Abstract
Patients with longstanding chronic ulcerative colitis are “at risk” of developing colorectal cancer. Approximately 1 in 6 patients will die as a result of colorectal malignancy, which can often be difficult to detect using conventional “white light” colonoscopy. New endoscopic techniques and technologies including the use of dye sprays, “chromoendoscopy”, high magnification chromoscopic colonoscopy and recently chromoscopic assisted confocal laser scanning in vivo endomicroscopy have now been introduced to improve the diagnostic yield of intraepithelial neoplasia at screening colonoscopy. This review details the true “risk” of colorectal cancer complicating ulcerative colitis, discusses the objective evidence to support current endoscopic screening guidelines, and describes the imminent technological paradigm shift about to occur in the endoscopic management and detection of intraepithelial neoplasia.
Burill Crohn and Herman Rosenberg reported the first case of adenocarcinoma complicating ulcerative colitis in 1925.1 Since that time, the role of cancer surveillance in the management of colitis has been the subject of much controversy. Cancer surveillance programmes are based on the hypothesis that repeated testing of a high‐risk population will identify patients who either have or are likely to develop cancer, where subsequent colectomy will allow cure.2 Since the introduction of endoscopic surveillance programmes in the past 10–20 years, results of a large number of studies have been published.3,4,5,6,7,8,9,10,11,12 However, the principle problem in assessing the impact of surveillance is the large number of patients and duration of study (15–20 years) required to demonstrate a significant effect on cancer stage and overall survival, given the problems with “lead‐time bias”.8,9,13,14,15 It has therefore been difficult to conduct controlled trials due to ethical issues in randomising to a control arm those patients at high risk of colorectal cancer (CRC).
CURRENT EVIDENCE BASE FOR COLORECTAL CANCER SCREENING IN CHRONIC ULCERATIVE COLITIS
CRC complicating chronic ulcerative colitis (CUC) is hypothesised to develop through a chronic inflammatory–dysplasia–carcinoma sequence.16,17 The incidence of CRC in CUC ranges from 7–30% and is primarily dependant on the extent, duration of disease and the presence of primary sclerosing cholangitis (PSC).2,18,19 Although a relatively low incidence of CRC is reported after 10 years of disease, a subsequent increase to approximately 9% and 18% after 20 and 30 years, respectively, is reported.20 Development of colon cancer in CUC accounts for 33% of ulcerative colitis related deaths.19 Based on the above data, colonoscopic cancer surveillance in patients with longstanding CUC is therefore recommended.
Studies examining the impact of cancer surveillance in ulcerative colitis have produced conflicting results, with data suggesting that surveillance leads to the detection of early‐stage cancer in only a minority of patients, resulting in a high cost‐to‐benefit ratio.4,8,10,11,14,21,22 Indeed, a number of patients develop interval cancers, despite current colonoscopic screening strategies.23 However, other surveillance studies have suggested improved mortality rates. Data from the Choi 18 year surveillance programme in the USA demonstrated that CRC was detected at an earlier stage in 15 of 19 patients (80%), compared with 9 of 22 (41%) non‐surveyed cancer patients.24 Additionally, the overall 5 year survival rate was 77% for the surveillance group compared with only 36% for the control group (p<0.03).24 Other studies addressing the outcome of patients receiving colonoscopic surveillance for CUC have also shown a beneficial trend.25,26
The hazard rate of surveillance colonoscopy with multiple biopsies is low.27 In Koobatian's analysis, the overall complication rate associated with CUC screening was 0.26%.27 The experience in the UK has shown similar results, with no incidence of complications recorded during 811 surveillance colonoscopies in the Lennard‐Jones series.3 The hazard rate therefore appears comparable to that of diagnostic colonoscopy.27 However, negative publication bias will play a significant part in the reporting of such data.
Current published data would suggest that early neoplastic change may be recognised by serial surveillance colonoscopies and thus ensure preventative proctocolectomy in CUC. However, Ransohoff's data suggests that only 20–50% of intraepithelial neoplasia (IN) can be detected using conventional colonoscopic methods.28 These data are unsurprising given that many cases of colorectal cancer complicating CUC adopt a diffusely infiltrative or flat morphology.16,28,29
Key points 1
Patients with longstanding chronic ulcerative colitis (CUC) are at an increased risk of developing colorectal cancer (CRC) (18% by 30 years symptom duration)
Risk factors for colitis associated CRC include duration >8 years, pan‐colorectal disease, family history of CRC, presence of primary sclerosing cholangitis, young age of inflammatory bowel disease (IBD) onset, backwash ileitis and failure to establish mucosal healing (that is, severity of inflammation)
Endoscopic findings can also aid in the risk stratification of patients at highest overall risk for colitis associated CRC development (that is, multiple pseudopolyps ± stricture formation)
BIOMARKERS FOR COLORECTAL CANCER COMPLICATING CHRONIC ULCERATIVE COLITIS
Dysplasia, defined as unequivocal neoplastic epithelium,30 is at present the most reliable biomarker of malignancy, being present in >70% of CUC patients with colorectal cancer.17 Additionally, dysplasia typically parallels the location of neoplasia, arising from chronically inflamed mucosa.29 Endoscopically, dysplasia can be challenging to identify with conventional endoscopic techniques, as subtle, flat mucosal lesions occur in addition to morphologically protuberant lesions.31 The term dysplasia‐associated lesion or mass (DALM) has been adopted to describe the group of “endoscopically visible” lesions, but in fact refers to a heterogeneous population of lesions which demonstrate plaque‐like, mass, stricture, sessile or pedunculated morphology.32 Indeed, sessile or pedunculated DALMs with conventional endoscopy can resemble sporadic adenomas.32,33 Accurate diagnosis of such lesions is of importance, as an adenoma‐like DALM is highly associated with the presence of invasive CRC.34 Many practitioners would view this as justification for colectomy, independent of the grade of DALM associated dysplasia.29,35 Alternatively, data now exist favouring polypectomy as an adequate therapeutic option for adenoma‐like dysplastic lesions (ALMs), either within or outside areas of documented colitis, followed by rigorous endoscopic surveillance.36 The optimal management strategy for ALMs, however, requires further clarification, particularly when considering diminutive, apparent “benign” adenomas endoscopically, within a colitis zone.29 Furthermore, there is now evidence that CUC‐associated non‐adenoma‐like DALMs have a discrete genotype in comparison to CUC‐related adenoma‐like DALMs and non‐CUC sporadic adenomas.32 Odze et al proposed that adenoma‐like lesions in CUC and non‐CUC patients may represent identical pathological entities. This was based on the observed similarity in genetic markers (on chromosome 3p) described in both groups.37 This is reflected by the apparent “safety” of polypectomy described in the treatment of adenoma‐like dysplastic lesions in CUC31 (figs 1 and 2). New data regarding the endoscopic management of ALM will be discussed below.
