutic equivalence of the two durations. If patients fail to respond to the initial course of treatment, an alternative antibacterial agent should be considered. Recommended second line antibacterials include macrolides, cephalosporins and, in severe cases, fluoroquinolones. Ideally, a nasal swab test should be conducted before initiating an alternative regimen. Lack of response to treatment at >72 h is an arbitrary time established to define treatment failures. Clinicians should monitor the response to antibiotic treatment, which may include instructing the patient to call the office or clinic if symptoms persist or worsen over the next few days.44 When a change in antibiotic treatment is made, the clinician should consider the limitations in coverage of the initial agent.
Thirty‐four comparative trials and five non‐comparative trials reported adverse events in using antimicrobial agents. Descriptions of adverse events were diverse among studies. It was not possible to make meaningful comparisons of adverse event rates across different antibiotic classes given the enormous variation in the reported rate of adverse events within the same antibiotic class. For example, the reported rate of diarrhoea with amoxicillin–clavulanate across different studies ranged from <2% to >30%. Overall, the most common adverse events involved the gastrointestinal and the central nervous systems. Severe adverse events were rare, occurring in <10% of any given study population.
TREATMENT OF ACUTE SINUSITIS OF DENTAL ORIGIN
Typical treatment of atraumatic odontogenic sinusitis is a 3–4 week trial of antibiotic treatment with adequate oral and sinus flora coverage. When indicated, surgical removal of the offending odontogenic foreign body (primary or delayed) or treatment of the odontogenic pathologic conditions combined with medical treatment is usually sufficient to cause resolution of symptoms. If an oroantral communication is suspected, prompt surgical management is recommended to reduce the likelihood of causing chronic sinus disease.
SURGICAL MANAGEMENT
Surgical treatment in acute rhinosinusitis is indicated primarily for two reasons: failed medical treatment and potential or actual development of an acute complication (table 3). Surgical treatment for acute rhinosinusitis includes the following.
Antral washout
Antral washout was the mainstay surgical procedure in the past, especially in patients who failed to respond to medical treatment. Currently its use is limited only to severe cases of acute rhinosinusitis that result in abscess formation within the paranasal sinuses. Sinus puncture and irrigation techniques allow removal of thick purulent sinus secretions. It can also provide material for culture and sensitivity to guide antibiotic selection if empiric therapy has failed or antibiotic choice is limited. This is particularly important in patients who are immunocompromised or in intensive care. Sinusitis can be a prominent source of sepsis in these patients. In adults, sinus puncture can usually be achieved using local anaesthesia. It has no effect in cases of osteomeatal obstruction and chronic ethmoidal inflammation. Antral lavage is contraindicated in children <3 years of age, in undeveloped maxillary sinus, in cases of trauma to the orbital floor, and in acute febrile maxillary sinusitis untreated with antibiotics.
External frontoethmoidectomy
An external approach to the ethmoidal sinuses in acute rhinosinusitis is limited to cases of complications of acute ethmoiditis such as orbital cellulites/abscess. It allows decompression and drainage of the involved sinuses, including subperiosteal and retro‐orbital abscess. Nowadays, it can be accompanied endoscopically in most patients.
Frontal sinus trephination
The traditional approach to acute frontal sinusitis (empyema) that fails to respond to conservative treatment is to trephine the sinus, but management of acute frontal sinusitis with restoration of integrity of the nasal frontal duct using endoscopic sinus surgical techniques is an ideal alternative.
Functional endoscopic sinus surgery
The introduction of endoscopes in sinus surgery has brought a revolution in the approach to surgery of the sinuses through the nasal cavity. It allows ventilation and drainage of the inflamed/infected sinuses and restoration of their mucociliary clearance. The role of functional endoscopic sinus surgery in acute rhinosinusitis is limited mainly to management of acute complications; however, it is often not the first choice for managing complications as there would be a greater tendency to bleeding. It has proven very effective in managing recurrent acute or chronic sinusitis.55,56
CONCLUSION
Acute rhinosinusitis is one of the most common disorders encountered in the primary care setting. It usually starts as a self‐limiting viral infection of the sinonasal mucosa. Causes of acute sinus inflammation include infection, allergy and local irritants. Cases due to allergy and irritants can usually be distinguished from infection on the basis of a careful history. Secondary bacterial infection may supervene with symptoms persisting for >10 days. Bacterial and viral rhinosinusitis is difficult to differentiate on clinical grounds. Most common bacteria implicated in acute rhinosinusitis are S pneumoniae, H influenzae and Moraxella catarrhalis. Most cases of acute rhinosinusitis are treated symptomatically, with antibiotics reserved for moderate or severe cases. Amoxicillin–clavulanate is more effective than cephalosporins in the short‐term follow‐up. There are no significant differences between other classes of antibiotics. There is a lack of studies that compare newer antibiotics with inexpensive agents such amoxicillin and trimethoprim–sulfamethoxazole. Surgery has a very limited role to play in acute rhinosinusitis and is mainly offered in cases of failed medical treatment or where there is a potential for onset of acute complications.
