Abstract
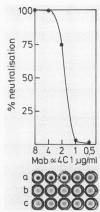
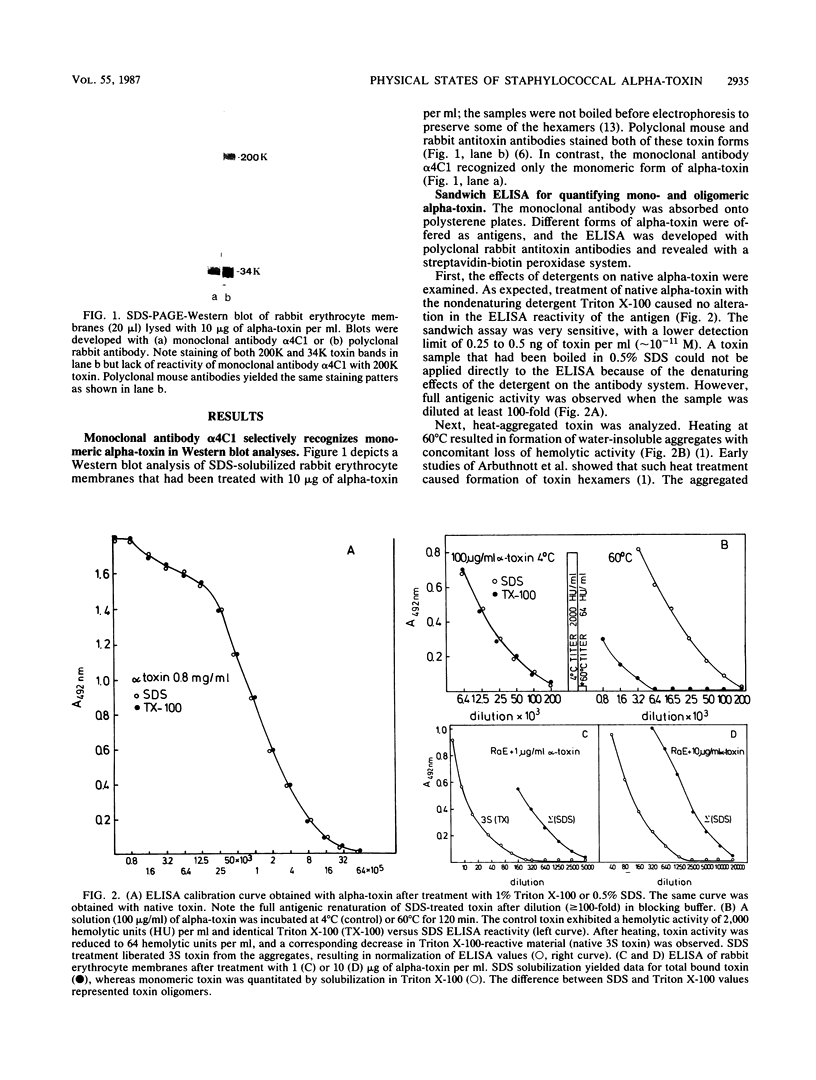
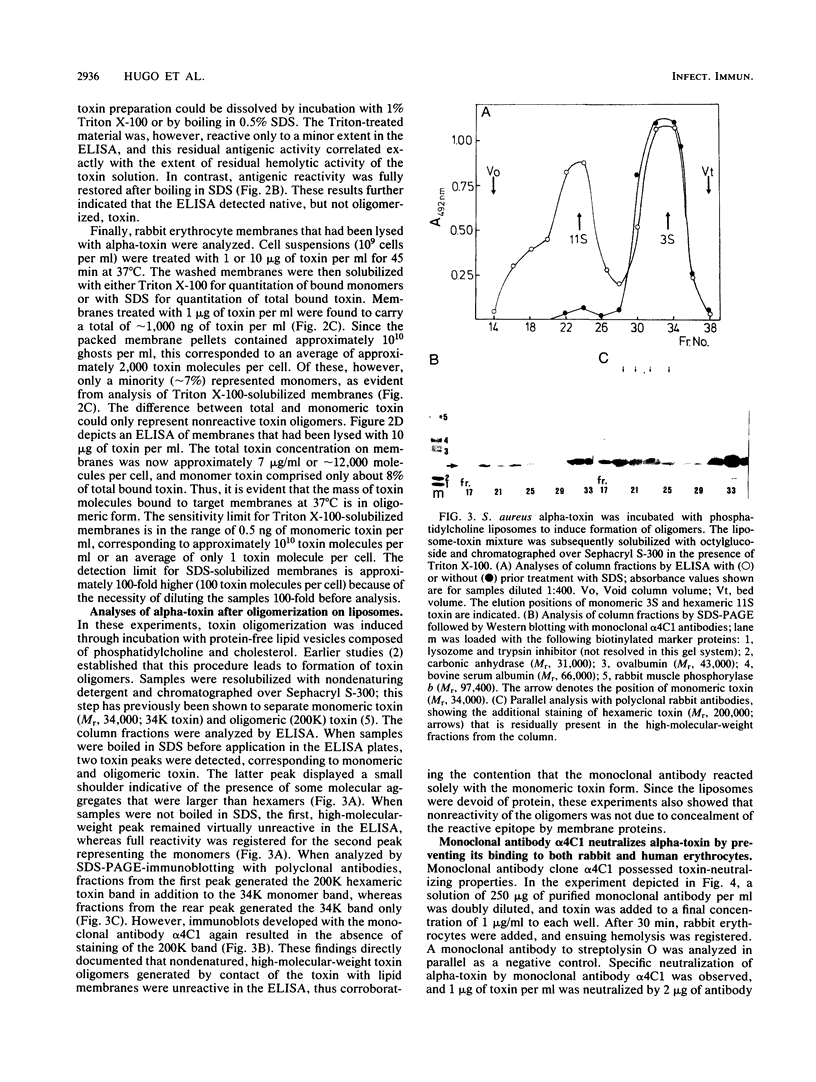
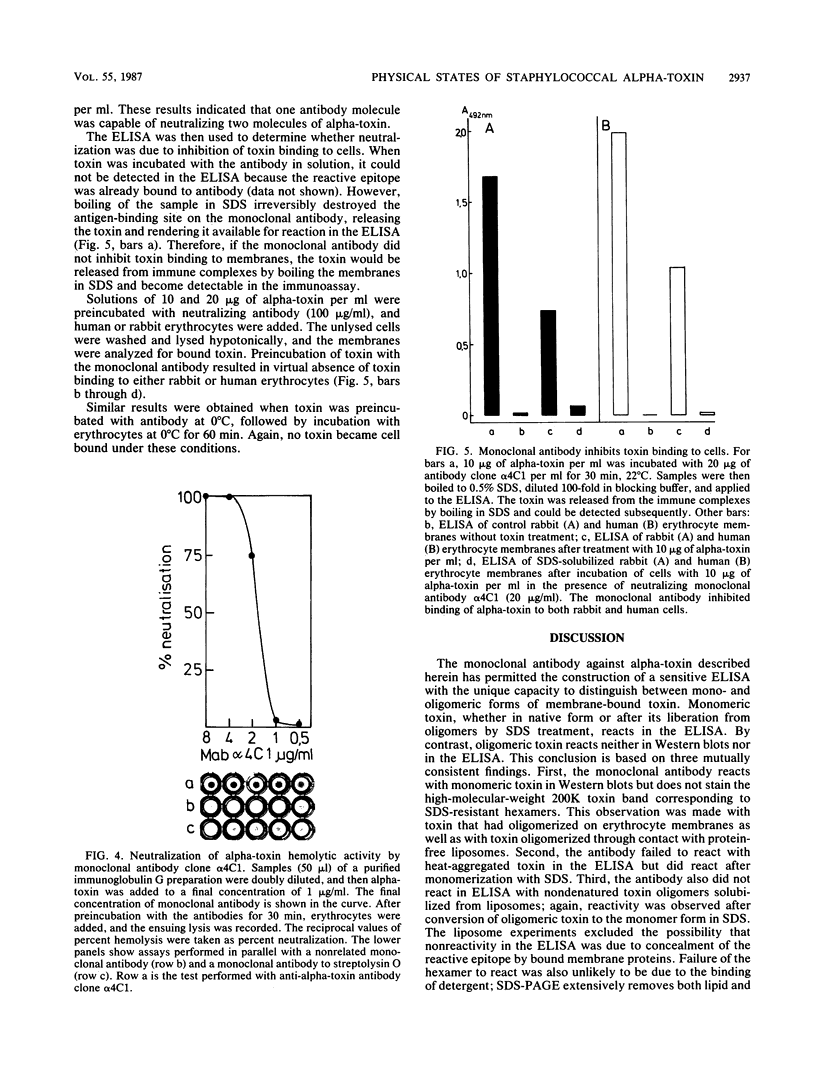
A murine monoclonal antibody generated against staphylococcal alpha-toxin was shown to react only with the monomeric (native), 3S form of the toxin. A sensitive sandwich enzyme-linked immunosorbent assay (ELISA) constructed with this antibody permitted detection of 0.25 to 0.5 ng of native toxin per ml. Toxin oligomers formed either by heat aggregation in solution, on target erythrocyte membranes, or on phosphatidylcholine-cholesterol liposomes were unreactive in the ELISA when membranes were solubilized with the nondenaturing detergent Triton X-100. After dissociation of the oligomers by boiling in sodium dodecyl sulfate, however, the ELISA reactivity of the liberated 3S toxin was fully restored. Parallel determinations of membrane-bound toxin with sodium dodecyl sulfate and Triton X-100 solubilization thus permitted direct quantitation of total and monomeric toxin, respectively; the difference between these two values was represented by toxin oligomers. The detection limits for membrane-bound oligomeric and monomeric toxin on erythrocyte membranes are in the order of 100 molecules and 1 molecule per cell, respectively. Using this ELISA, we show that over 90% of alpha-toxin molecules bound to target membranes at 37 degrees C are in oligomeric form. Evidence is given that the monoclonal antibody neutralizes alpha-toxin by inhibiting its binding to both rabbit and human erythrocytes. This ELISA is the first assay that quantitatively discriminates between mono- and oligomeric forms of a pore-forming protein on target cell membranes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arbuthnott J. P., Freer J. H., Bernheimer A. W. Physical states of staphylococcal alpha-toxin. J Bacteriol. 1967 Oct;94(4):1170–1177. doi: 10.1128/jb.94.4.1170-1177.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuthnott J. P., Freer J. H., Billcliffe B. Lipid-induced polymerization of staphylococcal -toxin. J Gen Microbiol. 1973 Apr;75(2):309–319. doi: 10.1099/00221287-75-2-309. [DOI] [PubMed] [Google Scholar]