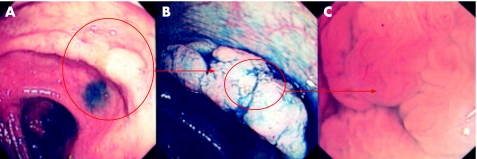
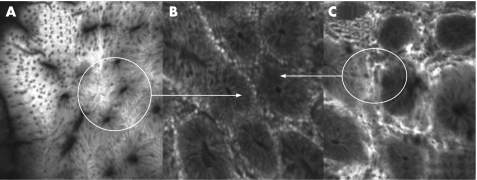
Figure 1 (A) Conventional “white light” endoscopic view of the distal transverse colon in a patient with longstanding pan‐colitis. There is patchy vascular net loss, mucosal pallor and focal thickening of the mucosal fold. (B) Indigo carmine 0.5% chromoscopy clearly delineates a lateral spreading tumour (NG‐type) with normal adjacent mucosal architecture. (C) High‐magnification imaging reveals of crypt type IV/IIIL suggestive of a low grade dysplastic tubulovillous adenoma. Endoscopically this lesion is classified as an adenoma‐like mass (ALM). Endoscopic resection is therefore indicated.
Figure 2 (A) Conventional “white light” endoscopic view of the distal sigmoid colon in a patient with longstanding pan‐colitis. There is focal mucosal pallor and nodularity. (B) Indigo carmine 0.5% chromoscopy shows a flat (Paris type 0‐IIb) lesion. The crypt pattern is a type I. On table diagnosis would favour a benign hyperplastic/metaplastic lesion. Endoscopic resection is therefore not indicated for this lesion.
Key points 2
IBD‐associated dysplasia can occur in endoscopic “normal” appearing mucosa (that is, flat) or as an elevated or protruberant mass
Adenoma‐like mass (ALM) may be managed by endoluminal resection (both flat and protruberant) if centres have local expertise in the adjunctive techniques
Dysplasia‐associated lesion or mass (DALM) should mandate colectomy due to the high risk of synchronous CRC
CURRENT ENDOSCOPIC SURVEILLANCE PROTOCOLS: LIMITATIONS AND CONTROVERSY
As IN and colitis‐associated cancer can occur in macroscopically normal mucosa, random biopsies at 10 cm intervals throughout the colorectum are currently recommended during screening colonoscopy.19 The probability for detection of neoplastic changes correlates with the number of biopsies taken and 40–50 biopsies are currently recommended for routine surveillance for those patients with established pan‐colitis.38 However, sampling error is a significant problem in any CUC surveillance programme as it is now well recognised that dysplastic change is usually patchy and difficult to distinguish from the surrounding mucosa with established chronic inflammatory changes.19 The consequence is that even with multiple biopsies, only a small proportion of the colonic mucosa will be sampled and thus dysplasia not revealed.
Furthermore, the overall significance of dysplasia in CUC has carried much controversy, as the likelihood of progression to cancer is difficult to predict. When considering Bernstein's analysis of 1225 patients who had undergone conventional colonoscopic surveillance with subsequent immediate colectomy following a diagnosis of CUC associated DALM formation, cancer was revealed in 43% of patients regardless of dysplasia grade (low grade dysplasia (LGD) or high grade dysplasia (HGD)).34 Other studies have offered impressive corroborative data for this finding where upon identification of HGD, carcinoma was revealed in 42–67% of colectomy specimens.25,34 If HGD is found at some time after the initial evaluation, 32% of such patients prove to have CRC.2 Thus, whenever a DALM or IN lesion is identified and confirmed by two independent gastrointestinal (GI) pathologists, there is a strong indication for colectomy. In the report by Bernstein et al, 69 patients were identified as having LGD on initial colonoscopy.34 Of these, 29% underwent progression at some time to HGD, DALM or cancer. Overall, 210 patients eventually developed LGD during surveillance with 17 subsequently evolving to CRC (8.1%). Moreover, the St Marks Hospital surveillance study indicates that the 5 year predictive value for HGD or cancer in patients with LGD was highly significant at 54%.25 This may be taken as evidence that the presence of IN (of any grade), particularly in flat mucosal change, should be considered as much an indication for colectomy as those with histological evidence of HGD or DALM without the requirement for confirmatory colonoscopy (clinical examples are shown in figs 3–5). However, even if we accept that dysplasia of any grade (IN) is a valid marker of colorectal cancer complicating CUC, further problems exist when addressing the accuracy and reliability of histopathological diagnosis.
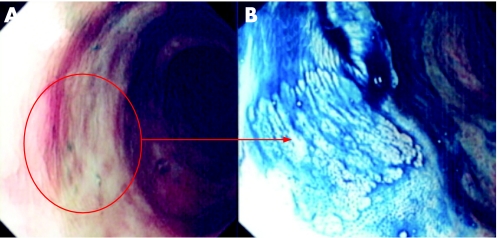
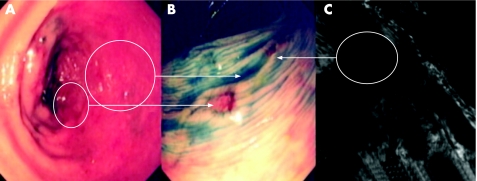
Figure 3 (A) Conventional “white light” view of the distal ascending colon in a patient with pan‐colitis of >20 years duration. There is focal nodularity and interruption of the vascular net pattern. (B) Indigo carmine 0.5% chromoscopy shows an irregular mucosal architecture with a central neoplastic crypt architecture. The lesion endoscopically is suggestive of a dysplasia‐associated lesion mass. Endoscopic resection in this case is contraindicated. Pan‐proctocolectomy is the treatment of choice due to the high risk of colitis associated colorectal cancer.