Footnotes
Conflict of interest: None declared.
References
- 1.Ashworth M A, Charlton J, Ballard K.et al Variations in antibiotic prescribing and consultation rates for acute respiratory infection in UK general practices 1995–2000. Br J Gen Pract 200555603–608. [PMC free article] [PubMed] [Google Scholar]
- 2.Gonzales R, Steiner J F, Lum A.et al Decreasing antibiotic use in ambulatory practice: impact of a multidimensional intervention on the treatment of uncomplicated acute bronchitis in adults. JAMA 19992811512–1519. [DOI] [PubMed] [Google Scholar]
- 3.Institut fur Medizinische Statistik Verordnungsindex Pharmazeutika 2003
- 4.Groupe d'Etude des Sinusites Infectieuses Current approaches to community‐acquired acute maxillary rhinosinusitis or sinusitis in France and literature review. Rhinol 200117(Supp1)1–38. [PubMed] [Google Scholar]
- 5.Osguthorpe J D, Hadley J A. Rhinoinusitis. Current concepts in evaluation and management. Otolaryngology for the internist. Med Clin N Am 19998327–41. [DOI] [PubMed] [Google Scholar]
- 6.Ray N F, Baraniuk I N, Thamer M.et al Healthcare expenditures for sinusitis in 1996: contributions of asthma, rhinitis, and other airway disorders. J Allergy Clin Immunol 1999103408–414. [DOI] [PubMed] [Google Scholar]
- 7.Schappert S M. Ambulatory care visits to physician offices, hospital outpatient departments, and emergency departments: United States, 1996. Vital Health Stat 19981341–37. [PubMed] [Google Scholar]
- 8.Kunin C M. Resistance to antimicrobial drugs ‐ a worldwide calamity. Ann Intern Med 1993118557–561. [DOI] [PubMed] [Google Scholar]
- 9.Mehra P, Murad H. Maxillary sinus disease of odontogenic origin. Otolaryngol Clin North Am 200437347–364. [DOI] [PubMed] [Google Scholar]
- 10.Lindberg S. Morphological and functional studies of the mucociliary system during infections of the upper airways. Arch Otolaryngol (Stockh) 199451522–25. [DOI] [PubMed] [Google Scholar]
- 11.Legert K G, Zimmerman M, Stierna P. Sinusitis of odontogenic origin pathophysiological implications of early treatment. Acta Otolaryngol 2004124655–663. [DOI] [PubMed] [Google Scholar]
- 12.Hinni M L, McCaffrey T V, Kasperbauer L L. Early mucosal changes in experimental sinusitis. Otolaryngol Head Neck Surg 1992107537–548. [DOI] [PubMed] [Google Scholar]
- 13.Iiuma T, Hirota Y, Kase Y. Radioopacity of the paranasal sinuses. Conventional views and CT. Rhinology 199432134–136. [PubMed] [Google Scholar]
- 14.Klossek J M, Dubreuil L, Richet H.et al Bacteriology of the adult middle meatus. J Laryngol Otol 1996110847–849. [DOI] [PubMed] [Google Scholar]
- 15.Dubin M G, Ebert C S, Coffey C S.et al Concordance of middle meatal swab and maxillary sinus aspirate in acute and chronic sinusitis: a meta‐analysis. Am J Rhinol 200519462–470. [PubMed] [Google Scholar]
- 16.Jonas I, Mann W. Misleading x‐ray diagnosis due to maxillary sinus asymmetries [author's translation]. Laryngol Rhinol Otol (Stuttg) 197655905–913. [PubMed] [Google Scholar]
- 17.McAlister W H, Lusk R, Muntz H R. Comparison of plain radiographs and coronal CT scans in infants and children with recurrent sinusitis. Am J Roentgenol 19891531259–1264. [DOI] [PubMed] [Google Scholar]
- 18.Low D E, Desrosiers M, McSherry J.et al A practical guide for the diagnosis and treatment of acute sinusitis. Can Med Assoc J 199715651–54. [PubMed] [Google Scholar]
- 19.Smith M B, Feldman W. Over‐the‐counter cold medications. A critical review of clinical trials between 1950 and 1991. JAMA 19932692258–2263. [DOI] [PubMed] [Google Scholar]
- 20.Zeiger R S. Prospects for ancillary treatment of sinusitis in the 1990s. J Allergy Clin Immunol 199290478–495. [DOI] [PubMed] [Google Scholar]
