Abstract
Age‐related macular degeneration (AMD) is the commonest cause of blindness in the population over 60 years of age and accounts for over 50% of those registered blind in the UK. The incidence is increasing and as older generations live longer a growing number of patients will be affected in the future. Affected patients lose central vision, important in all aspects of everyday life. This review outlines risk factors for AMD, clinical features, treatment and management strategies for patients, families and physicians caring for those with AMD. Recent trials are included along with practical clinical advice. While there is no curative treatment at present, intervention can reduce the risk of developing AMD and limit disease progression if it occurs. These modalities are discussed here. As new discoveries in the field of genetics and novel therapies emerge, a brighter future seems certain for the ageing population.
Keywords: age‐related macular degeneration, blindness, choroidal neovascular membrane, elderly, photodynamic therapy, quality of life
Vision is the primary sense and is a major determinant of quality of life. Good vision enables independent functioning and assumes greater importance with increasing age. Poor vision limits driving ability and is associated with falls and hip fractures. Age‐related macular degeneration (AMD) accounts for 50% of blind registrations in the UK1 and is increasing in incidence.2 This article addresses risk factors, clinical features, treatment, and the impact of the disease on patients and communities.
Prevalence of AMD
After the age of 60 the prevalence of AMD rises sharply,3 with 3.5% of those aged over 75 visually impaired due to this disease.2 Some studies estimate visual impairment from AMD to be up to 16% in a slightly older age group. Geographical variations in AMD exist because of genetic and environmental factors such as smoking, diet and possibly sunlight.
Risk factors for AMD
The pathogenesis of AMD is unclear. A number of potential risk factors have been implicated, but age and smoking are the only factors that seem to definitely increase risk.
Age
As well documented by epidemiological studies the prevalence of AMD increases with age.3
Gender
Meta‐analysis of several large studies suggests no statistical difference in prevalence of early AMD between men and women.2 Controversy exists over whether blindness from AMD is higher in females, although this may be due to increased life expectancy and over‐representation of females in these studies.
Smoking
Smoking has been associated with a relative risk of 2–4 for developing AMD.4,5,6,7 The increased risk may persist even 20 years after stopping smoking.6 Other studies suggest no increased risk for developing late AMD in past smokers compared to those who never smoked, suggesting benefits for current smokers who give up.7
Genetic influences
There appears to be an increased incidence of AMD in patients with a family history,8 although inheritance is unclear. This may be more important in earlier‐onset disease. In a number of families with dominant pattern, late‐onset disease (L‐ORD), the gene mutation has been identified on chromosome 1. Other candidate genes include fibulin 5, where a missense mutation is causal in a small number of people with AMD.9 Recently, genetic variation in a major regulator of the alternative complement pathway, factor H (HF1), has been proposed to underlie a major proportion of AMD.10 HF1 is synthesised by the retinal pigment epithelium and accumulates in drusen. While a strong genetic component in some families with AMD exists, environmental factors play a role and the story is far from complete.
Ethnic group
AMD is more prevalent in the US in non‐Hispanic white as compared to black or Mexican American populations.11
Hypertension
There are conflicting reports on the effect of uncontrolled blood pressure on the initial development of AMD.12 In patients in whom one eye is affected by AMD, the risk of second eye involvement seems to be higher with uncontrolled hypertension.13 The 10 year incidence figures from the Beaver Dam study show that when other factors are controlled for, persons with previously diagnosed, controlled hypertension at baseline were twice as likely and those with uncontrolled hypertension thrice as likely, to develop exudative AMD as normotensive individuals.12 This is consistent with other studies.13 However, no such relationship was found with blood pressure and AMD in other large studies.14
Diet
Foods rich in carotenoids (an important constituent of the retinal pigments) have a protective effect.15 A large prospective randomised controlled trial looked at the effect of high dose antioxidants and minerals in those with early AMD and found a reduction in progression to late‐stage disease of 20–25%.16 Fat intake has been associated with the risk of progression of AMD.17 Several clinical studies have yielded contradictory results regarding the role of cholesterol as a risk factor for AMD, and the possible protective benefit of HMG‐CoA reductase inhibitors (statins).18 There is not enough evidence at present to recommend statins as a preventative measure in all patients with AMD, but there are theoretical advantages for their use in AMD patients with elevated cholesterol.
Sunlight
There is no universal agreement on the role of light exposure in the development of AMD. A 10 year prospective study has shown that prolonged exposure to sunlight may be associated with increased risk of earlier development of AMD but not AMD progression.19 This study also found a protective effect of the use of a hat and sunglasses in reducing the incidence of early AMD. However, a large Australian case control study has failed to find a link between sunlight exposure and AMD.20 One difficulty of such studies is the reliability and accuracy of methods to quantify the cumulative light exposure of different individuals who live at the same geographical location.