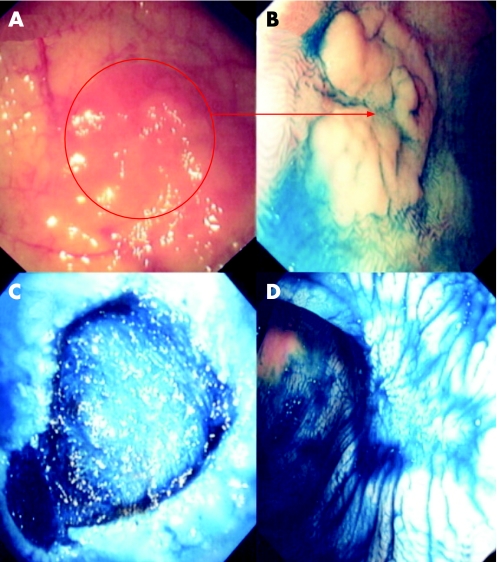
Figure 4 (A) Conventional “white light” view of the proximal sigmoid colon in a patient with distal colitis of >25 years duration. Note the focal mucosal erythema and loss of vascular net pattern. (B) Indigo carmine 0.5% chromoscopy clearly delineates a flat elevated circumscribed lesion with an area of central depression (Paris classification 0‐IIa+IIc). The adjacent mucosal architecture is within normal limits. The lesion endoscopically is an adenoma‐like mass (ALM). Endoluminal resection is indicated. (C) Endoscopic submucosal dissection using cap assistance has been performed. The lesion has been resected en bloc. Note the exposed underlying muscularis propria. (D) Post‐resection chromoscopic views of the lesion at 1 month. Note the depressed resection crater but complete re‐epitheliasation. There is no evidence of neoplastic crypt architecture indicating curative resection.
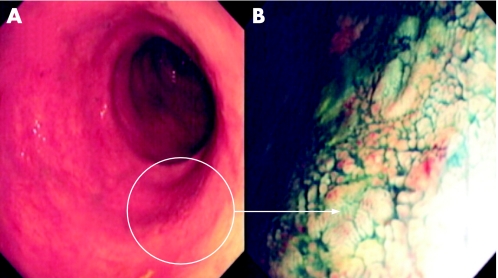
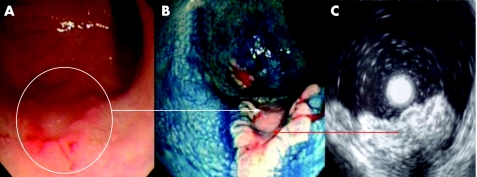
Figure 5 (A) Conventional “white light” view of the posterior‐lateral rectum in a patient with longstanding pan‐colitis. Note the focal nodularity and erythema. (B) Indigo carmine 0.5% chromoscopy delineated a flat elevated lesion with deep central depression (Paris class 0‐IIa + IIc). The lesion is highly suggestive of a submucosally invasive carcinoma. Endoscopic “through the scope” mini probe ultrasound is therefore used to locally stage the lesion. (C) 12.5 MHz mini probe “through the scope” ultrasound shows complete disruption of the third and fourth hypoechoic layers with infiltration of the muscularis. The lesion is a stage T2 carcinoma. Pan‐proctocolectomy is indicated.
It has long been recognised that there is a wide range of inter‐ and intra‐observer variability in assessing whether a lesion fulfils criteria for dysplasia or not, with further dilemmas occurring when pathologists attempt to grade degree of dysplastic change.39 In 1994 Connell et al reported their experience of dysplasia as diagnosed using standardised Riddell criteria.17,25 Intra‐observer agreement between two experienced pathologists blindly reviewing 301 specimens occurred in only 42% and 43% of cases respectively, where HGD and LGD were concerned. Many clinicians therefore remain unwilling to accept IN as a “gold standard” on which to base major clinical decision‐making, such as elective colectomy.
Despite these caveats, dysplasia of any grade or IN, diagnosed histologically, remains the cornerstone of CRC surveillance in ulcerative colitis. Recently, new endoscopic techniques have been developed by both Japanese and Western groups to optimise the detection of IN in this proposed “high risk” cancer cohort. Such techniques include high magnification imaging, chromoscopic colonoscopy and recently confocal endomicroscopy. Furthermore, recent data from the UK may suggest that flat dysplasia in CUC can now be treated endoscopically without the requirement for mandatory proctocolectomy.40 However, all adjunctive colonoscopic imaging techniques such as high magnification chromoscopic colonoscopy (HMCC) can do little to abolish observer variation at a histopathological level, but may significantly improve diagnostic yield, where timely diagnosis of IN and colitis‐associated CRC remains the prime clinical objective. These new techniques for targeting IN detection in CUC are discussed below.
CHROMOSCOPIC COLONOSCOPY AND HIGH MAGNIFICATION IMAGING AS AN ADJUNCTIVE SCREENING TOOL IN COLITIS ASSOCIATED CANCER SCREENING
In addition to the detection of sporadic neoplastic lesions of the colorectum, chromoscopic colonoscopy (use of dye spray) has now been described for the detection of IN in CUC.41,42,43 Either targeted or pan‐chromoscopy using either methylene blue (MB) or indigo carmine (IC) is used to improve the diagnostic yield of IN in conjunction with the SURFACE endoscopic guidelines44—that is, used to “unmask” circumscribed lesions during ongoing colonoscopy and enhance targeted biopsy accuracy or guide subsequent endoluminal resection strategy (table 1).
