Abstract
Aim
To investigate the inhibitive effects of triptolide (TPL) combined with 5‐fluorouracil (5‐FU) on colon carcinoma HT‐29 cells in vitro and in vivo and their side effects.
Methods
HT‐29 cells were cultured with RPMI 1640 medium. The single or combined effects of TPL and 5‐FU on HT‐29 cells were examined by MTT assay, flow cytometry. The combined effects were evaluated by the median‐effect principle. The model of tumour xenografts was established in nude mice. TPL 0.25 mg/kg/day and 5‐FU 12 mg/kg/day, either in combination or on their own, were injected into mice and the inhibitive effects and side effects were observed.
Results
TPL and 5‐FU either combined or alone inhibited significantly the proliferation of HT‐29 cells and induced obvious apoptosis. Mean (SD) growth inhibition rate reached 94.92 (2.76)% and the apoptic rate at 48 h reached 41.71 (1.38)%. The combined effects were synergistic (CI<1) at lower concentrations. TPL or 5‐FU alone inhibited significantly the growth of tumour xenografts and the inhibition rates were 78.53% and 84.16%; the drugs combined had more significant effect, the tumour inhibition rate reaching 96.78%. During the course of chemotherapy, no obvious side effect was observed.
Conclusion
The combined effects of TPL and 5‐FU on the growth of colon carcinoma in vitro and in vivo were superior to the effects when the agents were used individually. TPL combined with 5‐FU had synergistic effects at lower concentrations and promoted apoptosis, but did not increase the side effects of chemotherapy.
Colon carcinoma is one of the most common malignant diseases worldwide.1,2 Generally, approximately half of all patients with colon cancer can be cured with surgical resection of the primary tumour, while the remainder will eventually succumb to the predominant distant disease. Metastasis may already occur before the primary tumour can be detected. This characteristic of the disease has prevented any remarkable improvement in the cure rates in spite of the advances in surgical techniques. In order to improve the prognosis, chemotherapy is often used in a variety of clinical situations. The toxicity of these chemotherapeutic agents to normal tissues has been one of the major obstacles to successful cancer chemotherapy. Therefore, combined treatments with several chemotherapy regimens or even chemopreventive medicine are often used not only to enhance the treatment effect, but also to reduce the toxicity of these drugs.
Over the past 40 years, 5‐fluorouracil (5‐FU) has been the major chemotherapeutic agent for treating colorectal carcinoma; however, response rates have been around 20–35% with median overall survival no more than 1 year.3,4,5 So finding new anti‐cancer drugs with high therapeutic effect which can be used in combination with existing agents may provide an important way forward in the treatment of colorectal carcinoma.
Triptolide (TPL) is a diterpenoid triepoxide derived from the herb Tripterygium wilfordii that has been used as a natural medicine in China for many years. TPL exerts both anti‐inflammatory and antifertility activities through its ability to inhibit the proliferation of both activated monocytes and spermatocytes.6,7,8,9 Several reports have indicated that TPL also inhibits the proliferation of cancer cells in vitro and reduces the growth of some tumours and sensitises them to chemotherapy.10,11,12,13,14 In addition, clinical trials in China showed that TPL could achieve a total remission rate of 71% in mononucleocytic leukaemia and 87% in granulocytic leukaemia, which was more effective than any other chemotherapeutic agent currently available.13,15
In this study, we examined the effects of TPL combined with 5‐FU in regard to their activity against colon carcinoma in vitro and in vivo.
MATERIALS AND METHODS
Chemicals and reagents
TPL was obtained from Beijing fan‐China Biotechnology Co, Ltd (Beijing, China) and was 98% pure by reverse phase high‐performance liquid chromatography. It was stored in a stock solution of 1 mg/ml in dimethyl sulfoxide (DMSO) at −20°C and diluted to various concentrations with serum‐free culture medium when used. 5‐FU was obtained from Sigma (New York, NY, USA) and was diluted to various concentrations with serum‐free culture medium. Annexin V‐FITC kit was obtained from Beijing Biosea Biotechnology Co, Ltd.
Cell line and cell culture
The colon carcinoma cell line HT‐29 was obtained from the Department of Oncology, Zhongnan Hospital of Wuhan University, China. The cells were cultured in RPMI1640 (Sigma, USA), 10% fetal bovine serum (FBS, GibcoBRL Gaithersburg, MD, USA), 50 μg/ml streptomycin, 50 IU/ml penicillin, and 2 mM glutamine in a 5% humidified CO2 atmosphere at 37°C.