Cataract surgery
The natural, crystalline lens absorbs UV light (300–400 nm wavelength) throughout life. As it yellows with age, visible light is also absorbed, particularly in the blue spectrum (400–500 nm). UV and blue light are more phototoxic to the retina than longer wavelength light. Cataract surgery to remove an aged, yellow, natural lens therefore increases the amount of harmful light reaching the retina. Two major epidemiological studies have found an increased risk of late AMD in patients who had prior cataract surgery.21,22 It is usual practice to replace a cataractous lens with an intraocular lens (IOL). IOLs are colourless, but most have UV blocking chromophores incorporated. A new yellow IOL that also absorbs blue light has recently received US FDA approval. Some authors recommend this yellow IOL with few reservations, but others caution that too much blue light, which is important for night vision, may be absorbed.
Clinical presentation
Most patients with AMD have a slow and gradual decline in central vision, usually manifesting initially as difficulty reading smaller print. Less commonly, patients may report distortion of central vision. They may describe straight lines as appearing “bent” or “wavy”. Distortion in an elderly person is highly suggestive of the wet form of AMD and requires prompt referral to an ophthalmologist. Distortion may represent fluid build up under the macula with disturbance of photoreceptors. These symptoms can be differentiated from other common visual conditions in the elderly such as cataract, characterised by a gradual decline in both distance and near vision, and chronic glaucoma, which is usually asymptomatic. Later stages of the disease result in a central scotoma.
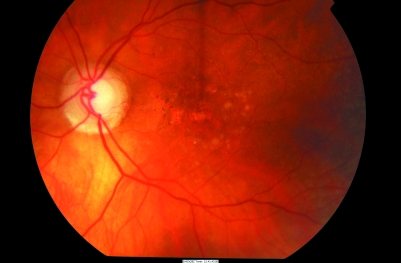
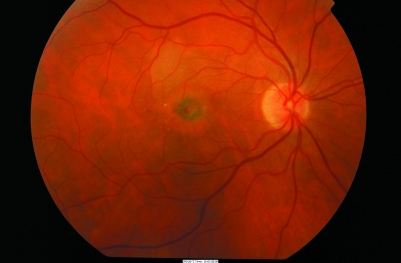
On examination, pigmentary changes are seen at the macula. A spectrum of changes may be present depending on the stage and type of AMD. Dry AMD shows atrophy of the retinal pigment epithelium, often with focal clumping (fig 1), while in wet AMD, subretinal fluid, haemorrhage and scarring may be seen (fig 2).
Figure 1 Colour photograph of the left eye shows dry AMD. Mottled discolouration of the macula is seen with areas of hyperpigmentation and hypopigmentation along with yellow drusen deposits.
Figure 2 Colour photograph of the right eye shows wet AMD. A circumscribed area in the macula shows subretinal blood and exudate. This patient will have experienced a rapid decline in central vision.
Management of patients with AMD
Referral to ophthalmology
Patients noticing sudden visual loss or new distortion should be referred for urgent ophthalmological review. Early assessment of exudative AMD is essential as all currently available treatments aim to preserve rather than restore vision. Patients with a slow decline in central vision should also be referred, but on a non‐urgent basis.
Investigations in the eye clinic
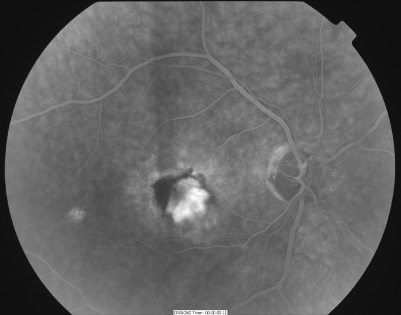
The near and distance acuity of the patient is recorded, and slit‐lamp fundal examination is carried out. If, as in the majority of cases, the AMD is of the dry type, no further investigations are necessary. If wet AMD is detected, a fluorescein angiogram (fig 3) may be indicated to further determine if the lesion is treatable with laser.
Figure 3 Fluorescein angiography confirms wet AMD. The fluorescent dye is seen in the retinal blood vessels. An abnormal area of hypofluorescence (subretinal blood) and contiguous hyperfluorescence (choroidal neovascular membrane) is seen at the macula.
Dry AMD: conservative management
Explanation and education
For most patients with AMD, little specific treatment beyond risk factor control is available. We advise patients to stop smoking, ensure blood pressure is controlled and eat a diet rich in fruit and fresh green leafy vegetables, especially spinach (which contains carotenoids). It is also prudent to recommend the use of a hat and sunglasses when outside in bright conditions.
