Abstract
Acute heart failure occurs in children following the operative correction of a congenital anomaly, as an acute change in a child with a congenital anomaly, or in a structurally normal heart with acute myocarditis. Acute heart failure in children justifies aggressive treatment because of the high potential for complete recovery. The options for providing mechanical support to the failing heart in a child include extracorporeal membrane oxygenation, left ventricular assist devices and the use of the intra‐aortic balloon pump (IABP). The principles of intra‐aortic balloon pump usage are described, and the literature regarding the indications and outcome of its use in children is reviewed.
Keywords: circulatory assistance, temporary; Congenital heart disease (CHD); postoperative care
The management of paediatric cardiac diseases has seen many advances in recent decades. Acute heart failure can occur in children either in the period following the operative correction of the congenital heart disease, as an acute change in a patient with congenital heart disease having myocardial disease, or in a structurally normal heart with acute myocarditis.1 When present, acute heart failure in children justifies aggressive treatment because of the high potential for complete recovery. The options for providing mechanical support include extracorporeal membrane oxygenation (ECMO), left ventricular assist devices (VAD) and the use of the intra‐aortic balloon pump (IABP). This article describes the essential principles and methods of IABP usage in children.
Intra‐aortic balloon counterpulsation
The IABP is a commonly used method of temporary circulatory support in adults. IABP usage in a paediatric population was first described in 1989.2 However, despite the availability of paediatric size balloons, the usage of IABP for temporary circulatory support in children has not been widespread.
The principle
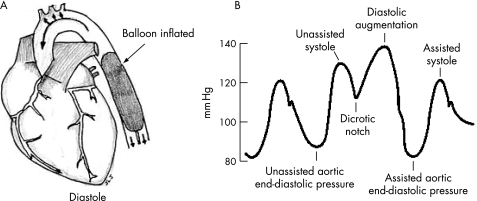
The IABP is basically an expandable balloon that is inserted into the descending aorta, just distal to the origin of the left subclavian artery (fig 1A). The balloon is periodically filled and emptied of helium gas, which is supplied to the balloon from a cylinder attached to the control console of the pump.
Figure 1 (A) Ideal location of the balloon. (B) Effect of augmentation on the aortic arterial trace.
It is essential to understand balloon inflation and deflation. Balloon deflation is timed to the opening of the aortic valve, in systole. Balloon inflation is timed to the closing of the aortic valve, in diastole. The inflated balloon can be considered to be a space‐occupying lesion in the descending thoracic aorta. If the balloon were inflated during systole and intra‐aortic space occupied, ventricular contraction and outflow would be impeded by the space occupied by the balloon, and hence afterload would increase. In reality, however, the IABP deflates in systole, at the onset of ventricular contraction and with the opening of the aortic valve. The space occupied by the balloon in the aorta is suddenly released at the onset of ventricular emptying. This causes a vacuum effect, or negative pressure in the aorta, thereby reducing ventricular afterload and ventricular wall stress, and improving cardiac performance.
General indicators of the need for mechanical support for a child with low cardiac output
Hypotension, persistent metabolic acidosis, low urine output, clinical signs of poor peripheral perfusion
despite maximal pharmacological support
in the setting of a reversible disease process
optimum surgical correction performed.
The converse occurs during diastolic inflation of the IABP. During the normal cardiac cycle, following the systolic aortic pressure wave, there is a diastolic elastic recoil that closes the aortic valve as well as provides the pressure head to perfuse the coronary arteries. Inflation of the balloon in diastole creates a space‐occupying lesion in the aorta in diastole displacing blood both proximally and distally. This increases the aortic diastolic pressure and hence coronary perfusion. Figure 1B illustrates these events.
In paediatric usage, it is the systolic unloading of the left ventricle during balloon deflation that is important. Owing to the resultant afterload reduction, the left ventricle can contract more efficiently and cardiac output can increase by up to 20%. However, it merits emphasis that the IABP only augments cardiac output; some amount of intrinsic function of the left ventricle is required and the IABP augments this. Hence, it is a modality that cannot be used in terminal cardiac failure. It is also to be noted that the right ventricle is not supported by IABP.
Basic principles governing IABP function
A gas‐filled balloon with volume varying with the weight of the child is placed in the descending aorta.
The balloon is sequentially inflated and deflated at different points in the cardiac cycle.
Deflation at the onset of systole greatly reduces the afterload and hence ventricular performance improves.
Inflation in diastole raises diastolic aortic pressure and hence coronary perfusion pressure increases.
In paediatric practice, it is the systolic unloading of the ventricle that is the important mechanism.
Clinical methods
Proper placement and timing of inflation and deflation are the key factors in the efficient utilisation of IABP.