Cell growth inhibition studies
In vitro growth inhibition effect of TPL on HT‐29 cells was determined by measuring MTT (3‐[4, 5‐dimethylthiazol‐2‐yl]‐2, 5‐diphenyltetrazolium bromide) dye absorbance of living cells. Briefly, cells (2×104 cells per well) were seeded in 96‐well microtitre plates. After exposure to TPL (1.25, 2.50, 5.00, 10.00, 20.00 ng/ml), 5‐FU (25, 50, 100, 200, 400 μg/ml) or TPL+5‐FU for 48 h, 20 μl MTT solution (5 mg/ml in phosphate buffered saline (PBS)) was added to each well and the plates were incubated for an additional 4 h at 37°C. The MTT solution in medium was aspirated off. To achieve solubilisation of the formazan crystal formed in viable cells, 200 μl DMSO was added to each well, and then the optical density at 570 nm was determined by a multiwell spectrophotometer reader (Tecan, Grödig/Salzburg, Austria). Each essay was performed in triplicate. Then the results were expressed as the inhibition rate (IR):
IR = (A−B)/A×100%
where A and B were the absorbance of the control and sample groups after 48 h incubation, respectively.
Evaluation of the combined effects of TPL and 5‐FU
The following equations described by Chou and Talalay16,17 were used to evaluate the nature of the interaction between TPL and 5‐FU:
D = Dm[fa/(1−fa)]1/m
where D is the dose, fa is the fractions of the system affected, respectively, by the dose D, Dm is the dose required to produce the median effect (analogous to the more familiar IC50 values), and m is a Hill‐type coefficient signifying the sigmoidicity of the dose–effect curve.
We obtained the confidence interval (CI) values using the Biosoft calcusyn written in BASIC for automatic graphing of CI with respect to fa. The two drugs were either mutually non‐exclusive or mutually exclusive.
When CI<1, synergism is indicated.
When CI = 1, summation is indicated.
When CI>1, antagonism is indicated.
Annexin V/PI staining
To quantify the percentage of cells undergoing apoptosis, we used the Annexin V‐FITC kit as described by the manufacturer. Briefly, HT‐29 cells were incubated for 24 h and 48 h with TPL (5 ng/ml) and 5‐FU (100 μg/ml) alone or in combination. Then the cells were washed twice with cold PBS and resuspended in binding buffer at a concentration of 1×106 cells/ml. After incubation, 100 μl of the solution was transferred to a 5 ml culture tube, and 5 µl of Annexin V‐FITC and 10 μl of Propidium Iodide (PI) were added. The tube was gently contrifuged and incubated for 15 min at room temperature in the dark. At the end of incubation, 400 μl of binding buffer was added, and the cells were analysed immediately by flow cytometry (Partec, Münster, Germany). Flow cytometry analysis was performed using the Cell Quest software.
Effect of TPL and 5‐FU on tumour development in nude mice
Fifty‐five male BALB/c‐nu/nu nude mice, 5–6 weeks old, were purchased from the Laboratory Animal Central of Wuhan University; they weighed 16–18 g at the time of experimentation. The mice were maintained in autoclaved filter‐top micro‐isolator cages with autoclaved water and sterile food ad libitum. The cages were kept in an isolator unit provided with filtered air. Tumour cells used for inoculation were grown in culture and harvested as described above. Forty‐eight mice were inoculated subcutaneously with injections of 1×107 cells/mouse; a further seven mice inoculated with saline were regarded as the control group. Tumour sizes were determined using micrometer callipers and mice with similar sized tumours were randomly divided into four groups (with 7 mice/group): saline tumour control group; TPL 0.15 mg/kg/day group; 5‐FU 12 mg/kg/day group; and TPL + 5‐FU combination group. At the end of 3 weeks, the mice were killed, and the tumour xenografts were removed and measured. Tumour volume (TV) was calculated using the following formula:
TV (mm3) = d2×D/2
where d and D are the shortest and the longest diameters, respectively.
This study was approved by the Institutional Review Board of Zhongnan Hospital of Wuhan University.
Evaluation of side effects
The livers and kidneys of different groups were fixed in 10% buffered formalin, and the preserved tissues were cleaned in running water, processed for histological examination according to the conventional methods, and stained with haematoxylin and eosin. The morphology of any lesions observed was classified and registered by an observer who was blind with respect to the treatment groups.
Blood was collected by cardiac puncture using heparin rinsed 1 ml syringes (20 gauge needles). Beckman 700 (Beckman, Chicago, USA) was used to detect the activity of alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN) and serum creatinine (Cr), the biomarkers of liver and renal injury.
