Abstract
Pneumothorax is a relatively common clinical problem which can occur in individuals of any age. Irrespective of aetiology (primary, or secondary to antecedent lung disorders or injury), immediate management depends on the extent of cardiorespiratory impairment, degree of symptoms and size of pneumothorax. Guidelines have been produced which outline appropriate strategies in the care of patients with a pneumothorax, while the emergence of video‐assisted thoracoscopic surgery has created a more accessible and successful tool by which to prevent recurrence in selected individuals. This evidence based review highlights current practices involved in the management of patients with a pneumothorax.
Pneumothorax is the presence of air between the parietal and visceral pleura. It is a relatively common respiratory disorder and can occur in a variety of clinical settings and in individuals of any age. The presentation of a pneumothorax varies between minimal pleuritic chest discomfort and breathlessness to a life‐threatening medical emergency with cardiorespiratory collapse requiring immediate intervention and subsequent prevention.1,2,3 This evidence based review article outlines the causes, diagnosis and current management of a pneumothorax. All authors performed a comprehensive literature search using Medline, Clinical Evidence, Cochrane Library and Embase up to November 2006. The following key words were used in the search: pneumothorax, causes, diagnosis, management, pleurodesis, diving, flying, tension, surgery and video assisted thoracoscopic surgery (VATS); we then selected and extracted articles that we felt to be of relevance to practising clinicians.
CLASSIFICATION AND PATHOGENESIS
Pneumothorax can be categorised as primary, secondary, iatrogenic or traumatic according to aetiology. Occasionally, individuals may develop a concomitant haemothorax due to bleeding caused by shearing of adjacent subpleural vessels when the lung collapses.
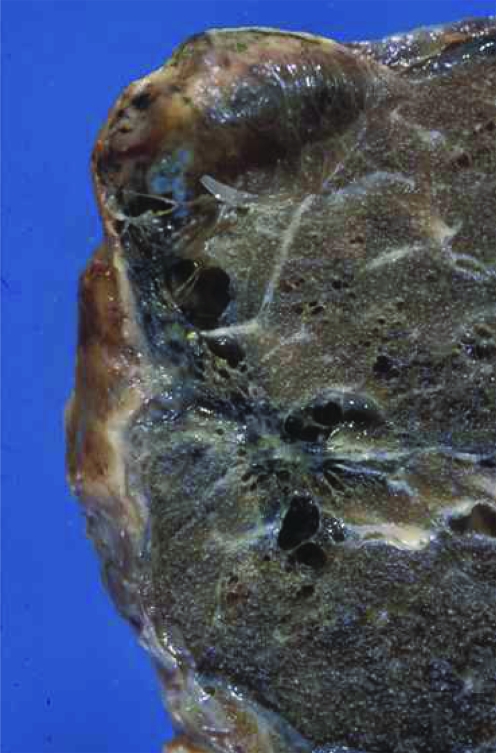
Primary spontaneous pneumothoraces occur most commonly in young, tall, thin males with no predisposing lung disease or history of thoracic trauma, although rupture of an underlying small subpleural bleb or bulla is thought to be responsible in many cases (fig 1).4,5 Moreover, current cigarette smoking greatly increases the risk of developing a pneumothorax by as much as nine times, with evidence of a dose–response relationship.6 The exact incidence of primary spontaneous pneumothorax is uncertain, although the yearly frequency in healthy individuals has been reported to be approximately 18–28/100 000 for males and 1.2–6/100 000 for females.7,8
Figure 1 A lung bleb.
Secondary pneumothoraces occur when there is an underlying lung abnormality. Conditions predisposing to the development of a secondary pneumothorax are shown in box 1, although chronic obstructive pulmonary disease is the most common.