Visual rehabilitation should begin early. A simple, but crucial intervention is a good light. Magnifiers are useful as reading aids, but they can be difficult to use at first. A wide range of visual aids, such as telescopes, can greatly increase vision for motivated patients. We routinely refer patients with AMD to a low vision clinic where a specially trained optometrist and nurse can address individual patient needs.
Blind registration
Visual deterioration in AMD often progresses and we register patients when they meet the criteria for partial sighted or blind registration. This initiates contact with social services and other organisations. The Royal National Institute for the Blind (RNIB) is one of the many organisations that provide services to low sighted people. They distribute large‐print information leaflets, organise visits to assess the patient's home and provide patients with practical devices to make day‐to‐day living easier. Many local organisations and self‐help groups exist throughout the country and the RNIB facilitate patients to contact other support groups.
Dietary supplements
Antioxidants may limit damage from free radicals produced during light absorption by retinal cells. Visual loss and the development of advanced AMD are reduced with high‐dose antioxidants and minerals,16 which include beta‐carotene, vitamin C, E, zinc and copper. Lung cancer risk may be increased with high dose beta‐carotene, so smokers and ex‐smokers should omit this vitamin. Another antioxidant, lutein, is the subject of a randomised controlled trial23 and results to date suggest some visual improvement in dry AMD.
Many health boards and Primary Care Trusts do not consider such dietary supplements prescription items and thus they are not universally available on the NHS.
Wet AMD: management
As for dry AMD, the first step in treatment lies in patient education. Risk factors should be addressed and, if appropriate, patients should be registered as having low sight and put in contact with support organisations. However, unlike dry AMD, treatment is available for some patients to modify disease progression and reduce the extent of resultant visual loss.
Current therapy
In the context of current UK treatment guidelines, about one third of newly diagnosed exudative AMD lesions are amenable to treatment with either photodynamic or conventional laser therapy.
Photodynamic therapy (PDT) is the current laser treatment of choice for subfoveal disease and shows a significant reduction in severe visual loss.24 A photosensitising dye (Verteporfin) is infused intravenously and passes to the choroid where it is preferentially absorbed by the rapidly dividing vessels of the neovascular membrane. A diode laser activates the dye and free radicals are released causing endothelial damage and thrombosis of affected vessels. The membrane is thus selectively destroyed, sparing the surrounding retina. Although there is a resultant scotoma, this is less than would be produced by conventional laser or by scarring from untreated disease. The disadvantages of PDT include the need for fluorescein angiography at each treatment to localise and exactly delineate the choroidal neovascular membrane (CNV), as well as during follow‐up visits. Patients require close monitoring after treatment and are generally seen at 3 monthly intervals as there is a high rate of membrane recurrence. Most patients need an average of three to five treatments over an 18 month to 2 year period.
Conventional argon laser treatment for AMD was first reported in the 1980s.25 Overall, treatment facilitated a 20% reduction in severe visual loss, but results were disappointing for more severe (subfoveal) disease. Ablating the neovascular membrane with an argon laser damages the overlying retina causing severe central visual loss and a resultant scotoma or blind spot in the vision. Thus, this treatment is useful only for neovascular membranes outside the fovea (extra‐foveal).
Newer therapies
Anti‐angiogenic treatments
Vascular endothelial derived growth factor (VEGF) is a potent mitogen and vascular permeability factor that plays a pivotal role in neovascularisation. The role of VEGF in AMD is less clear, but increased levels are present in neovascular membranes.26 Putative mechanisms in the pathogenesis of AMD include induction of choroidal new vessels, increasing vascular permeability with the formation of subretinal fluid or acting as a pro‐inflammatory agent causing leucocyte margination and damage to retinal endothelial cells.
Anecortave acetate (Retaane, Alcon) is a synthetic cortisone that blocks angiogenesis and inhibits VEGF. The advantages of this drug are 6 monthly injection intervals and less risk of infection, as it is not administered directly into the eye but into the sub‐Tenon's (peri‐ocular) space. There is increased visual stability in treated patients at 1 year, with 84% of treated eyes maintaining vision compared to 50% of controls.27 This drug represents an interesting development, as peri‐ocular administration is less invasive and safer than intra‐vitreal therapy. However, another study failed to demonstrate any significant advantage of anecortave over PDT.28 One problem with this route of administration is reflux of the drug from the sub‐Tenon's space, so further work is currently being carried out to address this problem.
