Abstract
Background
P‐POSSUM (Physiological and Operative Severity Score for the enumeration of Mortality and morbidity) predicts mortality and morbidity in general surgical patients providing an adjunct to surgical audit. O‐POSSUM was designed specifically to predict mortality and morbidity in patients undergoing oesophagogastric surgery.
Aim
To compare P‐POSSUM and O‐POSSUM in predicting surgical mortality in patients undergoing elective oesophagogastric cancer resections.
Methods
Elective oesophagogastric cancer resections in a district general hospital from 1990 to 2002 were scored by P‐POSSUM and O‐POSSUM methods. Observed mortality rates were compared to predicted mortality rates in six risk groups for each model using the Hosmer–Lemeshow goodness‐of‐fit test. The power to discriminate between patients who died and those who survived was assessed using the area under the receiver–operator characteristic (ROC) curve.
Results
313 patients underwent oesophagogastric resections. 32 died within 30 days (10.2%). P‐POSSUM predicted 36 deaths (χ2 = 15.19, df = 6, p = 0.019, Hosmer–Lemeshow goodness‐of‐fit test), giving a standardised mortality ratio (SMR) of 0.89. O‐POSSUM predicted 49 deaths (χ2 = 16.51, df = 6, p = 0.011), giving an SMR of 0.65. The area under the ROC curve was 0.68 (95% confidence interval 0.59 to 0.76) for P‐POSSUM and 0.61 (95% confidence interval 0.50 to 0.72) for O‐POSSUM.
Conclusion
Neither model accurately predicted the risk of postoperative death. P‐POSSUM provided a better fit to observed results than O‐POSSUM, which overpredicted total mortality. P‐POSSUM also had superior discriminatory power.
Oesophagogastric cancers continue to be a major cause of cancer mortality. Scotland currently has one of the highest incidences of oesophageal cancers in Europe and within the UK.1 Surgical resection continues to be the mainstay for treatment of oesophagogastric cancers. Postoperative mortality following oesophagogastric resections is significant and varies between 1.4–23%.2,3 In the UK, mortality for oesophagogastric cancer is higher than in the rest of Europe.4 In Scotland, patients with oesophagogastric cancers have a poor prognosis in comparison with other European countries.1 Postoperative mortality has continued to decline over the last decade mainly due to improved case selection, specialised provision of services and multidisciplinary involvement.
Preoperative risk assessment and informed consent play a vital role in the management of oesophagogastric cancers. It is essential for both the patient and surgeon to have an assessment preoperatively of the probability of success of a major surgical procedure. This should take into account the surgeon's performance, hospital performance, physiological status of the patient, and multidisciplinary involvement including interventional radiologists. This will enable a fully informed consent to be obtained from the patient. In addition this will identify patients who are at high risk from the operative procedure. In this group, preventive measures can be instituted and postoperative complications may be predicted to enable early recognition and institution of appropriate treatment, which may result in a better outcome.
Predicting postoperative mortality and risk assessment before surgery continues to be a challenge. Over the last decade the Physiological and Operative Severity Score for the enumeration of Mortality and morbidity (POSSUM),5 and its modifications such as P‐POSSUM6,7 have been used in general surgery and allied specialities to predict postoperative mortality with varying degree of success. POSSUM and P‐POSSUM both use a four grade, 12 factor Physiological Score and a six factor Operative Severity Score to predict operative mortality. These scoring systems, when used appropriately, can be useful in providing an estimation of postoperative mortality for an individual patient.7
O‐POSSUM was derived to provide a dedicated scoring system to predict postoperative mortality specifically for oesophageal and gastric surgery.8 This system was based on the methods used by POSSUM and P‐POSSUM, the primary end point being in‐hospital mortality. In O‐POSSUM, the risk factors were selected on the basis of their clinical relevance. Operative blood loss and number of procedures, which describe structure and process of care, were excluded from multivariate analysis.
The aim of our study was to compare the predictive accuracy of P‐POSSUM and O‐POSSUM in patients undergoing elective oseophagogastric resections for cancer.