Box 1: Conditions predisposing to the development of a secondary pneumothorax
-
Obstructive airway disease
-
-
chronic obstructive pulmonary disease
-
-
asthma
-
-
-
Suppurative lung disease
-
-
bronchiectasis
-
-
cystic fibrosis
-
-
-
Malignant disease
-
-
lung cancer
-
-
-
Interstitial lung disease
-
-
pulmonary fibrosis
-
-
extrinsic allergic alveolitis
-
-
sarcoidosis
-
-
lymphangioleiomyomatosis
-
-
histiocytosis X
-
-
-
Infections
-
-
pneumonia (for example, due to Staphylococcus aureus or Pneumocystis jiroveci)
-
-
tuberculosis
-
-
-
Miscellaneous
-
-
adult respiratory distress syndrome
-
-
Marfan syndrome
-
-
Ehlors Danlos syndrome
-
-
catamenial
-
-
rheumatoid arthritis and other connective tissue diseases
-
-
An iatrogenic pneumothorax is most commonly caused by central vein cannulation (subclavian more commonly so than internal jugular vein), pleural tap or biopsy, transbronchial biopsy, fine needle aspiration, and has occasionally been caused by acupuncture. Intravenous drug users who try and locate central veins are also at risk of developing a pneumothorax in the community.9 Intubated patients being mechanically ventilated may develop an iatrogenic pneumothorax due to high inspiratory inflation pressures causing pulmonary barotrauma. Before the widespread use of effective chemotherapy, artificial pneumothoraces were created by clinicians treating tuberculosis in an attempt to collapse and “rest” the affected lung and help heal cavitating disease. Traumatic pneumothorax occurs following direct injury to the thorax; common causes include penetrating chest injury or a fractured rib lacerating the visceral pleura.
Tension pneumothorax can occur due to any aetiology and is defined as any size of pneumothorax causing mediastinal shift and cardiovascular collapse. In individuals with advanced lung disease, even a small pneumothorax can cause significant respiratory failure and cardiovascular instability.
DIAGNOSIS
Clinical features
It is often possible to diagnose a pneumothorax—or include it in a list of possible diagnoses—on the basis of a consistent history and examination findings. Patients typically report an abrupt onset of pleuritic pain and breathlessness. Examination findings may vary according to the size of the pneumothorax and presence of limited cardiorespiratory reserve. Typical signs include reduced breath sounds, reduced ipsilateral chest expansion and hyperresonant percussion note. Tracheal shift away from the affected side, tachycardia, tachypnoea and hypotension occur in a tension pneumothorax. Contrary to traditional teaching, it has been recently suggested that in tension, lateralising signs are an inconsistent finding, although general features such as acute onset and rapid cardiovascular instability are universal.10
Imaging
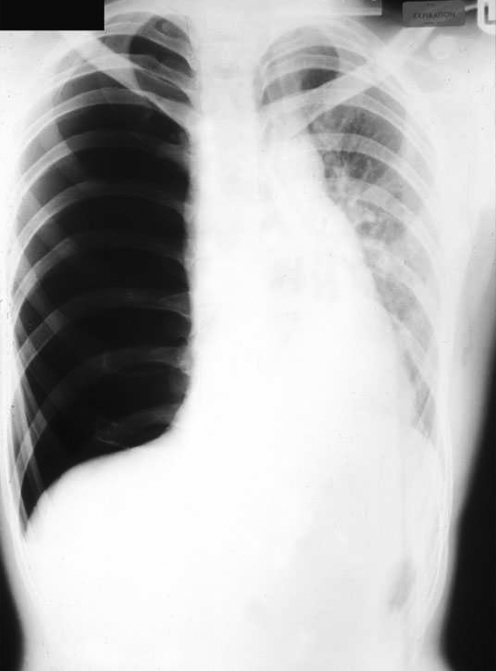
The postero‐anterior chest radiograph shows absent lung markings extending from the edge of visceral pleura to chest wall, although it is possible to confuse a pneumothorax with a lung bulla, edge of the scapula or artefact such as a piece of clothing. Care should be taken in the evaluation of the chest radiograph, especially portable films taken in accident and emergency. There is generally no need to request an expiratory film, although lateral views can sometimes provide additional information if it is uncertain whether a pneumothorax is present or not.11 Mediastinal shift is usually evident in individuals with a tension pneumothorax (fig 2). In patients undergoing transbronchial needle biopsy, transthoracic ultrasound has been proposed as being a useful and sensitive bedside test to detect a post‐intervention pneumothorax or hydropneumothorax.12
Figure 2 Chest radiograph showing a tension pneumothorax.