Pegaptanib (Macugen, EyeTech/Pfizer) is a highly selective inhibitor of VEGF and is the first anti‐VEGF drug approved for use in AMD in the US. The National Institute for Clinical Excellence (NICE) are reviewing the drug at present and will publish guidance in August 2007. Pegaptanib is an aptamer that binds to VEGF‐165, the most important of the five subtypes in the eye. It is injected directly into the vitreous and no systemic side effects have been reported.29 Promising results include a sustained reduction in visual loss at 2 years, with 70% of those treated compared to 55% of controls losing less than three lines of vision on the standard acuity chart.29 There was also an increase in vision in over 20% of treated patients.29 Disadvantages are the need for repeat injections at 6 weekly intervals and risk of infection (endophthalmitis rate of 0.03% in the study). Fluorescein angiography shows slowing of CNV growth, decreased lesion size and less leakage in the treated group. Pegaptanib is effective in all subgroups of wet AMD (ie, occult and classic) and appears more beneficial the earlier in the course of the disease it is administered.29
Ranibizumab (Lucentis, Genentech) is another new anti‐angiogenic drug. It is a mouse/human monoclonal antibody fragment that binds and blocks all forms of VEGF (at the VEGF receptor binding site). Clinical trials have shown that ranibizumab is effective in preventing visual loss, with 95% of treated eyes losing less than three lines on the standard acuity chart at 1 year compared to 62% of controls.30 Furthermore, this drug appears to be capable of significantly improving visual acuity in patients with wet AMD, with up to 34% of patients experiencing a significant visual gain as compared to 5% of the control group at 1 year after treatment.30 Angiography also confirms decreased CNV size and leakage as compared to eyes treated with PDT alone.31
Bevacizumab (Avastin, Roche) is a whole humanised mouse antibody that binds non‐selectively to all forms of VEGF (at the VEGF receptor binding site). It has been developed and licensed for use in humans as an intravenous treatment for metastatic colorectal cancer. It has significant systemic side effects when used in this way, including uncontrolled hypertension and an increased incidence of thrombo‐embolic events. It was originally investigated as a systemic treatment for AMD and showed promise in terms of visual improvement.32 However, a significant number of patients developed systemic side effects and this has prompted the increasing use of bevacizumab as an intravitreal injection. However, although the intravitreal use of this drug has been reported to be safe and effective,33,34 to date no large scale clinical trials have addressed this issue. Concerns with intravitreal administration include the long half‐life (100‐fold slower metabolism than ranibizumab), which may result in greater systemic absorption than other intravitreal agents. Systemic levels of bevacizumab after intravitreal injection have still to be determined. Furthermore, as the intraocular safety profile of the drug remains unknown, the long half‐life may potentiate any possible retinal toxicity. Pharmacologically, bevacizumab differs from ranibizumab in that it is a whole antibody and this may lead to greater antigenicity and inflammation. Lastly, there are different manufacturing standards for intraocular and intravenous injections and this too may be a cause of concern.
However, in spite of these concerns, bevacizumab has become an increasingly popular treatment worldwide for AMD as it was available before, and is much cheaper than, pegaptanib and ranibizumab. Currently, PDT can only be offered to a small proportion of patients depending on the angiographic appearance of the membrane. NICE are withholding judgement on pegaptanib and ranibizumab and will release their findings in August 2007. In the current climate where many treatments are not available on the NHS, many ophthalmologists are turning to those treatments they can access despite lack of robust evidence to support their use.
Experimental treatments
Angiogenesis is the result of the interplay of factors that promote angiogenesis and those that inhibit it. Pigment epithelium derived growth factor (PEDF) is a natural anti‐angiogenic agent which has been used experimentally by gene transfer in animal models to both halt progression and cause regression of laser‐induced CNVs.35 A phase 1 clinical trial looking at this is currently underway.36 These possibilities may herald a future of exciting new therapies for AMD.
New non‐pharmacological therapies
Transpupillary thermotherapy
Transpupillary thermotherapy uses modified laser treatment, which minimally elevates the temperature of the target neovascular membrane, destroying it with little collateral damage. It may be effective is some cases, but controversy still exists over its benefits.37,38,39
Diode feeder vessel photocoagulation
Diode feeder vessel photocoagulation involves an indocyano‐green dye infusion with laser directed at the stalk of the abnormal vessels. This is promising in some cases,40 but requires a high level of expertise, expensive equipment and further trials to assess the long‐term results.41
Surgery
Two main types of surgery for AMD have been carried out.
Submacular surgery removes the neovascular membranes from beneath the retina. Large trials show that for patients with subfoveal membranes and extensive haemorrhage, there is less severe visual loss at 2 years compared to controls.42 However, as most patients derive no benefit, this surgery is generally not performed in AMD.
Macular translocation surgery is still experimental and is radical retinal surgery. The macula is detached from the underlying diseased retinal pigment epithelium and choroid and reattached in a healthier part of the eye. Theoretically, this surgery preserves foveal photoreceptor function. Despite promising results,43,44,45,46 there is a steep learning curve for the surgeon46 and a considerable risk of retinal detachment. Furthermore, to avoid diplopia, patients must undergo extensive muscle surgery and this is a long and arduous undertaking for any patient.