PATIENTS AND METHODS
Patients who underwent elective oesophagogastric resections for cancer were studied from 1990 to 2002. All patients underwent staging investigations. These included upper gastrointestinal endoscopy and biopsy, computed tomographic scan and, in some, staging laparoscopy. All patients underwent preoperative evaluation which included a chest radiograph, electrocardiogram, respiratory function tests and echocardiogram if indicated. All surgical resections were performed with an intent to cure.
Crosshouse Hospital is a district general hospital in the county of Ayrshire, Scotland, with an estimated population of 220 000. Four surgeons were operating for the first half of the decade (1990 to 1995), two of whom had a special interest in the upper gastrointestinal system and performed all oesophagogastric surgery from 1996.
The data were recorded on a standard datasheet and then transferred to SPSS version 10.0 (SPSS Inc, Chicago, USA). The risk of death, R, was calculated using the P‐POSSUM equation using linear analysis as follows6:
P‐POSSUM, ln[R/(1‐R)] = −9.065+(0.1692* physiological score) + (0.1550* operative score)
where the physiological and operative scores were calculated as described by Copeland and colleagues.4 Three per cent of the data was missing, and the variables corresponding to missing data were assigned a score of 1 (representing a normal result). The O‐POSSUM8 score was calculated from the physiological score, operative severity score, pathology details and mode of surgery for individual patient as described on the website (www.riskprediction.org.uk). Mortality was determined at 30 days. The demographic distribution and pattern of patients scored are shown in table 1.
Table 1 Patient demographics.
| Oesophagus n = 110 | Stomach n = 203 | |
|---|---|---|
| Age (median, range) (years) | 69 (34–91) | 69 (32–92) |
| Sex | ||
| Male | 72 (65%) | 116 (57%) |
| Female | 38 (35%) | 87 (43%) |
| Surgery | ||
| Ivor/Lewis | 55 (50%) | |
| Transhiatal | 27 (25%) | |
| Others | 28 (25%) | |
| Total gastrectomy | 51 (25%) | |
| Subtotal gastrectomy | 37 (18%) | |
| Partial gastrectomy | 78 (38%) | |
| Others (incl palliative GJ) | 37 (18%) | |
| Histology | ||
| Adenocarcinoma | 80 (73%) | 193 (95%) |
| Squamous | 29 (26%) | 1 (1%) |
| Others | 1 (1%) | 9 (4%) |
GJ, gastrojejunostomy.
The variables used for calculations of P‐POSSUM and O‐POSSUM are shown in table 2.8
Table 2 Variables used in P‐POSSUM and O‐POSSUM equations.
| Physiological score | Operative severity score |
|---|---|
| Age (years)* | Operative severity |
| Cardiac signs/chest radiograph | Multiple procedures† |
| Respiratory history/chest radiograph | Total blood loss (ml)† |
| Systolic blood pressure (mm Hg) | Peritoneal soiling† |
| Pulse (beats/min) | Presence of malignancy |
| Glasgow Coma Scale | Mode of surgery |
| Haemoglobin | |
| White cell count (×1012/l) | |
| Urea (mmol/l) | |
| Sodium (mmol/l) | |
| Potassium (mmol/l) | |
| Electrocardiogram |
*Age was regressed independently from the Physiological and Operative severity score for the enumeration of Mortality and morbidity (POSSUM).
†Risk factors not used in scoring specific for upper gastrointestinal surgery (O‐POSSUM) (used from reference 8).
The patients were stratified into six risk groups based on survival probability to allow comparison between predicted and observed deaths. This was analysed by the Hosmer–Lemeshow (H–L) goodness of fit test to assess the calibration of the model.9 In this analysis a value of p<0.05 was considered to show a significant lack of fit by the model. The discrimination of the model was measured by the area under the receiver–operator characteristic (ROC) curve (AUC). A value of 0.5 represents chance performance and 1.0 represents perfect prediction. Values between 0.7–0.8 suggest reasonable discrimination and more than 0.8 suggest good discrimination.
RESULTS
A total of 313 patients were scored using the P‐POSSUM and O‐POSSUM equations. Thirty‐two patients died within 30 days of surgery. The overall observed mortality rate was 10.2%.