Table 1 The SURFACE guidelines for chromo‐colonoscopy in chronic ulcerative colitis surveillance.
| Strict patient selection |
| Patients with histologically confirmed ulcerative colitis and at least 8 years duration in clinical remission |
| Avoid patients with active disease |
| Unmask the mucosal surface |
| Optimise bowel preparation |
| Remove mucus and remaining fluid in the colon |
| Reduce peristaltic waves |
| On extubation a spasmolytic agent should be used if necessary |
| Full‐length staining of the colon (pan‐chromoscopy) |
| Augmented detection with dyes |
| Intravital staining with 0.4% indigo carmine or 0.1% methylene blue should be used to unmask flat lesions (Paris 0‐II) more frequently than is possible with conventional colonoscopy |
| Crypt architecture analysis |
| All lesions should be analysed according to the pit pattern classification. |
| Whereas pit patterns type I and II suggest the presence of non‐neoplastic lesions, staining patterns III‐V suggest the presence of intraepithelial neoplasia (IN) ± cancer. |
| Endoscopic targeted biopsies |
| Targeted biopsies should be taken of all mucosal alterations, particularly of circumscribed lesions with staining patterns including intraepithelial neoplasias and suspected carcinomas (crypt type III‐V) |
CLASSIFICATION OF SUPERFICIAL INTRAEPITHELIAL NEOPLASIA: A CONSENSUS WORKSHOP APPROACH
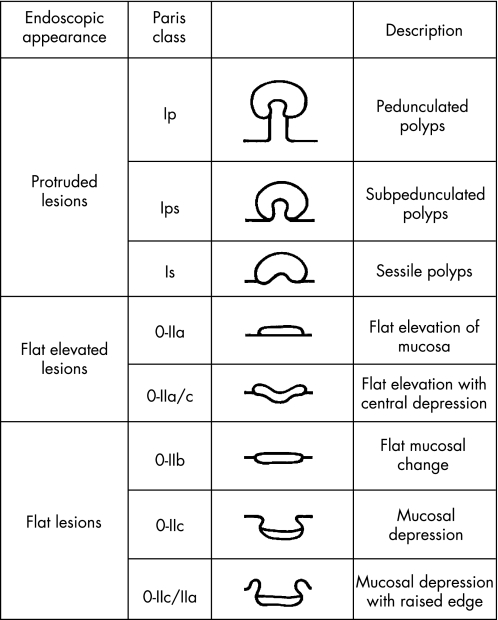
Detailed morphological assessment of a lesion at endoscopy is derived from both quantitative and qualitative criteria where initially size and anatomical location should be documented. The Paris workshop consensus guidelines now provide an endoscopic morphological reporting “framework” which reduces ambiguity in reporting45 (fig 6). Detailed morphological assessment (which is a key component to the SURFACE guidelines44) requires the localised application of a contrast chromoscopic agent, which in the colorectum is usually IC 0.1–0.5% solution or MB 0.1% locally applied to the lesion using the syringe push technique or via a trans‐portal diffusion catheter.46 Importantly, at this stage in morphological grading the macroscopic classification is made only from the gross appearance and should not be influenced by adjunctive clinical information or supplementary histopathological findings (that is, a lesion demonstrable of a type 0 morphology may subsequently be “up‐staged” to an advanced neoplasm at histopathology using the p‐TNM classification or indeed the reverse).45 Hence, in most Japanese studies, superficial lesions are classified according to subtypes of type 0 morphology that can be subgrouped into three distinct types (0‐I, polypoid/0‐II, non‐polypoid and non‐excavated/0‐III, non‐polypoid with a frank ulcer). Group I can again be segmentalised to include type 0‐Ip (pedunculated) and 0‐Is (sub‐pedunculated). Also, type 0‐II lesions include three distinct subgroups (0‐IIa, elevated/0‐IIb, completely flat with the mucosa/0‐IIc, slight depression without an ulcer crater). A depressed lesion with central depression is classified as a type 0‐IIc+IIa in contrast to a primary elevated lesion with a central depression at its apex (0‐IIa+IIc)—in the latter class the relative depression as a rule does not extend below the level of the adjacent normal mucosa. Such morphological differentiation, although complex, is clinically of utmost importance as type 0‐IIa+IIc lesions have a poor prognosis with an increased risk of deep submucosal invasion and hence LNM and associated lympho‐venous involvement and mucinous/poorly differentiated histopathological features47 (fig 5). Hence, detailed chromoscopic morphological assessment at index endoscopy is mandatory in guiding the most appropriate and safe endoluminal management complemented by HMCC crypt architecture analysis.
Figure 6 Paris workshop guidelines for the gross morphological classification of colorectal lesions.
PIT PATTERN CLASSIFICATION AND MAGNIFICATION CHROMOSCOPIC APPEARANCES
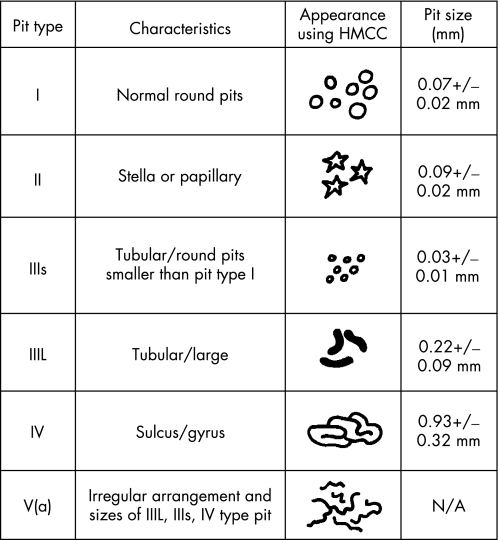
Establishment of the neoplastic characteristics, including the potential for deep submucosal invasion, using stereomicroscopy is a well‐established histopathological practice. Kosaka first reported the stereomicroscopic observation of the pit pattern in Paris class Ip/s lesions.48 Subsequently, Nishizawa reported stereomicroscopic findings of minute early superficial neoplasms describing the absence of glandular orifices or non‐structural pit patterns.49 Kudo later conducted stereomicroscopic observations in approximately 1600 lesions enabling the creation of the established pit classification, now used in Japanese and European colonoscopic practice. Kudo validated pit pattern comparison between stereomicroscopic chromoscopy and magnification video‐chromoscopy in 200150 followed by a large prospective UK analysis in 2004.51 These data demonstrated a high correlation rate between in vivo magnification chromoscopy as compared to ex vivo stereomicroscopy for “sporadic” colorectal lesions. Within the Kudo class five types of pit pattern are described according to macroscopic morphology and size (fig 7).50
Figure 7 The modified Kudo criteria for the classification of colorectal crypt architecture in vivo using high magnification chromoscopic colonoscopy. HMCC, high magnification chromoscopic colonoscopy.