Computed tomography (CT) imaging of the chest is occasionally performed when diagnostic uncertainty exists—for example, in order to distinguish a pneumothorax from large bulla or when the lung field is obscured by surgical emphysema. It is also often carried out before a contemplated surgical procedure, or when an underlying lung abnormality—such as interstitial lung disease, lymphangioleiomyomatosis or histiocytosis—is considered a possibility.
MANAGEMENT
The management of a pneumothorax depends on the severity of symptoms, its size, and presence of underlying lung disease. Chest radiographs are notoriously poor at assessing the volume of pneumothorax, although recent guidelines published by the British Thoracic Society suggest that the size of a pneumothorax should be categorised according to the amount of air visible between the lung edge and chest wall2:
Small pneumothorax: <2 cm rim present between the lung edge and chest wall
Large pneumothorax: ⩾2 cm rim present between the lung edge and chest wall.
Oxygen
High flow oxygen (>28%) should usually be given to individuals with a pneumothorax in order to maintain adequate oxygenation (saturation >92%) to vital organs. This also lowers the partial pressure of nitrogen, which may in turn accelerate the rate of absorption of air from the pleural cavity and hasten lung re‐expansion. However, care should be taken in individuals with chronic obstructive pulmonary disease who may retain carbon dioxide.
Primary spontaneous pneumothorax
Patients with a small spontaneous pneumothorax with few symptoms do not require active intervention. Most of these individuals do not require admission to hospital, but should be given written instructions to return to hospital if symptoms such as worsening breathlessness or chest pain develop. A follow up appointment within 1–2 weeks for repeat chest radiograph should be arranged before discharge. In some cases, it might be appropriate to admit patients if they live remote from medical access or if concerns exist regarding follow up care or attendance.
According to British Thoracic Society guidelines, symptomatic individuals with a large primary spontaneous pneumothorax should initially undergo needle aspiration with subsequent chest radiograph and observation.2 Thereafter, if needle aspiration is unsuccessful, a chest drain is usually required. This is in contrast to US guidelines where simple aspiration is not advocated and chest drain insertion is considered more appropriate.1 In a randomised trial of 137 patients with first episode of primary spontaneous pneumothorax, the effects of simple aspiration versus chest drain insertion were assessed.13 Immediate success was obtained in 62% assigned to undergo aspiration versus 68% having a chest drain inserted, while the 1 week success rates were similar in both group (88% vs 89%, respectively). Recurrence rates at 1 and 2 years were 22% and 31%, respectively, for patients who had simple aspiration, and 24% and 25%, respectively, for patients who had a chest drain insertion. In another study of 91 patients with primary spontaneous pneumothorax who underwent needle aspiration, recurrence occurred in 18% over the subsequent year.14
Current guidelines do not generally advocate that a surgical procedure to prevent recurrence after the first spontaneous episode is undertaken.1,2 However, it is important to inform patients that the recurrence rate for primary pneumothorax is more than 20% after the first episode13 and even greater after the second episode and tends to be more likely in women, tall men and smokers.15 It is conceivable that given the relatively high recurrence rate, perhaps in the future, greater numbers of individuals with first episode of spontaneous primary pneumothorax will proceed to have surgical intervention. Further randomised controlled studies, incorporating patient preference, cost and short and long term outcomes, are required to establish whether such an approach is merited.
Secondary pneumothorax
Patients with a small secondary pneumothorax with few symptoms require overnight observation. Individuals who are symptomatic from a larger pneumothorax require chest drain insertion, as needle aspiration is less likely to be successful, especially in older patients.16,17 Since many of these patients experience a further pneumothorax, it is advisable that an attempt is made to prevent recurrence with pleurodesis.