Blood samples for blood cell counts were obtained from eye sockets of mice. Erythrocytes, leucocytes and platelets were counted by Medonic CA620 VET automated cell counter (Boule Medical AB, Stockholm, Sweden). Blood marrow obtained from femurs isolated from mice were made into smears, and then observed by light microscopy after trypan blue staining.
Statistical analysis
The values for mean (SD) were calculated from raw data and then subjected to non‐parametric analysis (Mann–Whitney rank sum test). A value of p<0.05 was considered significant. Statistical analyses were performed using SPSS 11.5 software.
RESULTS
Effects of single drug exposure on the growth of colon carcinoma cell line HT‐29
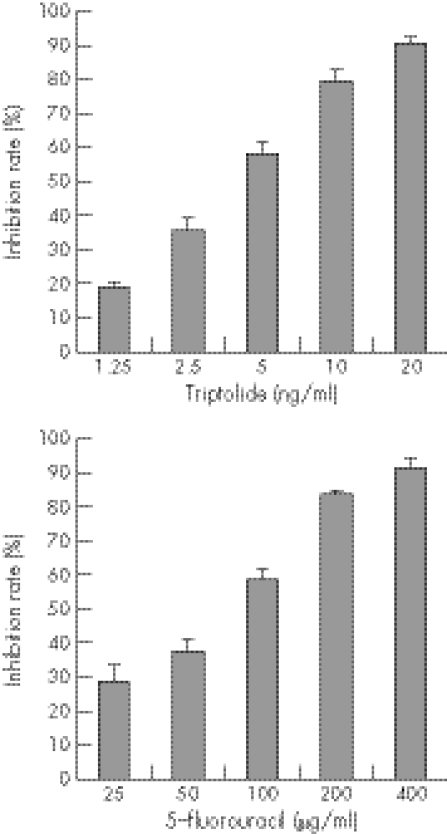
The inhibition of proliferation by TPL and 5‐FU in the HT‐29 human colon carcinoma cell line was assessed after 48 h of drug exposure, following 24 h culture in drug‐free medium. As shown in fig 1, after 48 h of treatment, growth of the HT‐29 cells was significantly inhibited in a dose‐dependent manner in vitro (p<0.01). The mean (SD) inhibition rate was 19.14 (1.37)% at the concentration of 1.25 ng/ml of TPL, and 90.92 (1.62)% at the concentration of 20 ng/ml. 5‐FU at 25 μg/ml had an inhibition rate of 28.99 (4.12)%, while the rate was 91.78 (2.88)% at 400 μg/ml.
Figure 1 Effect of triptolide (TPL) and 5‐fluorouracil (5‐FU) alone on proliferation of HT‐29 cells. HT‐29 tumour cells were treated with TPL at different concentrations (1.25, 2.50, 5.00, 10.00, 20.00 ng/ml) for 48 h, and then MTT was added to the culture for 4 h. The cells were harvested and the optical density was determined by spectrophotometer reader, then the results were expressed as inhibition rate. The proliferation of HT‐29 cells was inhibited by TPL in a dose dependent manner (p<0.01). Similar results were obtained for 5‐FU (p<0.01).
Combined effects of TPL and 5‐FU on the growth of HT‐29 cells
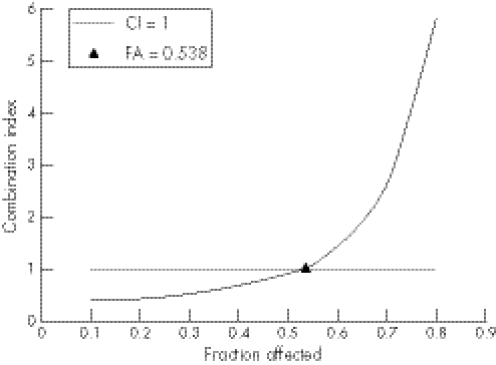
When the combination effects were studied, the cells were exposed for 48 h to both of the two drugs concurrently at a fixed molar ratio (TPL:5‐FU ratios were 1:361). The proliferation–inhibitory effect was assessed by the MTT assay. Then the inhibition rates were analysed by the method of Chou and Talalay.16 The experiments were repeated at least in triplicate. As shown in fig 2, the CI values were <1 when the fractions affected were lower than 0.538. It is indicated that TPL and 5‐FU had a synergistic effect on inhibiting proliferation of HT‐29 cells at lower concentrations.