The mortality rates predicted by the P‐POSSUM and O‐POSSUM equations compared to the observed mortality rate are shown in tables 3 and 4.
Table 3 Observed and predicted deaths in risk categories as defined by the P‐POSSUM score.
| Predicted risk | Number of patients | Predicted deaths | Observed deaths |
|---|---|---|---|
| 0–10 | 198 | 9 | 13 |
| >10–20 | 57 | 8 | 9 |
| >20–30 | 29 | 7 | 5 |
| >30–40 | 16 | 5 | 3 |
| >40–50 | 7 | 3 | 0 |
| 50–100 | 6 | 4 | 2 |
| 0–100 | 313 | 36 | 32 |
Table 4 Observed and predicted deaths in risk categories as defined by the O‐POSSUM score.
| Predicted risk | Number of patients | Predicted deaths | Observed deaths |
|---|---|---|---|
| 0–10 | 125 | 7 | 8 |
| >10–20 | 103 | 14 | 8 |
| >20–30 | 46 | 11 | 10 |
| >30–40 | 19 | 7 | 1 |
| >40–50 | 10 | 4 | 2 |
| 50–100 | 10 | 6 | 3 |
| 0–100 | 313 | 49 | 32 |
P‐POSSUM predicted 36 deaths, yielding a standardised mortality ratio (SMR) of 0.89. The H–L goodness of fit test, applied to the P‐POSSUM equation, indicated a significant lack of fit with the observed deaths (χ2 = 15.19, 6 df, p = 0.019).
O‐POSSUM predicted 49 deaths, yielding an SMR of 0.65. The H–L test applied to these data indicated a lack of fit (χ2 = 16.51, 6 df, p = 0.011).
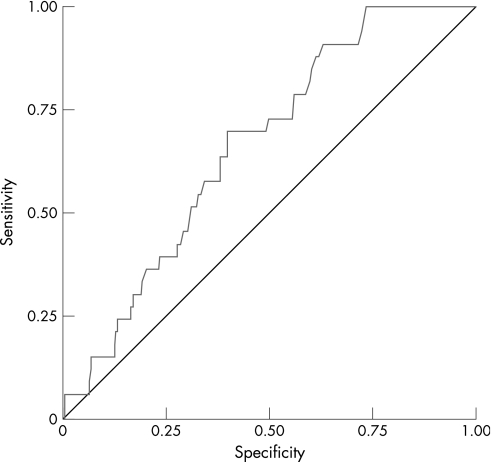
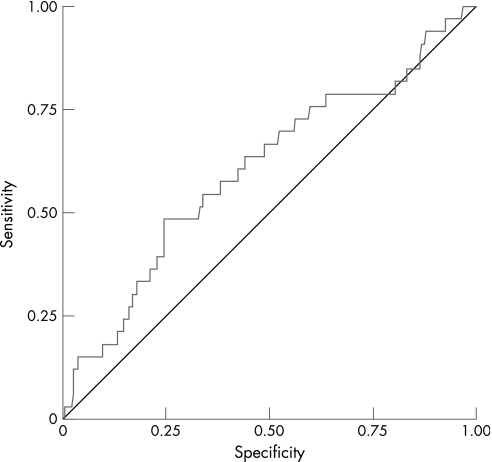
The ROC curve analysis applied to the P‐POSSUM scores showed poor discriminatory capability for mortality, as shown in fig 1, although significantly better than chance (AUC 0.68, 95% confidence interval 0.59 to 0.76). ROC curve analysis of O‐POSSUM scores (fig 2) revealed an even poorer discriminatory power for mortality (AUC 0.61, 95% confidence interval 0.50 to 0.72).
Figure 1 Receiver–operator characteristic (ROC) curve for mortality (P‐POSSUM).
Figure 2 Receiver–operator characteristic (ROC) curve for mortality (O‐POSSUM).