- Belmonte G., Cescatti L., Ferrari B., Nicolussi T., Ropele M., Menestrina G. Pore formation by Staphylococcus aureus alpha-toxin in lipid bilayers. Dependence upon temperature and toxin concentration. Eur Biophys J. 1987;14(6):349–358. doi: 10.1007/BF00262320. [DOI] [PubMed] [Google Scholar]
- Bernheimer A. W., Rudy B. Interactions between membranes and cytolytic peptides. Biochim Biophys Acta. 1986 Jun 12;864(1):123–141. doi: 10.1016/0304-4157(86)90018-3. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Füssle R., Tranum-Jensen J. Staphylococcal alpha-toxin: oligomerization of hydrophilic monomers to form amphiphilic hexamers induced through contact with deoxycholate detergent micelles. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5475–5479. doi: 10.1073/pnas.78.9.5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Muhly M., Füssle R. Correlation between toxin binding and hemolytic activity in membrane damage by staphylococcal alpha-toxin. Infect Immun. 1984 Nov;46(2):318–323. doi: 10.1128/iai.46.2.318-323.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Mechanism of complement cytolysis and the concept of channel-forming proteins. Philos Trans R Soc Lond B Biol Sci. 1984 Sep 6;306(1129):311–324. doi: 10.1098/rstb.1984.0092. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Membrane damage by pore-forming bacterial cytolysins. Microb Pathog. 1986 Feb;1(1):5–14. doi: 10.1016/0882-4010(86)90027-6. [DOI] [PubMed] [Google Scholar]
- Buckelew A. R., Jr, Colacicco G. Lipid monolayers. Interactions with staphylococcal alpha-toxin. Biochim Biophys Acta. 1971 Mar 9;233(1):7–16. doi: 10.1016/0005-2736(71)90352-x. [DOI] [PubMed] [Google Scholar]
- Cassidy P., Harshman S. Studies on the binding of staphylococcal 125I-labeled alpha-toxin to rabbit erythrocytes. Biochemistry. 1976 Jun 1;15(11):2348–2355. doi: 10.1021/bi00656a016. [DOI] [PubMed] [Google Scholar]