INTRAEPITHELIAL NEOPLASIA DETECTION AND CHARACTERISATION IN CHRONIC ULCERATIVE COLITIS: CURRENT EVIDENCE BASED PRACTICE
As IN and colitis‐associated cancer can occur in macroscopically normal mucosa, random biopsies at 10 cm intervals throughout the colorectum are currently recommended during screening colonoscopy. Historically, the probability for detection of neoplastic change was thought to correlate with the numbers of biopsies taken. However, recent data suggests that pan‐chromoscopy using MB with HMCC targeting of biopsies can improve the detection of Paris class 0‐II and diminutive lesions in CUC when compared to conventional colonoscopic screening protocols alone. Kiesslich et al showed that chromoscopy permitted a more accurate diagnosis of extent and inflammatory activity in CUC but also enhanced the detection of IN and CRC in colitis (p = 0.0002 and p = 0.003), respectively.43 Within this randomised controlled study IN detection was increased more than threefold in the chromoendoscopy group when compared to patients undergoing “white light” video endoscopy and conventional biopsy protocols (32 vs. 10 INs, respectively). Hurlstone et al subsequently validated these data using a selective IC chromoscopic technique.41,52 In this prospective study, 162 patients with longstanding established pan‐colitis (⩾8 years) underwent colonoscopy‐using HMCC and was compared to a control group consisting of 162 disease, age and sex matched controls undergoing conventional screening colonoscopy. Targeted chromoscopy, rather than pan‐colorectal MB chromoendoscopy, was used after detection of subtle changes in mucosal architecture such as fold convergence, air‐induced deformation, innominate groove interruption or focal colour change before targeted intravital staining with IC and subsequent magnification imaging. In this study chromoendoscopy with magnification assistance increased the diagnostic yield for IN significantly compared to controls (more than fourfold). IN in flat mucosal change was observed in 37 lesions of which 31 only were detected using chromoendoscopy and HMCC.41
Rutter et al also demonstrated a strong trend towards statistically increased dysplasia detection following IC pan‐colorectal chromoscopy (p = 0.06), with a targeted biopsy protocol detecting dysplastic change in significantly more patients than a non‐targeted protocol (p = 0.02).53 Furthermore, no dysplasia was detected in 2904 non‐targeted biopsies in comparison to a targeted biopsy protocol utilising pan‐colonic IC chromoendoscopy.53 The latter protocol required fewer biopsies (157) yet detected nine dysplastic lesions, seven of which were only visible after IC application.53
The largest prospective dataset using HMCC and targeted biopsies has recently been published by the Sheffield group.41 In this series, a total of 350 patients with longstanding pan‐colitis (⩾8 years duration) underwent surveillance colonoscopy with quadrantic biopsies taken at 10 cm incremental extubation intervals (as per British Society of Gastroenterology protocols), but with the addition of targeted biopsies of abnormal mucosal areas (defined lesions were further evaluated using modified Kudo crypt pattern analysis at 100× magnification). These data were then compared to 350 disease duration and disease extent/matched controls who had undergone conventional colonoscopic surveillance. Importantly, more IN lesions were detected in the HMCC group as compared to controls (69 vs 24, p<0.0001). Furthermore, chromoscopy increased the number of Paris class 0‐II lesions detected with IN as compared to controls (p<0.001). Twenty IN lesions were detected from 12 950 biopsies using conventional colonoscopy (0.15%) with 49/622 (8%) in the HMCC targeted group, and from 12 482 biopsies taken in the control group, of which only 18 (0.14%) yielded IN. However, from the targeted biopsy group without HMCC imaging, the yield was modestly improved at 1.6% (6/369). Also, using modified Kudo criteria,50 the sensitivity and specificity was 93% and 88%, respectively, for differentiating neoplastic from non‐neoplastic lesions. Total procedure time was significantly longer in the magnification chromoscopic group as compared to controls (p<0.02). These data now suggest that chromoscopic colonoscopy is a valid tool for the detection and in vivo classification of IN in CUC. Chromoendoscopy has now been incorporated into the guidelines for CUC surveillance in the USA (Crohn's and Colitis Foundation of America Colon Cancer in IBD Study Group) and may now prompt changes to the current screening guidelines endorsed by the British Society of Gastroenterology that are labour intensive, time consuming and have a low diagnostic yield.
Key points 4
Chromoscopic pan‐chromoscopy can increase the diagnostic yield of IN between 3–4 fold
High magnification imaging can help characterise ALM and DALM in vivo
ENDOSCOPIC MANAGEMENT OF INTRAEPITHELIAL NEOPLASIA IN CHRONIC INFLAMMATORY BOWEL DISEASE
As previously indicated, the endoscopic and histopathological interpretation of colorectal dysplastic lesions complicating CUC has carried much controversy.4 The principle problem endoscopically is the reliable differentiation of sporadic ALM, DALM and identification of morphologically subtle flat dysplastic lesions (Paris 0‐II) that often demonstrate poor prognostic histopathological characteristics.54 Previous molecular and histopathological series have also been unable to reliably distinguish between these discrete entities that further adds to the clinician's difficulty when selecting patients at high risk of CRC requiring pan‐proctocolectomy.25 Previous studies have addressed the prevalence and “safety” of endoscopic polypectomy for exophytic adenoma‐like DALM in CUC. Rubin and colleagues36 performed simple polypectomy of 70 histopathologically confirmed dysplastic lesions in 48 patients with no demonstrable synchronous flat dysplasia. In this initial report there was no subsequent cancer or flat dysplastic change detected at a median follow‐up of 4.1 years, data similar to the CUC cohort of Engelsgjerd31 and Odze.55 Furthermore, data from Connel56 and Nugent's57 group who performed snare polypectomy of polypoid dysplastic lesions within a colitis zone revealed no emergence of flat dysplasia or adenocarcinoma at 2–13 years and 3–11 years (median 6) follow up, respectively.