Tension pneumothorax
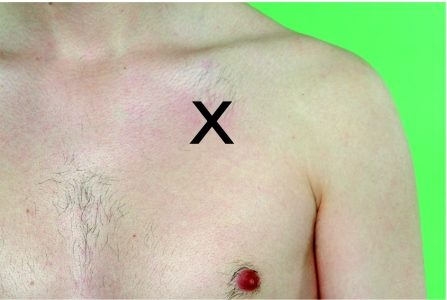
Tension pneumothorax is a medical emergency and clinicians should follow the ABC in terms of immediate management. In a life‐threatening situation, treatment may be necessary without a chest radiograph. A plastic cannula (Venflon) should be placed in the mid‐clavicular line in the second intercostal space and once the pleural space has been entered, a release of air should be heard when the internal needle is removed (fig 3). The cannula should be left in place until a chest drain is inserted and bubbling.
Figure 3 In patients with a tension pneumothorax, a plastic cannula (Venflon) should be inserted into the second intercostal space in the mid‐clavicular line. Informed consent was obtained for publication of this figure.
Chest drains
Since most hospital doctors will be expected to insert a chest drain at some point in their career, it is imperative that the safe technique of doing so and subsequent management is taught by an experienced operator.18 However, unnecessary and avoidable problems such as drain misplacement and inadequate attachment to the skin are frequently encountered. Indeed, in a recent survey of 55 junior doctors, 45% failed to identify correctly a safe position for insertion.19 Other problems encountered include bleeding, infection and empyema, damage to the neurovascular bundle, myocardium, mediastinal contents and lung parenchyma, surgical emphysema, and chest tube kinking and blockage.
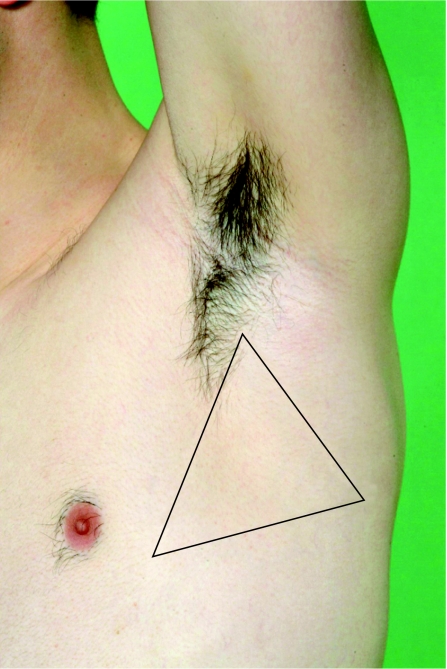
Chest drains are most easily inserted using the Seldinger technique (tube over wire) in the “safe triangle”—such as in the mid‐axillary line in the fifth intercostal space—with the patient sitting at 45° (fig 4). This minimises risk of injury to adjacent thoracic structures. The drain should be connected to an underwater seal and seen to be bubbling following insertion. In the treatment of a straightforward pneumothorax, a large bore chest drain is not usually required and a 10–14 calibre French gauge is adequate. In individuals who may have cervical spine instability—especially those involved in significant antecedent thoracic trauma—the chest drain should be placed with the patient lying supine. Drains should not be secured using a “purse‐string suture” as this has a poor cosmetic outcome and may be painful; a suture placed in the skin and then wrapped several times around the drain is usually adequate. Suction should not generally be applied to a chest drain within 48 h of insertion in order to avoid the possibility of re‐expansion pulmonary oedema.20 A chest radiograph should be arranged following insertion to check tube placement, although drains positioned either apically or basally can effectively drain a pneumothorax.
Figure 4 The “safe triangle” is the area bordered by the anterior border of the latissimus dorsi, the lateral border of the pectoralis major muscle, a line superior to the horizontal level of the nipple, and apex below the axilla. Informed consent was obtained for publication of this figure.