- Freer J. H., Arbuthnott J. P., Bernheimer A. W. Interaction of staphylococcal alpha-toxin with artificial and natural membranes. J Bacteriol. 1968 Mar;95(3):1153–1168. doi: 10.1128/jb.95.3.1153-1168.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freer J. H., Arbuthnott J. P., Billcliffe B. Effects of staphylococcal -toxin on the structure of erythrocyte membranes: a biochemical and freeze-etching study. J Gen Microbiol. 1973 Apr;75(2):321–332. doi: 10.1099/00221287-75-2-321. [DOI] [PubMed] [Google Scholar]
- Füssle R., Bhakdi S., Sziegoleit A., Tranum-Jensen J., Kranz T., Wellensiek H. J. On the mechanism of membrane damage by Staphylococcus aureus alpha-toxin. J Cell Biol. 1981 Oct;91(1):83–94. doi: 10.1083/jcb.91.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harshman S. Action of staphylococcal alpha-toxin on membranes: some recent advances. Mol Cell Biochem. 1979 Feb 9;23(3):143–152. doi: 10.1007/BF00219453. [DOI] [PubMed] [Google Scholar]
- Hugo F., Reichwein J., Arvand M., Krämer S., Bhakdi S. Use of a monoclonal antibody to determine the mode of transmembrane pore formation by streptolysin O. Infect Immun. 1986 Dec;54(3):641–645. doi: 10.1128/iai.54.3.641-645.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson P., Lindberg M., Haraldsson I., Wadström T. Virulence of Staphylococcus aureus in a mouse mastitis model: studies of alpha hemolysin, coagulase, and protein A as possible virulence determinants with protoplast fusion and gene cloning. Infect Immun. 1985 Sep;49(3):765–769. doi: 10.1128/iai.49.3.765-769.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato I., Watanabe M. Chemical studies on staphylococcal alpha-toxin and its fragments. Toxicon. 1980;18(3):361–365. doi: 10.1016/0041-0101(80)90018-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Menestrina G. Ionic channels formed by Staphylococcus aureus alpha-toxin: voltage-dependent inhibition by divalent and trivalent cations. J Membr Biol. 1986;90(2):177–190. doi: 10.1007/BF01869935. [DOI] [PubMed] [Google Scholar]