- 21.Mabry R L. Therapeutic agents in the medical management of sinusitis. Otolaryngol Clin North Am 199326561–570. [PubMed] [Google Scholar]
- 22.Malm L. Pharmacological background to decongesting and anti‐inflammatory treatment of rhinitis and sinusitis. Acta Otolaryngol 1994515(Suppl)53–55. [DOI] [PubMed] [Google Scholar]
- 23.Wiklund L, Stierna P, Berglund R.et al The efficacy of oxymetazoline administered with a nasal bellows container and combined with oral phenoxymethyl‐penicillin in the treatment of acute maxillary sinusitis. Acta Otolaryngol 1994515(Suppl)57–64. [DOI] [PubMed] [Google Scholar]
- 24.Bravo E L. Phenylpropanolamine and other over the counter vasoactive compounds. Hypertension 198811117–120. [DOI] [PubMed] [Google Scholar]
- 25.Benninger M S, Anon J, Mabry R L. The medical management of rhinosinusitis. Otolaryngol Head Neck Surg 1997117541–549. [DOI] [PubMed] [Google Scholar]
- 26.Sisson J H, Yonkers A J, Waldman R H. Effects of guaifenesin on nasal mucociliary clearance and ciliary beat frequency in health volunteers. Chest 1995107747–751. [DOI] [PubMed] [Google Scholar]
- 27.Rabago D, Zgierska A, Mundt M.et al Efficacy of daily hypertonic saline nasal irrigation among patients with sinusitis: a randomized controlled trial. J Fam Pract 2002511049–1055. [PubMed] [Google Scholar]
- 28.Heatley D G, McConnell K E. Kille n. Leverson GE. Nasal irrigation for the alleviation of sinonasal symptoms. Otolaryngol Head Neck Surg 200112544–48. [DOI] [PubMed] [Google Scholar]
- 29.Ovarnberg Y, Valtonen H, Laurikainen K. Intranasal beclomethasone dipropionate in the treatment of common cold. Rhinology 2001399–12. [PubMed] [Google Scholar]
- 30.Meltzer E O, Bachert C, Staudinger H. Treating acute rhinosinusitis: comparing efficacy and safety of mometasone furoate nasal spray, amoxicillin, and placebo. J Allergy Clin Immunol 20051161289–1295. [DOI] [PubMed] [Google Scholar]
- 31.Nayak A S, Settipane G A, Pedinoff A, Nasonex Sinusitis Group et al Effective dose range of mometasone furoate nasal spray in the treatment of acute rhinosinusitis. Ann Allergy Asthma Immunol 200289271–278. [DOI] [PubMed] [Google Scholar]
- 32.Puhakka T, Makela M J, Alanen A.et al Sinusitis in the common cold. J Allergy Clin Immunol 1998102403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puhakka T, Makela M J, Malmstrom K.et al The common cold: effects of intranasal fluticasone propionate treatment. J Allergy Clin Immunol 1998101726–731. [DOI] [PubMed] [Google Scholar]
- 34.Jackson J L, Peterson C, Lesho E. A meta‐analysis of zinc salts lozenges and the common cold. Arch Intern Med 19971572373–2376. [PubMed] [Google Scholar]
- 35.Hulisz D. Efficacy of zinc against common cold viruses: an overview. J Am Pharm Assoc 200444594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Douglas R M, Hemila H, D'Souza R.et al Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev 200418CD000980. [DOI] [PubMed] [Google Scholar]
- 37.Arroll B. Non‐antibiotic treatments for upper‐respiratory tract infections (common cold). Respir Med 2005991477–1484. [DOI] [PubMed] [Google Scholar]
- 38.Sasazuki S, Sasaki S, Tsubono Y.et al Effect of vitamin C on common cold: randomized controlled trial. Eur J Clin Nutr 2006609–17. [DOI] [PubMed] [Google Scholar]
- 39.Axelsson A, Chidekel N, Grebelius N.et al Treatment of acute maxillary sinusitis. A comparison of four different methods. Acta Otolaryngol 19707071–76. [DOI] [PubMed] [Google Scholar]