Figure 2 The combined effects of TPL and 5‐FU on the human colon carcinoma cell line HT‐29. HT‐29 cells were treated with TPL combined with 5‐FU at a constant molar ratio: 1:360. After 48 h treatment, the inhibition of proliferation was determined by MTT assay. The confidence interval (CI) values were determined using the pre‐described method. CI = 1 indicates an addictive effect; CI<1 indicates a synergistic effect; CI>1 indicates an antagonism effect. As shown here, the CI values are <1 when the fractions affected are lower than 0.538. It is indicated that TPL and 5‐FU had synergistic effects on inhibiting proliferation of HT‐29 cells at lower concentrations.
Apoptosis induced by TPL and 5‐FU
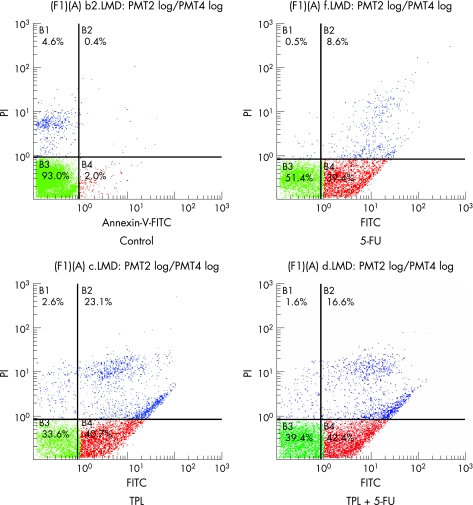
Apoptosis induced by TPL and 5‐FU was confirmed using Annexin V/PI staining to detect externalisation of phosphatidylserine on the cell membrane. As shown in fig 3, the proportion of Annexin V‐positive/PI‐negative cells increased progressively in HT‐29 cells incubated at low concentrations of TPL (5 ng/ml) and 5‐FU (100 μg/ml) for 48 h. TPL and 5‐FU alone significantly promoted apoptosis compared with group control (p<0.01, table 1 and fig 3) and the combined effects were stronger than the effects of TPL and 5‐FU alone (p<0.01, table 1 and fig 3).
Figure 3 TPL and 5‐FU either in combination or alone induced apoptosis of HT‐29 cells. HT‐29 cells were treated as described for 48 h. Annexin V‐positive/PI‐negative cells are in early stages of apoptosis and double positive cells are in late apoptosis, whereas Annexin V‐negative/PI‐positive cells are necrotic. In the text, we compared the rate of cells in early stage of apoptosis. TPL combined with 5‐FU or used individually significantly promoted apoptosis compared with control group (p<0.01), and the combined effect was stronger than the effect of TPL and 5‐FU alone (p<0.01).
Table 1 Apoptosis of HT‐29 cells exposed to TPL and/or 5‐FU for 24 h and 48 h.
| Group | Apoptosis rate (%) | |
|---|---|---|
| 24 h | 48 h | |
| TPL | 14.81 (1.07)†‡ | 36.37 (2.51)*‡ |
| 5‐FU | 15.71 (1.15)†‡ | 37.59 (2.75)*‡ |
| TPL+5‐FU | 26.03 (1.13)‡ | 41.71 (1.38)‡ |
| Control | 1.91 (0.21)† | 2.08 (0.33)† |
5‐FU; 5‐fluorouracil; TPL, triptolide.
Data presented as mean (SD); all data were carried out in triplicate.
*p<0.05, significantly different from TPL+5‐FU group; †p<0.01, significantly different from TPL+5‐FU group; ‡p<0.01, significantly different from control group.
Effect of TPL and 5‐FU on tumour development in nude mice
We examined the effects of TPL and 5‐FU on the growth of primary tumour xenografts in mice. During the whole course, no mouse died; 37 of 48 mice successfully grew tumour xenografts. Some nude mice of the 5‐FU group and the combination group developed erythema and papules, but the symptoms disappeared after treating them with benhydramin for 2–3 days. After growing for 7 days, the tumour xenografts reached a mean (SD) size of 138.53 (32.29) mm3. We chose 28 mice with tumour xenografts of around 100 mm3 in size and randomly divided them into four groups (with 7 mice/group) as described above. There were no statistical differences among the sizes of all the groups. Thereafter, the mice were given different treatments. The results showed that TPL combined with 5‐FU or when used individually had significant inhibitory effects on the growth of HT‐29 cells. The tumour volumes were all reduced compared with the saline chloride tumour control group (p<0.01, table 2), while degree of tumour reduction differed; the combination group's volume was less than TPL group and 5‐FU group (p<0.05, table 2). In the tumour control group, the tumours grew continuously and some appeared malignant with dilated vessels and volcanoid ulcer. The mean (SD) tumour volume was 482.03 (85.95) mm3 at the end of the experiment. When mice were treated with TPL combined with 5‐FU, the tumour inhibition rate was 96.78%, whereas the inhibition rates for those mice treated with TPL and 5‐FU alone were 78.53% and 84.16%, respectively (table 2). These results show that the antitumour effect of TPL combined with 5‐FU was superior to the effect of the drugs when used individually.