Odez and colleagues' long‐term follow‐up series validate these data where no significant difference was reported in the prevalence of polyp formation or cancer (mean follow‐up 82.1 months (17–156), 71.8 months (7–135) and 60.4 months (29–100) for the ALM (sporadic adenoma CUC group) and sporadic adenoma non‐CUC control group, respectively.55 However, there are fundamental problems with all of these studies which require addressing, given recent advancements in endoscopic technology: previous exclusion of patients with flat dysplastic mucosal disease and carpet (lateral spreading) tumours; no routine marking of polypectomy sites making the subsequent interpretation of post‐resection recurrence and metachronous lesional rates difficult to interpret; no routine use of chromoscopic‐assisted endoscopy which has now been shown to benefit the characterisation and detection of IN in CUC41; and variable definitions of non‐CUC “sporadic” control groups. Prevalence data concerning Paris 0‐II lesions within CUC has also never been included in these cohort studies with patients referred for pan‐proctocolectomy, given the lack of supportive data to justify endoluminal resection and surveillance.36
Previous published data by our group reported the anatomical “mapping”, histopathological characteristics and efficacy of endoscopic mucosal resection (EMR) for Paris 0‐II (flat) and Is (protuberant) lesions in a population assuming a “high” overall lifetime risk of colorectal neoplasia (entry criteria being previous HGD adenoma and colorectal neoplasia within the past 5 years).58 The Sheffield group have recently reported their experience of endoscopic IN management in CUC.40 The primary end points of this study were “safety” and clinical outcomes of CUC patients undergoing EMR for Paris class 0‐II and class 0‐I ALM as compared to sporadic controls using pan colonic IC chromoscopy and HMCC imaging for lesional characterisation. These data showed no significance was reached when comparing the histopathological characteristics (that is, prevalence of LGD/HGD/invasive neoplasia) and anatomical localisation of Paris 0‐II lesions between the CUC study group and non‐CUC controls. There was, however, a significant increase in Paris 0‐II lesion prevalence in the CUC group as compared to controls (82/155 (61%) vs 285/801 (35%)) (p<0.001). These data most likely reflect the chromoscopic technique used between these study cohorts (pan‐chromoscopy vs targeted), where previous randomised controlled data both from our group59 and Brooker et al60 have shown a significant increase in Paris 0‐II lesional detection when employing pan‐colorectal mucosal chromoscopy. Furthermore, of the 203 lesions detected throughout the duration of this study 167 (82%) were detected at index screening colonoscopy with 36/203 (18%) detected by subsequent surveillance. These data are almost inverse to the post‐index metachronous data reported by Rubin and colleagues36, with 71% of polyps being detected after a negative initial colonoscopy, but also represent a higher index lesional yield than those reported by Engelsgjerd's31 and Odze's55 group (42% and 58%, respectively). Even when assuming a colonoscopic lesion “miss rate” of 25–30% per procedure, according to the “back to back” data of Rex61 and Hixon,62 these higher index lesional frequency data and low metachronous rates at follow‐up most likely reflect the augmented chromoscopic technique used and importantly the removal of false positive “metachronous” rates, given the mandatory tattoo marking of lesions undergoing endoluminal resection—an important limitation of all previous studies.
Endoscopic mucosal resection in the context of Paris 0‐II colorectal lesions has not been described in the context of CUC before this study. Furthermore, these data showed no significant difference between patients undergoing EMR in the CUC group as compared to controls, with the primary end points being post‐resection recurrence, and complications (that is, procedure related haemorrhage/perforation). Recurrence rates in the CUC group were also lower than those described by other groups63,64 which most likely reflects the routine use of post EMR margin assessment65 using HMCC with combination post‐resection argon plasma coagulation margin ablation for those receiving piecemeal dissections.66
In summary, these data have shown for the first time that flat dysplastic lesions in the context of CUC can be managed by endoluminal resection using EMR, as in non‐colitis cohorts, and may potentially avoid the need for pan‐proctocolectomy in a selected group of patients. Furthermore, this study demonstrates how the management paradigm of IN complicating CUC can be fundamentally changed by employing new endoscopic technologies and endoluminal resection techniques.
THE FUTURE OF IMAGING IN CHRONIC ULCERATIVE COLITIS‐ASSOCIATED INTRAEPITHELIAL NEOPLASIA: CELLULAR RESOLUTION CONFOCAL ENDOMICROSCOPIC COLONOSCOPY
Recently, miniaturisation of a novel confocal laser endomicroscope (CLE) (Optiscan Pty, Notting Hill, Victoria, Australia) has permitted functional integration into the distal tip of a conventional video colonoscope (Pentax EC3870K, Pentax, Tokyo) enabling in vivo surface and subsurface (z‐axis) cellular resolution imaging during ongoing video endoscopy.67 Using CLE and intravenous sodium fluorescein as a peripheral contrast agent, the Mainz group reported cellular surface histological correlates between mucin‐containing goblet cells and columnar epithelial cells within the surface epithelial layer, in addition to contrast enhancement within the lamina matrix and microvascular net.67 Combination CLE imaging according to z‐axis subsurface structure and superficial crypt architecture could be graded into three discrete CLE groups, each predictive of IN in vivo67 (table 2). Using a paired proportional model, histologically verified IN ± invasive cancer was represented with an overall accuracy of 99.2% using Mainz CLE grading, data now validated by the Sheffield group.