- 40.Lau J, Zucker D, Engels E A.et al Diagnosis and treatment of acute bacterial rhinosinusitis. Evid Rep Technol Assess (Summ) 199991–5. [PMC free article] [PubMed] [Google Scholar]
- 41.Williams J W, Aguilar C, Cornell J.et al Antibiotics for acute maxillary sinusitis. Cochrane Database Syst Rev 2004 [DOI] [PubMed]
- 42.Ip S, Fu L, Balk E.et al Update on acute bacterial rhinosinusitis. Evid Rep Technol Assess (Summ) 20051241–3. [PMC free article] [PubMed] [Google Scholar]
- 43.Scheid D C, Hamm R M. Acute bacterial rhinosinusitis in adults: part II. Treatment. Am Fam Physician 2004701697–1704. [PubMed] [Google Scholar]
- 44.Anon J B, Jacobs M R, Poole M D.et al Antimicrobial treatment guidelines for acute bacterial rhinosinusitis. Otolaryngol Head Neck Surg 2004130(1 Suppl)1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karlowsky J A, Draghi D C, Thornsberry C.et al Antimicrobial susceptibilities of Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis isolated in two successive respiratory seasons in the US. Int J Antimicrob Agents 20022076–85. [DOI] [PubMed] [Google Scholar]
- 46.Hoban D, Felmingham D. The PROTEKT surveillance study: antimicrobial susceptibility of Haemophilus influenzae and Moraxella catarrhalis from community acquired respiratory tract infections. J Antimicrob Chemotherapy 20025049–59. [DOI] [PubMed] [Google Scholar]
- 47.Poole M D, Portugal L G. Treatment of rhinosinusitis in the outpatient setting. Am J Med 2005118(Suppl 7A)45–50. [DOI] [PubMed] [Google Scholar]
- 48.Anon J B. Current management of acute bacterial rhinosinusitis and the role of moxifloxacin. Clin Infect Dis 200541(Suppl 2)167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jacobs M R, Felmingham D, Appelbaum P C.et al The Alexander Project 1998–2000: susceptibility of pathogens isolated from community‐acquired respiratory tract infection to commonly used antimicrobial agents. J Antimicrob Chemother 200352229–246. [DOI] [PubMed] [Google Scholar]
- 50.Luterman M, Tellier G, Lasko B.et al Efficacy and tolerability of telithromycin for 5 or 10 days vs. amoxicillin/clavulanic acid for 10 days in acute maxillary sinusitis. Ear Nose Throat J 200382576–580. [PubMed] [Google Scholar]
- 51.Roos K, Brunswig‐Pitschner C, Kostrica R.et al Efficacy and tolerability of once‐daily therapy with telithromycin for 5 or 10 days for the treatment of acute maxillary sinusitis. Chemotherapy 200248100–108. [DOI] [PubMed] [Google Scholar]
- 52.Ferguson B J, Anon J, Poole M D.et al Short treatment durations for acute bacterial rhinosinusitis: five days of gemifloxacin versus 7 days of gemifloxacin. Otolaryngol Head Neck Surg 20021271–6. [DOI] [PubMed] [Google Scholar]
- 53.Henry D C, Riffer E, Sokol W N.et al Randomized double‐blind study comparing 3‐ and 6‐day regimens of azithromycin with a 10‐day amoxicillin‐clavulanate regimen for treatment of acute bacterial sinusitis. Antimicrobial Agents Chemother 2003472770–2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sher L D, McAdoo M A, Bettis R B.et al A multicenter, randomized, investigator‐blinded study of 5‐ and 10‐day gatifloxacin versus 10‐day amoxicillin/clavulanate in patients with acute bacterial sinusitis. Clin Ther 200224269–281. [DOI] [PubMed] [Google Scholar]
- 55.Stammberger H. Endoscopic endonasal sinus surgery: concepts in treatment of recurring rhinosinusitis. Part I. Anatomic and pathophysiologic considerations. Otolaryngol Head Neck Surg 198694134–136. [DOI] [PubMed] [Google Scholar]
- 56.Stammberger H, Posawetz W. Functional endoscopic sinus surgery: Concept, indications and results of the Messerklinger technique. Eur Arch Otorhinolarygol 199024063–76. [DOI] [PubMed] [Google Scholar]