Table 2 Inhibitory effects of TPL and 5‐FU on HT‐29 tumour in nude mice.
| Group | n | Volume (mm3) | Inhibition rate (%) |
|---|---|---|---|
| TPL | 7 | 103.50 (23.06)*† | 78.53 |
| 5‐FU | 7 | 76.35 (6.44)*† | 84.16 |
| TPL+5‐FU | 7 | 15.54 (2.08)† | 96.78 |
| Control | 7 | 482.03 (85.95) |
5‐FU; 5‐fluorouracil; TPL, triptolide.
Data presented as mean (SD) and are expressed as inhibition rate (%) = [1−mean of tumour volume of tests/mean of tumour volume of control]×100%. There were 7 mice per group.
*p<0.05, significantly different from TPL+5‐FU group; †p<0.01, significantly different from control group.
Evaluation of side effects
At the end of the experiment, the nude mice were necropsied. There was no obvious metastasis, peptic ulcer and haemorrhage, or injury of the liver and kidney that could be observed by the naked eye.
Hepatic toxicity was monitored by quantitative analysis of the ALT and AST activities that were used as the biochemical markers of liver injury.18 The hepatic toxicity induced by different treatment is shown in table 3. ALT and AST activities in the serum were not significantly raised (compared to the control group, p>0.05), and there was no difference among the combination group, TPL group and 5‐FU group (compared to the control group, p>0.05). At necropsy, the livers of mice in the treatment groups appeared smooth and normal in colour. There was no significant difference in liver weight between the treated groups and the control group; even the histological examination showed no obvious lesion. Similar results were obtained for renal injury.
Table 3 Effect of TPL combined with 5‐FU or alone on hepatic and renal function.
| Group | n | ALT (u/l) | AST(u/l) | BUN (μmol/l) | Cr (μmol/l) |
|---|---|---|---|---|---|
| TPL | 7 | 28.12 (3.16) | 106.76 (11.8) | 5.92 (0.67) | 52.87 (2.01) |
| 5‐FU | 7 | 27.50 (4.23) | 99.50 (27.65) | 5.83 (0.94) | 52.99 (5.86) |
| TPL+5‐FU | 7 | 29.67 (3.22) | 100.71 (17.18) | 5.86 (0.86) | 56.83 (3.00) |
| Tumour control | 7 | 27.38 (1.79) | 102.08 (17.69) | 5.44 (0.39) | 53.46 (2.53) |
| Normal control | 7 | 27.29 (2.32) | 101.02 (19.25) | 5.41 (0.53) | 52.59 (2.17) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; Cr, creatinine; 5‐FU; 5‐fluorouracil; TPL, triptolide.
Data are presented as mean (SD), with n = 7 mice/group. Groups were treated as follows: TPL (5 mg/kg/day); 5‐FU (12 mg/kg/day); TPL (5 mg/kg/day) + 5‐FU (12 mg/kg/day); Tumour control (saline of equal volume); Normal control. No differences were observed in the ALT, AST, BUN, and Cr among all groups (p>0.05).
The blood cell count of the nude mice demonstrated the inhibitive effects of TPL used either alone or in combination with 5‐FU (table 4). The results were similar to the marrow‐nucleated cell count. The results indicated that TPL combined with 5‐FU at lower concentrations did not enhance the haematological side effects.
Table 4 Effect of TPL combined with 5‐FU or alone on blood cell count.
| Group | n | Erythrocyte (×1012/l) | Leucocyte (×109/l) | Platelet (×109/l) |
|---|---|---|---|---|
| TPL | 7 | 8.55 (0.68) | 4.18 (2.43) | 815.13 (96.31) |
| 5‐FU | 7 | 8.46 (0.57) | 4.33 (3.59) | 817.78 (94.66) |
| TPL+5‐FU | 7 | 8.57 (0.25) | 4.36 (2.35) | 821.25 (109.32) |
| Tumour control | 7 | 8.23 (0.62) | 4.27 (1.36) | 809.36 (86.53) |
| Normal control | 7 | 8.47 (0.26) | 4.09 (1.58) | 810.13 (93.03) |
5‐FU; 5‐fluorouracil; TPL, triptolide.
Data are presented as mean (SD), with n = 7 mice/group. Groups were treated as follows: TPL (5 mg/kg/day); 5‐FU (12 mg/kg/day); TPL (5 mg/kg/day) + 5‐FU (12 mg/kg/day); Tumour control (saline of equal volume); Normal control. No differences were observed in the blood cell counts among groups (p>0.05).