DISCUSSION
Quality assurance and risk assessment is important in an era of public choice. Surgical mortality is often seen as a surrogate of performance, to enable comparison between individual surgeons and hospitals. This method of comparison can be misleading due to differences in case mix.10
Since the introduction of POSSUM,5 it has been possible to predict successfully postoperative mortality in patients undergoing general surgical procedures. This method and its subsequent modifications such as P‐POSSUM have been applied successfully in various general surgical procedures11,12,13,14,15,16,17,18,19 in the UK. More recently POSSUM has been successfully applied in populations outside the UK.20,21,22 POSSUM, when applied specifically to oesophageal resections, did not predict mortality and morbidity accurately23 and overpredicted mortality in patients with gastric cancer undergoing D2‐gastrectomy.24
O‐POSSUM was designed to provide a dedicated model for prediction of mortality after oesophagogastric resections. A recent article by Gocmen et al showed that O‐POSSUM predicted mortality more accurately than P‐POSSUM in patients undergoing resections for gastric cancer.25
In the present study, P‐POSSUM and O‐POSSUM models failed to predict postoperative mortality accurately following elective oesophagogastric cancer resections, but P‐POSSUM provided better estimation of overall mortality when compared to O‐POSSUM.
The failure of the O‐POSSUM model in this cohort could be due to a number of factors, such as the inclusion of only elective resections for cancer, the treatment of missing data and the consideration of only 30 day mortality. The overall mortality rate during the study period is comparable to published data26 with a decrease in mortality in the latter half of the decade. Whether this was due to improved perioperative care, provision of specialist services or improved patient selection is unclear.
As the original authors suggested, the O‐POSSUM equation should be tested and applied to various different populations for the assessment of its predictive power.8 When applied to this population, the O‐POSSUM equation overpredicted the overall mortality rate.
P‐POSSUM appeared to be a better fitting model, with superior discriminatory power. Predictive accuracy of both scoring systems may be limited in patients undergoing elective resections for cancer. This should be borne in mind when using these methods for comparative audit and patient information.
Abbreviations
AUC - area under the receiver–operator characteristic curve
H–L - Hosmer–Lemeshow
POSSUM - Physiological and Operative Severity Score for the enumeration of Mortality and morbidity
ROC - receiver–operator characteristic
SMR - standardised mortality ratio
Footnotes
Funding: none. Financial support: none
Competing interests: none
References
- 1.Gilbert F J, Park K G M, Thompson A M.Scottish Audit for Gastric and Oesophageal Cancer‐Report 1997–2000. www.show.scot.nhs.uk/crag/ (accessed 2 April 2007)
- 2.Jamieson G G, Mathew G, Ludemann R.et al Postoperative mortality following oesophagectomy and problems in reporting its rate. Br J Surg 200491943–947. [DOI] [PubMed] [Google Scholar]
- 3.Koshy M, Esiashvilli N, Landry J C.et al Multiple management modalities in esophageal cancer: epidemiology, presentation and progression, work‐up, and surgical approaches. Oncologist 20049137–146. [DOI] [PubMed] [Google Scholar]
- 4.Bachmann M O, Alderson D, Edwards D.et al Cohort study in South and West England of the influence of specialization on the management and outcome of patients with oesophageal and gastric cancers. Br J Surg 200289914–922. [DOI] [PubMed] [Google Scholar]
- 5.Copeland G P, Jones D, Walters M. POSSUM: a scoring system for surgical audit. Br J Surg 199178355–360. [DOI] [PubMed] [Google Scholar]
- 6.Prytherch D R, Whiteley M S, Higgins B.et al POSSUM and Portsmouth POSSUM for predicting mortality. Physiological and Operative Severity Score for the enUmeration of Mortality and morbidity. Br J Surg 1998851217–1220. [DOI] [PubMed] [Google Scholar]