Quality of life and AMD
The development of AMD has a profound effect on patients as well as their families. Untreated AMD and subsequent visual loss is associated with falls, poor quality of life scores,47 depression48 and restriction in daily activities.49 Elderly patients may find loss of vision difficult to adapt to and lose confidence in their ability to navigate and cope with everyday household tasks. Frequently, impaired hearing and mobility compound these problems.
The visual loss resulting from AMD can lead to inability to fulfil socially allocated roles, which in turn may cause a sense of worthlessness and depression. However, some patients assume their visual loss as a challenge, and struggle to find alternative ways of continuing their activities. This ability to adapt and accept allows them to overcome the frustration and hardships that come with losing vision. Loss of vision with AMD not only affects the patient but also family, friends and society. Family members may adopt a role the person with advanced AMD can no longer play, for example a spouse or neighbour may undertake cooking, washing, shopping, etc. For patients living alone, the intervention of social services is imperative, as well as support networks via the RNIB.
The late stages of both wet and dry AMD are usually associated with severe visual loss, which has profound effects on overall quality of life. It is not surprising that so much effort has been channelled into research on this disease over the last two decades. Currently, available treatments have only limited success and aim mainly to prevent severe visual loss. AMD requires a multi‐disciplinary approach whereby all of the patient's needs are addressed, with psychological and social support key to ensure patients' well being.
Key points
AMD is a leading cause of blindness in the elderly and is increasing.
Known risk factors include smoking and uncontrolled blood pressure, while a diet rich in fruits and green leafy vegetables may be protective. Where the diet lacks these components, vitamin and mineral supplements (beta‐carotene, vitamins C, E, zinc, copper and lutein) should be taken.
Elderly patients with new distortion or a sudden loss of vision should be referred promptly to the ophthalmology clinic.
Laser (photodynamic therapy) is the current mainstay of treatment, but only a proportion of patients are suitable.
Increased understanding of AMD genetics and new anti‐angiogenic therapies herald an exciting new era in AMD management.
Conclusion
New hope for old eyes is sustained by ongoing research on promising new AMD treatments. This, combined with exciting new discoveries identifying genetic and environmental susceptibility factors paves the way for a brighter future for older generations. As we enter a new era for AMD treatment, we need to continue to increase public awareness of AMD. We should encourage healthy eating and cessation of smoking, and educate patients about the symptoms of AMD, thus prompting earlier presentation to the ophthalmology service. A multi‐disciplinary approach is key in the management of this common and life‐altering disease and geriatricians are in an excellent position to help instrument and usher in these changes.
Key references for further reading
2 Owen CG, Fletcher AE, Donoghue M, et al. How big is the burden of visual loss caused by age related macular degeneration in the United Kingdom? Br J Ophthalmol 2003;87(3):312–7.
4 Klein R, Klein BE, Moss SE. Relation of smoking to the incidence of age‐related maculopathy. The Beaver Dam Eye Study. Am J Epidemiol 1998;147(2):103–10.
7 Tomany SC, Wang JJ, Van Leeuwen R, et al. Risk factors for incident age‐related macular degeneration: pooled findings from 3 continents. Ophthalmology 2004;111(7):1280–7.
16 A randomized, placebo‐controlled, clinical trial of high‐dose supplementation with vitamins C and E, beta‐carotene, and zinc for age‐related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol 2001;119(10):1417–36.
48 Rovner BW, Zisselman PM, Shmuely‐Dulitzki Y. Depression and disability in older people with impaired vision: a follow‐up study. J Am Geriatr Soc 1996;44(2):181–4.
Multiple choice questions (true (T); false (F); answers after the references)
Eating plenty of fresh vegetables is especially important in preventing AMD.
Symptoms of recent distortion should be referred to the ophthalmology clinic on a non‐urgent basis.
Most lesions of AMD are amenable to laser treatment.
Laser treatment improves the vision.
Provision of a reading light makes a large difference in AMD.
Glossary of terms
AMD (age‐related macular degeneration): degenerative disease of the macula resulting in loss of central vision. Two forms exist:
Dry (atrophic) AMD: is generally slowly progressive and is the most common. It causes moderate to severe visual loss (fig 1).
Wet (exudative) AMD: accounts for 10% of AMD but results in 90% of blindness. Severe rapid visual loss occurs. A neovascular membrane originating from the choroid breaks through Bruch's membrane and causes retinal scarring and irreversible photoreceptor damage (fig 2). Based on the angiographic appearance (see below), the membrane may amenable to laser treatment.