DISCUSSION
Colorectal carcinoma remains a major public health threat and accounts for approximately 13% of all cancers.19,20 More effective treatments and earlier detection have led to improved survival over recent decades. However, around 50% of newly diagnosed colorectal carcinoma patients will eventually progress due to micrometastases, and die of their disease, in spite of the advances in surgical techniques and radiotherapy. Therefore chemotherapy becomes one of the most important means of extending the survival of colorectal carcinoma patients.
Until 1985, 5‐FU was the sole agent available for the treatment of colorectal carcinoma. For advanced colon carcinomas, the drug had a response rate of around 10% and a median overall survival of 9–11 months. In the 1990s, randomised comparisons clearly showed that chemotherapy was indeed improving the condition of patients with metastatic colorectal carcinoma. Furthermore, this decade also saw the investigation of new drugs and combinations of chemotherapy.21,22
In recent years, the interest in exploiting traditional medicines for prevention or treatment of tumours has increased. The Chinese herb Tripterygium wilfordii Hook F (TWHF) has been used in traditional Chinese medicine for more than 2000 years. However, its potential value was recognised by Western medicine only after investigators observed the effectiveness of TWHF in the treatment of leprosy and rheumatoid arthritis. TPL, a highly oxygenated diterpene, was first isolated by Kupchan et al from an ethanol extract of TWHF on the basis of bioassay‐directed fraction.23 It has been identified as the major component of TWHF responsible for immunosuppressive and anti‐inflammatory effects.24
Many studies in vitro and in vivo showed that TPL was cytotoxic to various types of tumour cells and significant antiproliferative and apoptotic effects were observed in tumour cells treated by TPL.25,26,27,28 But Shamon et al point out that while TPL is capable of demonstrating some antitumour activity, it is certainly not curative, and the range of efficiency is narrow (ED50 values range from 1–20 ng/ml for human breast cancer cell lines).11
In this study, we investigated the inhibitive effect of TPL combined with 5‐FU on colon carcinoma in vitro and in vivo. We found that TPL and 5‐FU alone inhibited significantly the proliferation of colon carcinoma cells in a dose‐dependent manner (TPL 1.25–20 ng/ml; 5‐FU 25–400 μg/ml). The combined effect of TPL and 5‐FU on the growth of human colon carcinoma was superior to that of TPL or 5‐FU alone in vitro and in vivo, and the combined effect was synergistic at lower concentrations. The results are interesting and encourage further research into the mechanism of this synergistic effect. Then we examined the apoptosis induced by TPL (5 ng/ml) and 5‐FU (100 μg/ml). The results showed that TPL combined with 5‐FU at lower concentrations could induced apoptosis of HT‐29 cells and the effect was stronger than that of TPL and 5‐FU alone.
It has been reported that TPL potently inhibits the proliferation of both leukaemia and solid tumours by inducing apoptosis.10,25,29 However, the mechanisms underlying the apoptosis process are poorly understood. Apoptosis is a tightly regulated cellular process. The signals for apoptosis can be initiated from outside the cell (extrinsic) through the death‐receptor pathway, or from inside the cell (intrinsic) through the mitochondrial pathway.30,31 Wang et al found that TPL induced apoptosis of HeLa and PANC‐1 cells and it was likely that the two pathways operate in parallel to mediate the apoptosis cascade.32 5‐FU inhibits the thymic pyrimidine nucleotidase of tumour cells and affects DNA stability.33 Many experiments have shown that 5‐FU also induces apoptosis of gastroenteral carcinoma cells, including the attendance of p53, bcl‐2, caspase‐3 and caspase‐8.34,35,36,37 We found that TPL combined with 5‐FU at lower concentrations could prompt apoptosis, while the mechanism is still obscure.
TPL does have one major drawback as an antitumour agent—its toxicity. Adverse effects were reported including gastroenteral disturbances, amenorrhoea, kidney dysfunction, leucopenia, thrombocytopenia, and aplastic anaemia. So we designed the model of tumour xenografts in mice and gave chemotherapy with TPL and 5‐FU at lower concentrations. During the whole course of the experiment, there were no obvious side effects apart from the appearance of erythema and papules in some mice. So we may conclude that TPL combined with 5‐FU at lower concentrations would not increase the side effects of chemotherapy.
In conclusion, TPL combined with 5‐FU had a significantly greater anti‐colon cancer effect than TPL and 5‐FU used alone. In addition, this study found that toxicity did not increase when the drugs were used in combination, which indicates potential for combined use of the drugs in clinical treatment.