In children, an open surgical insertion of the balloon is employed.3 Usually, a femoral artery is used, although transthoracic insertion (after cardiac surgery) may be used and may be indicated in neonates. The balloon is usually inserted through a small polytetrafluoroethylene patch placed on the femoral arteriotomy. Proper placement of the balloon, just distal to the origin of the left subclavian artery, is essential for proper functioning of the IABP and for avoiding potentially catastrophic complications. The position of the balloon can be assessed by simple measurement, fluoroscopy, post‐insertion chest radiograph or preferably by echocardiography. Surgically placed balloons ranging from 2.5 to 7 ml are available for infants and children (up to 18 kg). The criterion used in selecting an appropriate‐size catheter has been detailed elsewhere.4
The timing of balloon inflation and deflation is extremely important. This may be performed by either using electrocardiographic tracing or echocardiography. It must be noted that smaller paediatric balloon catheters differ from adult catheters in that they do not have a central pressure‐monitoring lumen. Hence, timing of the balloon on the basis of aortic pressure waveform is not an option in smaller children. Using electrocardiography, inflation should occur along the ascending T wave (ventricular repolarisation = diastole), and deflation is timed before the R wave (which signifies the onset of ventricular contraction).
Theoretical controversies with the use of IABP in children
Elasticity of the paediatric aorta would alter the forces of diastolic augmentation and render IABP ineffective.
The high heart rates of children and the high incidence of arrhythmia would make timing of balloon inflation and deflation difficult.
The incidence of complications when using the IABP in children is prohibitive.
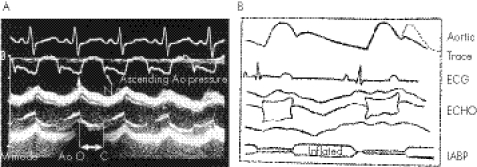
However, the timing of IABP using electrocardiography in children has been shown to have certain limitations.5 Recent reports have emphasised M‐mode echocardiography as the tool to ensure proper timing of balloon inflation and deflation.6 A parasternal echocardiographic image provides a good view of both the aortic valve leaflets and the balloon. Using aortic valve opening and closing as reference points, balloon inflation and deflation can be timed accurately (fig 2).
Figure 2 (A) Simultaneous recording of electrocardiogram and ascending aortic pressure. Millar and M‐mode echocardiogram showing that the upstroke of the arterial pressure curve (U) coincides with the aortic valve (Ao) opening (O) from the M‐mode recording and that the dicrotic notch (D) coincides with aortic valve closure (C). From Minich et al7; reproduced with permission of the publishers. (B) For clarity, schematic diagram of the same events.
The patient generally starts receiving intravenous heparin to prevent thromboembolic complications, to maintain an activated partial thromboplastin time of around 1.5–2 times the control.
The magnitude of augmentation may be adjusted in two ways. The pumping ratio can be changed; this is the ratio of augmented beats to unaugmented beats (for example, 1:1, 1:2, etc). Alternatively, the balloon inflation volume can be adjusted, which would hence alter the level of afterload reduction. In general, on initiation of IABP support, ratios of 1:1 or 1:2 are selected, along with the maximum balloon volume.
Weaning from the IABP begins when haemodynamic stability is maintained (as assessed by clinical, biochemical and echocardiographic parameters) following the reduction of pharmacological support to minimal levels. The pumping ratio is then gradually changed from 1:1 to 1:3 or 1:4. Then, after a period of observation, the balloon may be removed.
In the past, the use of IABP has been associated with much morbidity and complications. However, currently, increased familiarity with insertion technique, better instrumentation and awareness of possible complications have decreased the incidence of major complications. Some complications8 are related to groin dissection (haematoma, lymph leak, wound infections, pseudoaneurysm), distal limb ischaemia (thrombosis, embolism) or are due to misplacement of the balloon (intrathoracic/intra‐abdomenal aortic rupture, ischaemic damage to bowel/kidney/spinal cord).
In the past, three factors were thought to preclude the use of IABP in children. Firstly, the paediatric aorta was thought to have greater elasticity than the adult aorta. So most of the energy generated during balloon filling was thought to be transferred to the expansile aorta and, as a result, diastolic augmentation would be dampened. However, this has recently been disproved.9 Secondly, there were also concerns regarding the efficacy of using the IABP in the paediatric population with its higher incidence of arrhythmias, making optimal timing very difficult. Thirdly, it has generally been assumed that IABP usage in children is associated with an increased frequency of complications. Perhaps that was the case before the advent of catheters designed specifically for paediatric use. However, in the two most recent reports of IABP usage in children, the incidence of complications was only 6.9% and 12.5%.10,11
Advantages of IABP for providing left ventricular support
Less invasive than ECMO and VAD
Easily portable equipment
Simple to use
Less aggressive anticoagulation needed
No extracorporeal circuit is required
May be less expensive than the other modalities.