- 7.Neary W D, Heather B P, Earnshaw J J. The Physiological and Operative Severity Score for the enUmeration of Mortality and morbidity (POSSUM). Br J Surg 200390157–165. [DOI] [PubMed] [Google Scholar]
- 8.Tekkis P P, McCulloch P, Poloniecki J D.et al Risk‐adjusted prediction of operative mortality in oesophagogastric surgery with O‐POSSUM. Br J Surg 200491288–295. [DOI] [PubMed] [Google Scholar]
- 9.Lemeshow S, Le Gall J R. Modeling the severity of illness of ICU patients. A systems update. JAMA 19942721049–1055. [PubMed] [Google Scholar]
- 10.Markus P M, Martell J, Leister I.et al Predicting postoperative morbidity by clinical assessment. Br J Surg 200592101–106. [DOI] [PubMed] [Google Scholar]
- 11.Brooks M J, Sutton R, Sarin S. Comparison of surgical risk score, POSSUM and p‐POSSUM in higher‐risk surgical patients. Br J Surg 2005921288–1292. [DOI] [PubMed] [Google Scholar]
- 12.Brunelli A, Fianchini A, Xiume F.et al Evaluation of the POSSUM scoring system in lung surgery. Physiological and Operative Severity Score for the enUmeration of Mortality and Morbidity. Thorac Cardiovasc Surg 199846141–146. [DOI] [PubMed] [Google Scholar]
- 13.Brunelli A, Fianchini A, Gesuita R.et al POSSUM scoring system as an instrument of audit in lung resection surgery. Physiological and operative severity score for the enumeration of mortality and morbidity. Ann Thorac Surg 199967329–331. [DOI] [PubMed] [Google Scholar]
- 14.Copeland G P, Jones D, Wilcox A.et al Comparative vascular audit using the POSSUM scoring system. Ann R Coll Surg Engl 199375175–177. [PMC free article] [PubMed] [Google Scholar]
- 15.Irvine C D, Shaw E, Poskitt K R.et al A comparison of the mortality rate after elective repair of aortic aneurysms detected either by screening or incidentally. Eur J Vasc Endovasc Surg 200020374–378. [DOI] [PubMed] [Google Scholar]
- 16.Senagore A J, Delaney C P, Duepree H J.et al Evaluation of POSSUM and P‐POSSUM scoring systems in assessing outcome after laparoscopic colectomy. Br J Surg 2003901280–1284. [DOI] [PubMed] [Google Scholar]
- 17.Poon J T, Chan B, Law W L. Evaluation of P‐POSSUM in surgery for obstructing colorectal cancer and correlation of the predicted mortality with different surgical options. Dis Colon Rectum 200548493–498. [DOI] [PubMed] [Google Scholar]
- 18.Tekkis P P, Prytherch D R, Kocher H M.et al Development of a dedicated risk‐adjustment scoring system for colorectal surgery (colorectal POSSUM). Br J Surg 2004911174–1182. [DOI] [PubMed] [Google Scholar]
- 19.Sagar P M, Hartley M N, Mancey‐Jones B.et al Comparative audit of colorectal resection with the POSSUM scoring system. Br J Surg 1994811492–1494. [DOI] [PubMed] [Google Scholar]
- 20.Lam C M, Fan S T, Yuen A W.et al Validation of POSSUM scoring systems for audit of major hepatectomy. Br J Surg 200491450–454. [DOI] [PubMed] [Google Scholar]
- 21.Bennett‐Guerrero E, Hyam J A, Shaefi S.et al Comparison of P‐POSSUM risk‐adjusted mortality rates after surgery between patients in the USA and the UK. Br J Surg 2003901593–1598. [DOI] [PubMed] [Google Scholar]
- 22.Gotohda N, Iwagaki H, Itano S.et al Can POSSUM, a scoring system for perioperative surgical risk, predict postoperative clinical course? Acta Med Okayama 199852325–329. [DOI] [PubMed] [Google Scholar]
- 23.Zafirellis K D, Fountoulakis A, Dolan K.et al Evaluation of POSSUM in patients with oesophageal cancer undergoing resection. Br J Surg 2002891150–1155. [DOI] [PubMed] [Google Scholar]
- 24.Bollschweiler E, Lubke T, Monig S P.et al Evaluation of POSSUM scoring system in patients with gastric cancer undergoing D2‐gastrectomy. BMC Surg 200558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gocmen E, Koc M, Tez M.et al Evaluation of P‐POSSUM and O‐POSSUM scores in patients with gastric cancer undergoing resection. Hepatogastroenterology 2004511864–1866. [PubMed] [Google Scholar]
- 26.Gillison E W, Powell J, McConkey C C.et al Surgical workload and outcome after resection for carcinoma of the oesophagus and cardia. Br J Surg 200289344–348. [DOI] [PubMed] [Google Scholar]