ACKNOWLEDGEMENTS
We thank Shi‐quan LIU, Department of Oncology, Zhongnan Hospital of Wuhan University, for providing the HT‐29 cell line and the technical support of cell culture, and Dong XIA, Institute of Pathology, for exceptional technical assistance in pathological examination.
Abbreviations
ALT - alanine aminotransferase
AST - aspartate aminotransferase
BUN - blood urea nitrogen
CI - confidence interval
Cr - creatinine
DMSO - dimethyl sulfoxide
5‐FU - 5‐fluorouracil
TPL - triptolide
TWHF - Tripterygium wilfordii Hook F
Footnotes
Competing interests: none declared
References
- 1.Parker S L, Tong T, Bolden S.et al Cancer statistics, 1996. CA Cancer J Clin 1996465–27. [DOI] [PubMed] [Google Scholar]
- 2.Jessup J M, McGinnis L S, Winchester D P.et al Clinical highlights from the National Cancer Data Base: 1996. CA Cancer J Clin 199646185–192. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham D, Findlay M. The chemotherapy of colon cancer can no longer be ignored. Eur J Cancer 1993292077–2079. [DOI] [PubMed] [Google Scholar]
- 4.Labianca R, Pessi A, Facendola G.et al Modulated 5‐fluorouracil (5‐FU) regimen in advanced colorectal cancer: a critical review of comparative studies. Eur J Cancer 199632A(Suppl 5)S7–12. [DOI] [PubMed] [Google Scholar]
- 5.Levi F, Zidani R, Missel J L.et al Randomised multicentrr trial of chemotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. International Organization for Cancer Chronotherapy. Lancet 1997350681–686. [DOI] [PubMed] [Google Scholar]
- 6.Chen B J. Triptolide, a novel immunosuppressive and anti‐inflammatory agent purified from a Chinese herb Tripterygium wilfordii Hook F. Leuk Lymphoma 200142253–265. [DOI] [PubMed] [Google Scholar]
- 7.Chan M A, Kohlmeier J E, Branden M.et al Triptolide is more effective in preventing T cell proliferation and interferon‐gamma production than is FK506. Phytother Res 199913464–467. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y, Liu Z, Tolosa E.et al Triptolide induces apoptotic death of T lymphocyte. Immunopharmacology 199840139–149. [DOI] [PubMed] [Google Scholar]
- 9.Huynh P N, Hikim A P, Wang C.et al Long‐term effects of triptolide on spermatogenesis, epididymal sperm function, and fertility in male rats. J Androl 200021689–699. [PubMed] [Google Scholar]
- 10.Yang S, Chen J. Triptolide inhibits the growth and metastasis of solid tumors. Molecular Cancer Therapeutics 2003265–72. [PubMed] [Google Scholar]
- 11.Shamon L A, Pezzuto J M, Graves J M.et al Evaluation of the mutagenic, cytotoxic, and antitumor potential of TPL, a highly oxygenated diterpene isolated from Tripterygium wilfordii. Cancer Lett 1997112113–117. [DOI] [PubMed] [Google Scholar]
- 12.Tengchaisri T, Chawengkirttikul R, Rachaphaew N.et al Antitumor activity of TPL against cholangiocarcinoma growth in vitro and in hamsters. Cancer Lett 1998133169–175. [DOI] [PubMed] [Google Scholar]
- 13.Jiang X H, Wong B C, Lin M C.et al Functional p53 is required for triptolide‐induced apoptosis and AP‐1 and nuclear factor‐kappaB activation in gastric cancer cells. Oncogene 2001208009–8018. [DOI] [PubMed] [Google Scholar]
- 14.Fidler J M, Li K, Chung C.et al PG490‐88, a derivative of triptolide, causes tumor regression and sensitizes tumors to chemotherapy. Mol Cancer Ther 20032855–862. [PubMed] [Google Scholar]
- 15.Lu L H, Lian Y Y, He G Y.et al Clinical study of triptolide in treatment of acute leukemia. Clin Exp Investig Hematol 199231–3. [Google Scholar]
- 16.Chou T C, Talalay P. Quantitative analysis of dose effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 19842227–55. [DOI] [PubMed] [Google Scholar]
- 17.Chou T C, Motzer R J, Tong Y.et al Computerized quantitation of synergism and antagonism of Taxol, topotecan and cisplatin against human teratocarcinoma cell growth: a rational approach to clinical protocol design. J Natl Cancer Inst 1994861517–1524. [DOI] [PubMed] [Google Scholar]