Indications and results of IABP use in children
In the absence of widespread usage of IABP in paediatric cardiac centers worldwide, literature reports on paediatric IABP have been sporadic. In Pollock et al's2 study, which is the earliest experience of IABP use in children, there was a 43% survival among a group of 14 children. Webster and Veasy12 have reported on patients aged 5 days to 18 years; the smallest child weighed 4.2 kg. The survival in children <3 years of age was 75%. However, overall survival was only 25%. Park et al12 studied children with left ventricular dysfunction after cardiac surgery and reported 44% survival among those managed with IABP. Del Nido et al14 reported the successful utilisation of IABP support in a 2 kg infant. Among his series of patients, 37% were long‐term survivors. However, most of these reports dated from the years 1980–93.3,12,13,14,15 Much has evolved since then, especially advances in intensive care monitoring, treatment, antiobiotic availability and specifically intra‐aortic balloons and consoles specifically designed for paediatric use. Recent studies10,11,14,17 have reported much better results in the era 1988–2003. Table 1 summarises the results of these studies.
Table 1 Results of intra‐aortic balloon pump use among individual patient groups.
| Disease | Number | Hospital survival (%) | |
|---|---|---|---|
| Medical | Cardiomyopathy | 8 | 50 |
| Myocarditis | 2 | 100 | |
| HUS | 1 | 100 | |
| Blunt trauma | 2 | 100 | |
| Surgical | ALCAPA | 9 | 89 |
| TGA | 2 | 50 | |
| LVOTO | 1 | 100 | |
| AV/MV disease | 3 | 100 | |
| Single ventricle | 8 | 25 | |
| TOF | 5 | 20 | |
| VSD | 6 | 50 | |
| ASD | 5 | 100 | |
| Complex conduit repairs | 1 | 60 | |
| Total | 40 | 67.5% |
ALCAPA, anomalous origin of the coronary artery from the pulmonary artery; ASD, atrial septal defects; AV/MV, aortic valve/mitral valve; HUS, haemolytic uraemic syndrome; LVOTO, left ventricular outflow obstruction; TGA, transposition of great arteries; TOF, tetralogy of Fallot; VSD, ventricular septal defects.
While interpreting these results, it is important to bear in mind that IABP provides support only to the left ventricle. Hence, its use would be indicated in disease conditions in which left ventricular failure occurs. These include, for example, anomalous origin of the coronary artery from the pulmonary artery, transposition of great arteries, left ventricular outflow obstruction, aortic and mitral valve diseases, and IABP has been used with nearly 100% success in these patients. Where disease is biventricular in nature, with a component of pulmonary hypertension, or in which right ventricular pathology predominates—for example, tetralogy of Fallot or after complex conduit repairs—the success rates would be predictably lower, as table 1 shows. Although patient numbers are small, IABP has been used successfully to support children with acute myocarditis and cardiac trauma, which are self‐limiting causes of cardiac failure. And this experience compares well with the 80% survival of children with myocarditis managed by ECMO.18 The results of IABP use after a Fontan procedure has been disappointing. This is not surprising since the efficiency of the Fontan circulation is based on the postoperative level of pulmonary vascular resistance and not on systolic cardiac function. When its use has been successful after a Fontan procedure, it has been in those rare situations when the pulmonary vascular resistance has been normal and altered cardiac function proven to be the cause of the dysfunction.10,11
The rate of complication following IABP use has been acceptable. Pinkney et al10 reported an incidence of 6.9% (2 of 29 patients), including sepsis in one patient and transient ischaemia in the other. Neither complication required IABP removal. Kalavrouziotis et al11 reported a 12.5% (3 of 24 patients) incidence of IABP‐related complications—two involved transient limb ischaemia and one patient developed mesenteric ischaemia and subsequently died. Apart from this, they encountered septic and bleeding complications but did not relate these to IABP usage.
Conclusion
Most children with acute heart failure requiring mechanical support are currently being managed with ECMO or VAD (table 2).
Table 2 Comparision of the modalities of mechanical support in children.
| Parameter | IABP | ECMO | VAD |
|---|---|---|---|
| Ventricle supported | Left ventricle | Both ventricles | Mainly left ventricle |
| Complexity of equipment | Low | High | Moderate |
| Ease of use | Easy | Low | Moderate |
| Anticoagulation needed | Low | High | High |
| Complication rate | Moderate | High | Least |
| Duration of support | Days | Weeks | Weeks to months |
| Survival (%) | 56 | 40 | 40 |
ECMO, extracorporeal membrane oxygenation; IABP, intra‐aortic balloon pump; VAD, ventricular assist devices.