Amsler grid: a simple grid for patients to self‐test for early signs of distortion in their central vision.
Bruch's membrane: five‐layered membrane between the choroid and retina, incorporating the basement membranes of the innermost layer of the choroid (the choriocapillaris) and the outermost layer of the retina (the retinal pigment epithelium).
Drusen: deposits on Bruch's membrane, which serve as an early marker for AMD.
Fluorescein angiography: fluorescein dye is injected intravenously. A special camera projects blue light into the eye and the dye in the retinal and choroidal blood vessels fluoresces (emits green light) and is readily visible. Rapid sequential photographs are taken and abnormal leakage corresponding to a choroidal neovascular membrane (CNV) is identified (fig 3).
The fovea: most central part of the macula with the highest density of cone photoreceptors.
The macula: specialised area in the retina serving central vision. Contains the greatest concentration of photoreceptors, which depend on the underlying retinal pigment epithelium and vascular choroid for nutrition and metabolism.
Acknowledgements
We would like to acknowledge the help given to us by Mr Stuart Gairns and Ms Marion Brannan from the photography department in the Princess Alexandra Eye Pavilion. All illustrations are from a hospital bank of photographs and the patients have given full informed consent for publication and teaching.
Abbreviations
AMD - age‐related macular degeneration
CNV - choroidal neovascular membrane
IOL - intraocular lens
PDT - photodynamic therapy
RNIB - Royal National Institute for the Blind
VEGF - vascular endothelial derived growth factor
Answers (F, false; T, true)
T – Fresh vegetables especially spinach are very important in preventing AMD (contain carotenoids).
F – Distortion of recent onset may be a symptom of new onset wet AMD and should be referred urgently.
F – Currently, only approximately 15% of lesions are amenable to laser (PDT) treatment.
F – Laser stabilises but does not improve the vision.
T – Provision of a reading light makes reading much easier for these patients.
Footnotes
Funding: None.
Competing interests: None.
References
- 1.Evans J, Wormald R. Is the incidence of registrable age‐related macular degeneration increasing? Br J Ophthalmol 199680(1)9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Owen C G, Fletcher A E, Donoghue M.et al How big is the burden of visual loss caused by age related macular degeneration in the United Kingdom? Br J Ophthalmol 200387(3)312–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weih L M, VanNewkirk M R, McCarty C A.et al Age‐specific causes of bilateral visual impairment. Arch Ophthalmol 2000118(2)264–269. [DOI] [PubMed] [Google Scholar]
- 4.Klein R, Klein B E, Moss S E. Relation of smoking to the incidence of age‐related maculopathy. The Beaver Dam Eye Study. Am J Epidemiol 1998147(2)103–110. [DOI] [PubMed] [Google Scholar]
- 5.Christen W G, Glynn R J, Manson J E.et al A prospective study of cigarette smoking and risk of age‐related macular degeneration in men. JAMA 1996276(14)1147–1151. [PubMed] [Google Scholar]
- 6.Seddon J M, Willett W C, Speizer F E.et al A prospective study of cigarette smoking and age‐related macular degeneration in women. JAMA 1996276(14)1141–1146. [PubMed] [Google Scholar]
- 7.Tomany S C, Wang J J, Van Leeuwen R.et al Risk factors for incident age‐related macular degeneration: pooled findings from 3 continents. Ophthalmology 2004111(7)1280–1287. [DOI] [PubMed] [Google Scholar]
- 8.Klein B E, Klein R, Lee K E.et al Risk of incident age‐related eye diseases in people with an affected sibling: the Beaver Dam Eye Study. Am J Epidemiol 2001154(3)207–211. [DOI] [PubMed] [Google Scholar]
- 9.Stone E M, Braun T A, Russell S R.et al Missense variations in the fibulin 5 gene and age‐related macular degeneration. N Engl J Med 2004351(4)346–353. [DOI] [PubMed] [Google Scholar]
- 10.Hageman G S, Anderson D H, Johnson L V.et al A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age‐related macular degeneration. Proc Natl Acad Sci U S A 2005102(20)7227–7232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein R, Rowland M L, Harris M I. Racial/ethnic differences in age‐related maculopathy. Third National Health and Nutrition Examination Survey. Ophthalmology 1995102(3)371–381. [DOI] [PubMed] [Google Scholar]