Chest drains should be removed (preferably during expiration or when performing a Valsalva manoeuvre) providing the lung has re‐expanded on the chest radiograph and there is no evidence of air leak for around at least 24 h. However, in a study of 69 trauma patients (102 chest drains), a similar (8% vs 6%, p = 1.0) number of pneumothoraces occurred following removal during end‐inspiration and end‐expiration, respectively.21 Debate continues as to whether drains should be clamped and a subsequent chest radiograph arranged before removal,19 although doing so avoids the unnecessary trouble of re‐inserting a further chest drain if the lung collapses. If clamped, it is preferable to do so in a ward where nursing and medical staff are experienced in chest drain management and are aware that it should be unclamped if the patient becomes symptomatic. However, chest drains should never be clamped in patients where bubbling persists.
REFERRAL TO A THORACIC SURGEON
The basic principle behind surgical intervention lies in removing pleural bullae or suturing apical perforations, in addition to performing a pleurodesis, pleural abrasion or pleurectomy to prevent recurrence. Video assisted thoracoscopic surgery (VATS) has facilitated a less invasive means by which to access the pleural space, especially in more elderly patients with comorbidities.3 Some patients with poor performance status may not be fit for a surgical procedure (for example, those with advanced chronic obstructive pulmonary disease) and a chemical pleurodesis with talc (magnesium silicate) slurry is preferable. Ward based pleurodesis with talc is usually well tolerated, although it can be associated with pleural pain, mild fever or occasionally empyema; a rare complication is adult respiratory distress syndrome.2 The success rate of ward based talc slurry pleurodesis is between 80–90% and for surgical intervention (VATS stapling, pleurectomy or instillation of talc) is at least 95%.22,23,24,25 In one study of 861 cases of primary spontaneous pneumothorax, VATS talc pleurodesis with or without stapling of bullae was safe and resulted in a recurrence rate of only 1.7% over a 52 month follow up period.26 In the same study, recurrence was significantly associated (p = 0.037) with smoking. Other data have indicated that thoracoscopic pleural argon beam coagulation may have a role to play in the treatment of primary spontaneous pneumothorax, although further studies are required to investigate this.27
Referral to a thoracic surgeon should be considered in patients who have a first spontaneous pneumothorax and an “at‐risk” profession (such as aircraft pilot or diver). Other indications for consideration of a definitive surgical procedure to reduce chance of recurrence include second ipsilateral pneumothorax, bilateral pneumothorax, concomitant haemothorax or first contralateral pneumothorax. Individuals who have a persistent air leak (a bubbling chest drain) after 5 days of chest intubation should also be referred for surgical consideration.2
PNEUMOTHORAX IN SPECIAL CONDITIONS
Flying
As pressure falls during ascent in aircraft, an inversely proportional rise in gas volume occurs (Boyle's law). This causes expansion of air within gas filled bodily chambers such as in an undrained pneumothorax. Airline passengers with a closed pneumothorax may therefore experience difficulties due to gas expansion during ascent, and can develop a tension pneumothorax. As a consequence, individuals with an untreated pneumothorax must not fly in commercial aircraft. Providing 1 week (or 2 weeks in the case of a traumatic pneumothorax or thoracic surgery) has elapsed after resolution of a pneumothorax and the chest radiograph is normal, individuals may be permitted to fly (http://www.brit‐thoracic.org.uk/c2/uploads/FlightPCsummary04.pdf). Some individuals with a longstanding pneumothorax have flown without complication but only with careful pre‐flight assessment, including CT imaging and exposure to a hypoxic hypobaric environment in a decompression chamber.28
Diving
The development of a pneumothorax at depth is associated with potentially fatal consequences, since during ascent, the volume of gas within a closed pneumothorax will expand, in turn leading to a tension pneumothorax. Current guidelines suggest that a previous spontaneous pneumothorax is a contraindication to underwater diving unless treated by bilateral surgical pleurectomy in association with normal lung function and CT scan following surgery. A previous traumatic pneumothorax may not be an absolute contraindication providing it has healed and subsequent lung function and CT thorax scan are normal.29
Cystic fibrosis
Patients with cystic fibrosis who develop a pneumothorax should generally be managed in a manner similar to those without the disease, although needle aspiration is usually less successful. It may take longer for the lung to expand and concomitant infection—often with Pseudomonas aeruginosa—should be treated aggressively with intravenous antibiotics. Consideration should be given to prevention of subsequent pneumothorax by either surgical intervention or talc pleurodesis in order to prevent recurrence (which tends to be high without intervention).