However, recent advances in paediatric IABP technology have made its use feasible for children of all ages with acceptable morbidity. In properly selected groups of children with predominantly left ventricular failure, it has been shown to be an effective and lifesaving adjunct to conventional medical treatment of refractory low cardiac output. These developments are especially relevant to emerging countries where the cost of ECMO/VAD is prohibitively high; the use of IABP may be a more cost‐effective modality in properly selected patients.
Abbreviations
ECMO - extracorporeal membrane oxygenation
IABP - intra‐aortic balloon pump
VAD - ventricular assist devices
References
- 1.Aulsender M, Artman M. Overview of the management of pediatric heart failure. Prog Pediatr Cardiol 200011231–241. [DOI] [PubMed] [Google Scholar]
- 2.Pollock J, Charlton M C, Williams W G.et al Intra aortic balloon pumping in children. Ann Thorac Surg 198029522–528. [DOI] [PubMed] [Google Scholar]
- 3.Veasy L G, Webster H F, McGough E C. Intra‐aortic balloon pumping. Adaptation for pediatric use. Crit Care Clin 19862237–249. [PubMed] [Google Scholar]
- 4.Booker P D. Intra‐aortic balloon pumping in young children. Paediatr Anaesth 19977501–507. [DOI] [PubMed] [Google Scholar]
- 5.Pantalos G M, Minich L L, Tani L Y.et al Estimation of timing errors for intra aortic balloon pump use in pediatric patients. ASAIO J 199945166–171. [DOI] [PubMed] [Google Scholar]
- 6.Minich L L, Tani L Y, McGough E C.et al A novel approach to pediatric intra aortic balloon pump using M‐mode echocardiography. Am J Cardiol 199780367–369. [DOI] [PubMed] [Google Scholar]
- 7.Munich L L, Tani L Y, Pantalos G M.et al Neonatal model of intra‐aortic balloon pumping: improved efficacy using echocardiographic timing. Ann Thorac Surg 1998661527–1532. [DOI] [PubMed] [Google Scholar]
- 8.Minich L, Tani l, Hawkins J.et al In vitro evaluation of the effect of aortic compliance on pediatric intra‐aortic balloon pumping. Pediatr Crit Care Med 20012139–144. [DOI] [PubMed] [Google Scholar]
- 9.Eltchaninoff H, Dimas A P, Whitlow P L. Complications associated with percutaneous placement and use of intra aortic balloon counterpulsation. Am J Cardiol 199371328. [DOI] [PubMed] [Google Scholar]
- 10.Pinkney K A, Minich L L, Tani L Y.et al Current results with intraaortic balloon pumping in infants and children. Ann Thorac Surg 200273887–891. [DOI] [PubMed] [Google Scholar]
- 11.Kalavrouziotis G, Karunaratne A, Raja S.et al Intra‐aortic balloon pumping in children undergoing cardiac surgery: an update of the Liverpool experience. J Thorac Cardiovasc Surg 20061311382. [DOI] [PubMed] [Google Scholar]
- 12.Webster H, Veasy L G. The intra aortic balloon pump in children. Heart Lung 198514548–555. [PubMed] [Google Scholar]
- 13.Park J K, Hsu K T, Gersony W M. Intra aortic balloon pump management of refractory congestive heart failure in children. Pediatr Cardiol 19931419–22. [DOI] [PubMed] [Google Scholar]
- 14.Del Nido P J, Swan P R, Benson L N. Successful use of intra aortic balloon pumping in a 2‐kilogram infant. Ann Thorac Surg 198846574–576. [DOI] [PubMed] [Google Scholar]
- 15.Nawa S, Sugawaqra E, Murakami T.et al Efficacy of intra‐aortic balloon pumping for failing Fontan circulation. Chest 198893599–603. [DOI] [PubMed] [Google Scholar]
- 16.Akomea‐Agyin C, Kejriwal N K, Franks R.et al Intra aortic balloon pumping in children. Ann Thorac Surg 1999671415–1420. [DOI] [PubMed] [Google Scholar]
- 17.Pozzi M, Santoro G, Makundan S. Intra‐aortic balloon pump after anomalous origin of left coronary artery. Ann Thorac Surg 199865555–557. [DOI] [PubMed] [Google Scholar]
- 18.Duncan B W, Bohn D J, Atz A M. Mechanical circulatory support for the treatment of children with acute fulminant myocarditis. J Thorac Cardiovasc Surg 2001122440–446. [DOI] [PubMed] [Google Scholar]