- 12.Klein R, Klein B E, Tomany S C.et al The association of cardiovascular disease with the long‐term incidence of age‐related maculopathy: the Beaver Dam eye study. Ophthalmology 2003110(4)636–643. [DOI] [PubMed] [Google Scholar]
- 13.Macular Photocoagulation Study Group Risk factors for choroidal neovascularization in the second eye of patients with juxtafoveal or subfoveal choroidal neovascularization secondary to age‐related macular degeneration. Arch Ophthalmol 1997115(6)741–747. [DOI] [PubMed] [Google Scholar]
- 14.Vinding T, Appleyard M, Nyboe J.et al Risk factor analysis for atrophic and exudative age‐related macular degeneration. An epidemiological study of 1000 aged individuals. Acta Ophthalmol (Copenh) 199270(1)66–72. [DOI] [PubMed] [Google Scholar]
- 15.Seddon J M, Ajani U A, Sperduto R D.et al Dietary carotenoids, vitamins A, C, and E, and advanced age‐related macular degeneration. Eye Disease Case‐Control Study Group. JAMA 1994272(18)1413–1420. [PubMed] [Google Scholar]
- 16.Age‐Related Eye Disease Study Research Group A randomized, placebo‐controlled, clinical trial of high‐dose supplementation with vitamins C and E, beta‐carotene, and zinc for age‐related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol 2001119(10)1417–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seddon J M, Cote J, Rosner B. Progression of age‐related macular degeneration: association with dietary fat, transunsaturated fat, nuts, and fish intake. Arch Ophthalmol 2003121(12)1728–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guymer R H, Chiu A W, Lim L.et al HMG CoA reductase inhibitors (statins): do they have a role in age‐related macular degeneration? Surv Ophthalmol 200550194–206. [DOI] [PubMed] [Google Scholar]
- 19.Tomany S C, Cruickshanks K J, Klein R.et al Sunlight and the 10‐year incidence of age‐related maculopathy: the Beaver Dam Eye Study. Arch Ophthalmol 2004122(5)750–757. [DOI] [PubMed] [Google Scholar]
- 20.Darzins P, Mitchell P, Heller R F. Sun exposure and age‐related macular degeneration. An Australian case‐control study. Ophthalmology 1997104(5)770–776. [DOI] [PubMed] [Google Scholar]
- 21.Klein R, Klein B E, Wong T Y.et al The association of cataract and cataract surgery, with the long‐term incidence of age‐related maculopathy. Arch Ophthalmol 20021201551–1558. [DOI] [PubMed] [Google Scholar]
- 22.Wang J J, Klein R, Smith W.et al Cataract surgery and the 5 year incidence of late‐stage age‐related maculopathy: pooled findings from the Beaver Dam and Blue Mountains eye studies. Ophthalmology 20031101960–1967. [DOI] [PubMed] [Google Scholar]
- 23.Richer S, Stiles W, Statkute L. Double‐masked, placebo‐controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age‐related macular degeneration: the Veterans LAST study (Lutein Antioxidant Supplementation Trial). Optometry 200475(4)216–230. [DOI] [PubMed] [Google Scholar]
- 24. Photodynamic therapy of subfoveal choroidal neovascularization in age‐related macular degeneration with verteporfin: one‐year results of 2 randomized clinical trials ‐ TAP report. Treatment of age‐related macular degeneration with photodynamic therapy (TAP) Study Group. Arch Ophthalmol 1999117(10)1329–1345. [PubMed] [Google Scholar]
- 25.Macular Photocoagulation Study Group Argon laser photocoagulation for neovascular maculopathy. Three‐year results from randomized clinical trials.Arch Ophthalmol 1986104(5)694–701. [PubMed] [Google Scholar]
- 26.Matsuoka M, Ogata N, Otsuji T.et al Expression of pigment epithelium derived factor and vascular endothelial growth factor in choroidal neovascular membranes and polypoidal choroidal vasculopathy. Br J Ophthalmol 200488(1)809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D'Amico D J, Goldberg M F, Hudson H.et al Anecortave Acetate Study Group. Anecortave acetate as monotherapy for treatment of subfoveal neovascularisation in age‐related macular degeneration: twelve‐month clinical outcomes, Ophthalmology 20031102372–2383. [DOI] [PubMed] [Google Scholar]
- 28.Slakter J S, Bochow T W, D'Amico D J.et al Anecortave Acetate Clinical Study Group. Anecortave acetate (15 milligrams) versus photodynamic therapy for treatment of subfoveal neovascularisation in age‐related macular degeneration. Ophthalmology 20061133–13. [DOI] [PubMed] [Google Scholar]
- 29.Gragoudas E S, Adamis A P, Cunningham E T., Jret al VEGF Inhibition Study in Ocular Neovascularisation Clinical Trial Group. Pegaptanib for neovascular age‐related macular degeneration. N Engl J Med 2004351(27)2805–2816. [DOI] [PubMed] [Google Scholar]