Table 2 Mainz confocal endomicroscopy criteria for the prediction of intraepithelial colorectal neoplasia.
| Confocal endomicroscopy criteria | |||
|---|---|---|---|
| Normal | Regenerative | Neoplastic | |
| Vascular architecture | Hexagonal network delineating peri‐luminal crypt stroma | Hexagonal network with no or mild increase in capillary density | Distorted/dilated vascular architecture with elevated leakage. Irregular architecture with little/poor orientation to adjunct tissue |
| Crypt architecture | Regular luminal crypt openings/distribution. Goblet cells visualised at normal density | Star‐shaped luminal crypt openings ± focal aggregates of regular shaped crypts ± goblet cell depletion | Ridge‐lined irregular epithelial layer. Crypt/goblet cell attenuation. Irregular cell architecture with mucin depletion. |
Using combined MB pan‐chromoscopy to “unmask” circumscribed lesions according to SURFACE guidelines with targeted biopsies of lesions characterised using confocal laser scanning endomicroscopy at ongoing video colonoscopy, the Mainz group have recently reported their experience of IN detection using this novel technology in CUC cancer screening.68 In patients randomised to receive CLE imaging, circumscribed lesions detected by chromoendoscopy were further characterised using endomicroscopy, with particular reference to cellular and vascular changes (using peripheral intravenous fluoroscene coupling), and classified according to the established Mainz confocal pattern classification to predict IN, with targeted biopsies graded according to modified Vienna criteria. In the conventional surveillance “arm” random biopsies at 10 cm intervals with additional targeted biopsies of visible mucosal change using “white light” imaging only were performed. When considering the primary outcome analysis of histologically confirmed IN, chromoscopic endomicroscopy significantly improved the yield of IN as compared to controls (p<0.007) and was able to resolve discrete cellular structure in vivo.
Furthermore, the presence of neoplastic change in this study could be predicted with a high overall accuracy (sensitivity 94.7%, specificity 98.3%, accuracy 97.8%) and significantly reduced the number of biopsy specimens per patient without any decrease in yield of IN (21.2 biopsies/patient vs 42.2 biopsies/patient, p<0.05). Indeed, if only circumscribed lesions would have been biopsied using SURFACE guidelines and CLE targeting, the total number of biopsies could have been limited to only 3.9/patient without reducing the overall yield of IN; however, theoretically CLE could further decrease five times the number of biopsies required to diagnose IN if only “suspicious” in vivo mucosal architecture was biopsied (0.78 biopsies/patient).68 Clinical examples are shown in figs 8–10.
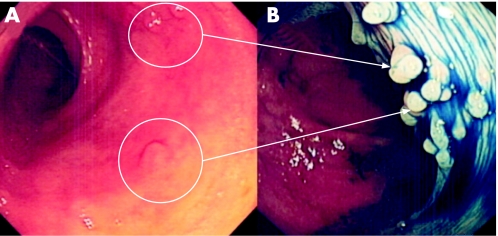
Figure 8 (A) Conventional “white light” views of the distal sigmoid colon in a patient with longstanding pan‐colitis. Note the focal mucosal pallor and nodularity of the mucosa. (B) Chromoscopy using 0.5% indigo carmine shows multiple sessile (Paris 0‐Is) lesions. The endoscopic diagnosis is most likely to be inflammatory polyps. Endoluminal resection is therefore not indicated. Further characterisation using high magnification imaging or confocal endomicroscopy would be beneficial.
Figure 9 (A) Superficial crypt architecture of the suspected inflammatory lesion shown in fig 8 using the Pentax confocal endomicroscope and intravenous fluoroscene. The crypt architecture at 10 μm z‐axis penetration is organised but with crypt elongation. There is no goblet cell depletion indicating an inflammatory aetiology. (B) Confocal laser scanning imaging at 100 μm in the z‐axis. The crypts are well aligned in a concentric architecture again indicating a non‐neoplastic nature. (C) Deep 250 μm laser scanning images shows a hexagonal per‐cryptic vascular net pattern with no extravasation of fluoroscene. The “black dots” represent individual red cells within the per‐cryptic capillary. This vascular pattern according to Mainz criteria is suggestive of hyperplasia or normal colonic mucosa.
Figure 10 (A) Conventional “white light” views of the distal descending colon in a patient with longstanding pan‐colitis. There is focal erythema suggestive of a potential lesion. (B) Indigo carmine 0.5% chromoscopy reveals a well circumscribed flat elevated lesion with central depression (Paris class 0‐IIa+IIc). Detailed morphological assessment of this lesion indicates a high probability of submucosally invasive carcinoma. (C) A repeat colonoscopy using the confocal laser scanning endomicroscope shows a dilated and tortuous vascular net pattern at 60–80 μm in the z‐axis with complete destruction of the normal cryptic architecture. The lesion fulfils neoplastic criteria using Mainz the confocal imaging classification. Subsequent proctocolectomy was performed which revealed a stage T2/N1 well differentiated adenocarcinoma with associated lympho‐venous infiltration.
ENDOSCOPIC TECHNOLOGIES IN DEVELOPMENT WITH APPLICABILITY TO INTRAEPITHELIAL NEOPLASIA DETECTION IN CHRONIC ULCERATIVE COLITIS CANCER SCREENING
Autofluorescence colonoscopy
Chromoscopy, high magnification imaging, high frequency endoscopic ultrasound and confocal endomicroscopy are techniques that have been introduced in an attempt to improve the sensitivity and specificity of “white light” colonoscopy for IN detection and characterisation. However, a new endoscopic technology that is fluorescence‐based is under evaluation by many groups.69,70 This technology exploits either the “autofluorescence” of naturally occurring molecules such as collagen, NADH, flavins and porphyrins or fluorescence caused by exogenously administered fluorescent drugs.