HIV infection
Previous data have shown that infection with tuberculosis or Pneumocystis jiroveci (previously carinii) can predispose to the development of a pneumothorax in patients with HIV infection.30 Indeed, in an HIV infected individual, P jiroveci infection should be considered as the most likely aetiological factor.31
CONCLUSION
Pneumothorax is a relatively common respiratory diagnosis and it is important that it is managed promptly and in an appropriate manner. Immediate management is largely determined by the extent of cardiorespiratory compromise, degree of symptoms and size of pneumothorax and may involve observation alone, needle aspiration or chest drain insertion. Since recurrence rates are relatively high, selected individuals should be considered for definitive surgical treatment (usually by VATS) or instillation of talc slurry in less fit individuals.
Junior doctors are frequently given responsibility to insert chest drains but should ideally receive a period of training and supervision before this. Indeed, with increased availability of clinical skills laboratories this procedure should form a core element of postgraduate training. This has the ultimate aim of reducing complications at insertion and subsequent aftercare of chest drains, which are not infrequently encountered in accident and emergency, and acute medical receiving and respiratory wards.
Footnotes
Competing interests and acknowledgements: none.
Informed consent was obtained for publication of figs 3 and 4
References
- 1.Baumann M H, Strange C, Heffner J E.et al Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest 2001119590–602. [DOI] [PubMed] [Google Scholar]
- 2.Henry M, Arnold T, Harvey J. BTS guidelines for the management of spontaneous pneumothorax. Thorax 200358(Suppl II)ii39–ii52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng C S, Lee T W, Wan S, Yim A P. Video assisted thoracic surgery in the management of spontaneous pneumothorax: the current status. Postgrad Med J 200682179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lesur O, Delorme N, Fromaget J M.et al Computed tomography in the etiologic assessment of idiopathic spontaneous pneumothorax. Chest 199098341–347. [DOI] [PubMed] [Google Scholar]
- 5.Donahue D M, Wright C D, Viale G.et al Resection of pulmonary blebs and pleurodesis for spontaneous pneumothorax. Chest 19931041767–1769. [DOI] [PubMed] [Google Scholar]
- 6.Bense L, Eklund G, Wiman L G. Smoking and the increased risk of contracting spontaneous pneumothorax. Chest 1987921009–1012. [DOI] [PubMed] [Google Scholar]
- 7.Melton L J, 3rd, Hepper N G, Offord K P. Incidence of spontaneous pneumothorax in Olmsted County, Minnesota: 1950 to 1974. Am Rev Respir Dis 19791201379–1382. [DOI] [PubMed] [Google Scholar]
- 8.Bense L, Wiman L G, Hedenstierna G. Onset of symptoms in spontaneous pneumothorax: correlations to physical activity. Eur J Respir Dis 198771181–186. [PubMed] [Google Scholar]
- 9.Miller D R, Harden J L, Currie G P. A case of self‐inflicted bilateral pneumothorax. Resuscitation 200671122–123. [DOI] [PubMed] [Google Scholar]
- 10.Leigh‐Smith S, Harris T. Tension pneumothorax—time for a re‐think? Emerg Med J 2005228–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glazer H S, Anderson D J, Wilson B S.et al Pneumothorax: appearance on lateral chest radiographs. Radiology 1989173707–711. [DOI] [PubMed] [Google Scholar]