- 30.Rosenfeld P J, Brown D M, Heier J S.et al Ranibizumab for neovascular age‐related macular degeneration. N Engl J Med . 2006;3551419–1431. [DOI] [PubMed]
- 31.Brown D M, Kaiser P K, Michels M.et al Ranibizumab versus verteporfin for neovascular age‐related macular degeneration. N Engl J Med . 2006;3551432–1444. [DOI] [PubMed]
- 32.Michels S. Systemic bevacizumab (Avastin) therapy for neovascular age‐related macular degeneration: twelve‐week results of an uncontrolled open‐label clinical study. Ophthalmology 20051121035–1047. [DOI] [PubMed] [Google Scholar]
- 33.Rosenfeld P J. Intravitreal Avastin: the low cost alternative to Lucentis? Am J Ophthalmol 2006142141–143. [DOI] [PubMed] [Google Scholar]
- 34.Bashshur Z F, Bazarbachi A, Schakal A.et al Intravitreal bevacizumab for the management of choroidal neovascularization in age‐related macular degeneration. Am J Ophthalmol 20061421–9. [DOI] [PubMed] [Google Scholar]
- 35.Mori K, Gelbach P, Ando A.et al Regression of ocular neovascularisation in response to increased expression of pigment epithelium‐derived factor. Invest Ophthalmol Vis Sci 2002432428–2434. [PubMed] [Google Scholar]
- 36.Rasmussen H, Chu K W, Campochiaro P.et al Clinical protocol. An open‐label, phase I, single administration, dose escalation of ADGVPEDF.11D (ADPEDF) in neovascular age‐related macular degeneration (AMD). Hum Gene Ther 2001122029–2032. [PubMed] [Google Scholar]
- 37.Myint K, Armbrecht A M, Mon S.et al Transpupillary thermotherapy for the treatment of occult CNV in age‐related macular degeneration: a prospective randomized controlled pilot study. Acta Ophthalmol Scand 200684(3)328–332. [DOI] [PubMed] [Google Scholar]
- 38.Stolba U, Krebs I, Lamar P D.et al Long term results after transpupillary thermotherapy in eyes with occult choroidal neovascularisation associated with age related macular degeneration. Br J Ophthalmol 200690(2)158–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maberley D A, Chew H, Ma P.et al Comparison of photodynamic therapy and transpupillary thermotherapy for subfoveal choroidal neovascularization due to age‐related macular degeneration. Can J Ophthalmol 200540(3)378–383. [DOI] [PubMed] [Google Scholar]
- 40.Shiraga F, Ojima Y, Matsuo T.et al Feeder vessel photocoagulation of subfoveal choroidal neovascularization secondary to age‐related macular degeneration. Ophthalmology 1998105(4)662–669. [DOI] [PubMed] [Google Scholar]
- 41.Flower R W. Optimizing treatment of choroidal neovascularization feeder vessels with age‐related macular degeneration. Am J Ophthalmol 2002134(2)228–239. [DOI] [PubMed] [Google Scholar]
- 42.Hawkins B S, Bressler N M, Miskala P H.et al Surgery for subfoveal choroidal neovascularisation in age‐related macular degeneration: ophthalmic findings: SST report no. 11. Ophthalmology 2004111(11)1967–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abdel‐Meguid A, Lappas A, Hartmann K.et al One year follow up of macular translocation with 360 degree retinotomy in patients with age related macular degeneration. Br J Ophthalmol 200387(5)615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lai J C, Lapolice D J, Stinnett S S.et al Visual outcomes following macular translocation with 360‐degree peripheral retinectomy. Arch Ophthalmol 2002120(10)1317–1324. [DOI] [PubMed] [Google Scholar]
- 45.Mruthyunjaya P, Stinnett S S, Toth C A. Change in visual function after macular translocation with 360 degrees retinectomy for neovascular age‐related macular degeneration. Ophthalmology 2004111(9)1715–1724. [DOI] [PubMed] [Google Scholar]
- 46.Toth C A, Freedman S A. Macular translocation with 360‐degree peripheral retinectomy: impact of technique and surgical experience on visual outcomes. Retina 200121(4)293–303. [DOI] [PubMed] [Google Scholar]
- 47.Armbrecht A M, Aspinall P A, Dhillon B. A prospective study of visual function and quality of life following PDT in patients with wet age related macular degeneration. Br J Ophthalmol 200488(10)1270–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rovner B W, Zisselman P M, Shmuely‐Dulitzki Y. Depression and disability in older people with impaired vision: a follow‐up study. J Am Geriatr Soc 199644(2)181–184. [DOI] [PubMed] [Google Scholar]
- 49.Stuck A E, Walthert J M, Nikolaus J.et al Risk factors for functional status decline in community‐living elderly people: a systematic literature review. Soc Sci Med 199948(4)445–469. [DOI] [PubMed] [Google Scholar]