- 18.Awad M E, Abdel‐Rahman M S, Hassan S A. Acrylamide toxicity in isolated rat hepatocytes. Toxicol in Vitro 199812699–704. [DOI] [PubMed] [Google Scholar]
- 19.Parkin D M, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin 19994933–64. [DOI] [PubMed] [Google Scholar]
- 20.Greenlee R T, Hill‐Harmon M B, Murray T.et al Cancer statistics 2001. CA Cancer J Clin 20015115–36. [DOI] [PubMed] [Google Scholar]
- 21.Nordic Gastrointestinal Tumor Adjuvant Project Expectancy or primary chemotherapy in patients with advanced asymptomatic colorectal cancer: a randomized trial. J Clin Oncol 199210904–911. [DOI] [PubMed] [Google Scholar]
- 22.Scheithauer W, Rosen H, Kornek G V.et al Randomized comparison of combination chemotherapy plus supportive care or supportive care alone in patients with metastatic colorectal cancer. BMJ 1993306752–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kupchan S M, Court W A, Dailey R G.et al Triptolide and tripdiolide, novel antileukemic ditepenoid tripoxides from Triterygium wilfordii. J Am Chem Soc 1972947194–7195. [DOI] [PubMed] [Google Scholar]
- 24.Tao X, Cai J J, Lipsky P E. The identity of immunosuppressive components of the ethyl acetate extracts and chloroform methanol extract of Tripterygium wilfordii Hook F. J Pharmacol Exp Ther 19952721305–1312. [PubMed] [Google Scholar]
- 25.Yinjun L, Jie J, Yungui W. Triptolide inhibits transcription factor NF‐kappaB and induces apoptosis of multiple myeloma cells. Leuk Res 20052999–105. [DOI] [PubMed] [Google Scholar]
- 26.Lou Y J, Jin J. Triptolide down‐regulates bcr‐abl expression and induces apoptosis in chronic myelogenous leukemia cells. Leuk Lymphoma 200445373–376. [DOI] [PubMed] [Google Scholar]
- 27.Chang W T, Kang J J, Lee K Y.et al Triptolide and chemotherapy cooperate in tumor cell apoptosis. A role for the p53 pathway. J Biol Chem 20012762221–2227. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Y X, Huang Y L, Xu Q N.et al Several monomes from Tripterygium wilfordii inhibit proliferation of glioma cells in vitro. Ai Zheng 2002211106–1108. [PubMed] [Google Scholar]
- 29.Chan E W, Cheng S C, Sin F W.et al Triptolide induced cytotoxic effects on human promyelocytic leukemia, T cell lymphoma and human hepatocellular carcinoma cell lines. Toxicol Lett 200112281–87. [DOI] [PubMed] [Google Scholar]
- 30.Chen M, Wang J. Initiator caspases in apoptosis signaling pathways. Apoptosis 20027313–319. [DOI] [PubMed] [Google Scholar]
- 31.Green D R. Apoptotic pathways: ten minutes to dead. Cell 2005121671–674. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Matta R, Shen G.et al Mechanism of triptolide‐induced apoptosis: effect on caspase activation and Bid cleavage and essentiality of the hydroxyl group of triptolide. J Mol Med 200684405–415. [DOI] [PubMed] [Google Scholar]
- 33.Yeh K H, Yeh S H, Hsu S H.et al Prolonged and enhanced suppression of thymidylate synthase by weekly 24‐h infusion of high‐dose 5‐fluorouracil. Br J Cancer 2000831510–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu X X, Kakehi Y, Mizutani Y. Activation of caspase‐3 in renal cell carcinoma cells by anthracyclines or 5‐fluorouracil. Int J Oncol 20011919–24. [DOI] [PubMed] [Google Scholar]
- 35.Ikebukuro K, Adachi Y, Toki J.et al Morphological change, loss of deltapsim and activation of caspases upon apoptosis of colorectal adenocarcinoma induced by 5‐FU. Cancer Lett 2000153101–108. [DOI] [PubMed] [Google Scholar]
- 36.Adachi Y, Taketani S, Oyaizu H.et al Apoptosis of colorectal adenocarcinoma induced by 5‐FU and/or IFN‐gamma through caspase‐3 and caspase‐8. Int J Oncol 1999151191–1196. [DOI] [PubMed] [Google Scholar]
- 37.Matsuhashi N, Saio M, Matsuo A.et al The evaluation of gastric cancer sensitivity to 5‐FU/CDDP in terms of induction of apoptosis: time‐ and p53 expression‐dependency of anti‐cancer drugs. Oncol Rep 200514609–615. [PubMed] [Google Scholar]