- 12.Reissig A, Kroegel C. Accuracy of transthoracic sonography in excluding post‐interventional pneumothorax and hydropneumothorax. Comparison to chest radiography. Eur J Radiol 200553463–470. [DOI] [PubMed] [Google Scholar]
- 13.Ayed A K, Chandrasekaran C, Sukumar M. Aspiration versus tube drainage in primary spontaneous pneumothorax: a randomised study. Eur Respir J 200627477–482. [DOI] [PubMed] [Google Scholar]
- 14.Chan S S, Rainer T H. Primary spontaneous pneumothorax: 1‐year recurrence rate after simple aspiration. Eur J Emerg Med 20061388–91. [DOI] [PubMed] [Google Scholar]
- 15.Sadikot R T, Greene T, Meadows K.et al Recurrence of primary spontaneous pneumothorax. Thorax 199752805–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Archer G J, Hamilton A A, Upadhyay R.et al Results of simple aspiration of pneumothoraces. Br J Dis Chest 198579177–182. [PubMed] [Google Scholar]
- 17.Ng A W, Chan K W, Lee S K. Simple aspiration of pneumothorax. Singapore Med J 19943550–52. [PubMed] [Google Scholar]
- 18.Laws D, Neville E, Duffy J. BTS guidelines for the insertion of a chest drain. Thorax 200358(Suppl II)ii53–ii59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hyde J, Sykes T, Graham T. Reducing morbidity from chest drains. BMJ 1997314914–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tariq S M, Sadaf T. Images in clinical medicine. Reexpansion pulmonary edema after treatment of pneumothorax. N Engl J Med 20063542046. [DOI] [PubMed] [Google Scholar]
- 21.Bell R L, Ovadia P, Abdullah F.et al Chest tube removal: end‐inspiration or end‐expiration? J Trauma 200150674–677. [DOI] [PubMed] [Google Scholar]
- 22.Kennedy L, Sahn S A. Talc pleurodesis for the treatment of pneumothorax and pleural effusion. Chest 19941061215–1222. [DOI] [PubMed] [Google Scholar]
- 23.Baumann M H, Strange C. Treatment of spontaneous pneumothorax: a more aggressive approach? Chest 1997112789–804. [DOI] [PubMed] [Google Scholar]
- 24.Ayed A K, Al‐Din H J. The results of thoracoscopic surgery for primary spontaneous pneumothorax. Chest 2000118235–238. [DOI] [PubMed] [Google Scholar]
- 25.Hatz R A, Kaps M F, Meimarakis G.et al Long‐term results after video‐assisted thoracoscopic surgery for first‐time and recurrent spontaneous pneumothorax. Ann Thorac Surg 200070253–257. [DOI] [PubMed] [Google Scholar]
- 26.Cardillo G, Carleo F, Giunti R.et al Videothoracoscopic talc poudrage in primary spontaneous pneumothorax: a single‐institution experience in 861 cases. J Thorac Cardiovasc Surg 2006131322–328. [DOI] [PubMed] [Google Scholar]
- 27.Bobbio A, Ampollini L, Internullo E.et al Thoracoscopic parietal pleural argon beam coagulation versus pleural abrasion in the treatment of primary spontaneous pneumothorax. Eur J Cardiothorac Surg 2006296–8. [DOI] [PubMed] [Google Scholar]
- 28.Currie G P, Kennedy A M, Paterson E.et al A chronic pneumothorax and fitness to fly. Thorax 200762187–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.British Thoracic Society British Thoracic Society guidelines on respiratory aspects of fitness for diving. Thorax 2003583–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tumbarello M, Tacconelli E, Pirronti T.et al Pneumothorax in HIV‐infected patients: role of Pneumocystis carinii pneumonia and pulmonary tuberculosis. Eur Respir J 1997101332–1335. [DOI] [PubMed] [Google Scholar]
- 31.Spivak H, Keller S. Spontaneous pneumothorax in the AIDS population. Am Surg 199662753–756. [PubMed] [Google Scholar]