- O'Reilly M., de Azavedo J. C., Kennedy S., Foster T. J. Inactivation of the alpha-haemolysin gene of Staphylococcus aureus 8325-4 by site-directed mutagenesis and studies on the expression of its haemolysins. Microb Pathog. 1986 Apr;1(2):125–138. doi: 10.1016/0882-4010(86)90015-x. [DOI] [PubMed] [Google Scholar]
- Phimister G. M., Freer J. H. Binding of 125I-alpha toxin of Staphylococcus aureus to erythrocytes. J Med Microbiol. 1984 Oct;18(2):197–204. doi: 10.1099/00222615-18-2-197. [DOI] [PubMed] [Google Scholar]
- Reichwein J., Hugo F., Roth M., Sinner A., Bhakdi S. Quantitative analysis of the binding and oligomerization of staphylococcal alpha-toxin in target erythrocyte membranes. Infect Immun. 1987 Dec;55(12):2940–2944. doi: 10.1128/iai.55.12.2940-2944.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelestam M., Möllby R. Sensitive assay for detection of toxin-induced damage to the cytoplasmic membrane of human diploid fibroblasts. Infect Immun. 1975 Aug;12(2):225–232. doi: 10.1128/iai.12.2.225-232.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelestam M., Möllby R., Wadström T. Effects of staphylococcal alpha-, beta-, delta-, and gamma-hemolysins on human diploid fibroblasts and HeLa cells: evaluation of a new quantitative as say for measuring cell damage. Infect Immun. 1973 Dec;8(6):938–946. doi: 10.1128/iai.8.6.938-946.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobkes N., Wallace B. A., Bayley H. Secondary structure and assembly mechanism of an oligomeric channel protein. Biochemistry. 1985 Apr 9;24(8):1915–1920. doi: 10.1021/bi00329a017. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Tomita T., Yasuda T. Membrane-damaging action of staphylococcal alpha-toxin on phospholipid-cholesterol liposomes. Biochim Biophys Acta. 1987 Apr 23;898(3):257–265. doi: 10.1016/0005-2736(87)90065-4. [DOI] [PubMed] [Google Scholar]