The detection of neoplastic lesions using autofluorescence depends on subtle changes in the concentration or distribution in depth of endogenous fluorophores, changes in tissue micro‐architecture, and altered mucosal thickness or blood concentrations.71 All these factors affect the fluorescence intensity or spectrum due to wavelength‐specific light absorption.71 Using exogenous fluorophores, the detection of lesions is based on selective drug uptake or target tissue retention relative to uptake by normal tissue.71
Kapadia et al, using ultraviolet (UV) light in the colon, were able to discriminate normal mucosa, adenomas and hyperplastic lesions with accuracies of 100%, 100% and 94%, respectively.70 In the first in vivo human colonic spectroscopic study by Cothren et al, differentiation between adenoma and non‐adenomatous lesions was achieved in 97% of cases.72 Cotheran et al in the first blinded study also identified colonic dysplasia with 90% sensitivity and 95% specificity.73
However, the clinical potential of spectroscopy in routine endoscopic practice and as applicable to CRC screening in CUC has yet to be clarified. Chwirot et al performed multi‐spectral autofluorescence imaging in the colon of polyps ex vivo,74 with Wang recently describing a prototype fluorescence imaging system applied ex vivo to detect and localise adenomas in colectomy specimens from patients with familial adenomatous polyposis (FAP).75 A second generation fluorescence imaging system was described by Wang in 1999 for the in vivo detection of colorectal adenomas in which UV light was transmitted through a fibreoptic bundle inserted through the side port of the endoscope.76 A videocolonoscope was then used to capture the fluorescence light within the wavelength band 400–700 nm.76 False‐colour images were then overlaid onto the conventional white light images, displaying regions where the fluorescence intensity exceeded threshold values relative to a moving image.76 In this study of 30 patients, 18 lesions visible with white light imaging were examined where all six hyperplastic lesions were correctly identified with sensitivity for dysplasia reported as 83%.76 However, despite these encouraging initial data, further studies are required before such technology can be implemented to CUC endoscopic screening.
Key references
Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta‐analysis. Gut 2001;48:526–35.
Lynch DA, Lobo AJ, Sobala GM, et al. Failure of colonoscopic surveillance in ulcerative colitis. Gut 1993;34:1075–80.
Kiesslich R, Fritsch J, Holtmann M, et al. Methylene blue‐aided chromoendoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology 2003;124:880–8.
Hurlstone DP, Sanders DS, Hunter MD, et al. Endoscopic mucosal resection for flat neoplasia in chronic ulcerative colitis: can we change the endoscopic management paradigm? Gut 2006;56:838–46.
Kiesslich R, Goetz M, Lammersdorf K. Chromoscopy‐guided endomicroscopy increases the diagnostic yield of intraepithelial neoplasia in ulcerative colitis with reduced number of biopsies. Gastroenterology 2007;132:874–82.
CONCLUSIONS
The data presented in this review have summarised the key studies addressing novel techniques for targeted IN and CRC screening in CUC, while also addressing the clinical and technological shortfalls of currently operative endoscopic screening policies. However, there are many issues to address before the “routine” implementation of these new technologies into current endoscopic practice, such as learning curve characteristics, advanced level endoscopic interpretation and histopathological training—a mandatory requirement for confocal endomicroscopic colonoscopy. Furthermore, the majority of published data suggests an overall expansion to procedure times which raises important economic issues, given the current pressures experienced by many centres regarding optimising list scheduling and limited endoscopic and histopathological manpower. However, given the current level of evidence, chromoendoscopy to target IN during colonoscopy with or without further characterisation using endomicroscopy, magnification imaging or autofluorescence now represents the new “gold standard” surveillance practice. All of these technologies, either used as single modalities or as complementary duel modality imaging, aim to optimise early cancer and IN detection in CUC which is the cornerstone of surveillance management. Accurate in vivo assessment of dysplasia in this “at risk” CRC cohort can only serve to improve prognosis and optimise the cost‐effectiveness and long‐term sustainability of endoscopic surveillance in CUC.
MULTIPLE CHOICE QUESTIONS (TRUE (T)/FALSE (F); ANSWERS AFTER THE REFERENCES)
1. The increased risk of patients with longstanding ulcerative colitis developing dysplasia and colorectal cancer is:
4% at 20 years
10% at 30 years
18% at 30 years
No significant increase as compared to an age/sex matched individual
2. Conventional “white light” surveillance colonoscopy for the detection of dysplasia in chronic ulcerative colitis screening (according to British Society of Gastroenterology (BSG) guidelines) requires:
Quadrantic biopsies every 10 cm throughout the colo‐rectum including targeted biopsies to any “suspicious” mucosal change
Quadrantic biopsies every 10 cm throughout the colo‐rectum only
No biopsies are normally required
Targeted biopsies to actively inflamed areas only
3. IBD‐associated dysplasia only occurs in:
Strictured areas
Flat mucosal change
Protruberant lesions
Can occur in any of the above mucosal change
4. Chromoscopic colonoscopy (use of dye spray) as part of colorectal cancer endoscopic screening in chronic ulcerative colitis:
Improves the detection of intraepithelial neoplastic lesions 3–4 fold as compared to conventional BSG protocols
Cannot provide any useful information regarding inflammatory activity and extent of disease
SURFACE guidelines are not applicable when performing this procedure
Should only be performed when there is active inflammation in the colorectum
5. Adenoma‐like mass in chronic ulcerative colitis:
Is an indication for urgent colectomy
Can be treated by endoscopic resection in all cases
Should only be treated by endoluminal resection if there is no adjacent flat mucosal dysplasia
Is the same pathological entity as a dysplasia‐associated lesion or mass
Abbreviations
ALM - adenoma‐like mass
CLE - confocal laser endomicroscope
CRC - colorectal cancer
CUC - chronic ulcerative colitis
DALM - dysplasia‐associated lesion or mass
FAP - familial adenomatous polyposis
GI - gastrointestinal
HGD - high grade dysplasia
HMCC - high magnification chromoscopic colonoscopy, LGD, low grade dysplasia
IBD - inflammatory bowel disease
IC - indigo carmine
IN - intraepithelial neoplasia
MB - methylene blue
PSC - primary sclerosing cholangitis
UV - ultraviolet
ANSWERS
(A) F (B) F (C) T (D) F
(A) T (B) F (C) F (D) F
(A) F (B) F (C) F (D) T
(A) T (B) F (C) F (D) F
(A) F (B) F (C) T (D) F
Footnotes
Competing interests: None stated
References
- 1.Crohn B, Rosenberg H. The sigmoidoscopic picture of chronic ulcerative colitis (non‐specific). Am J Med Sci 1925170220–228. [Google Scholar]
- 2.Eaden J A, Abrams K R, Mayberry J F. The risk of colorectal cancer in ulcerative colitis: a meta‐analysis. Gut 200148526–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lennard‐Jones J E, Melville D M, Morson B C.et al Precancer and cancer in extensive ulcerative colitis: findings among 401 patients over 22 years. Gut 199081800–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blackstone M O, Riddell R H, Rogers B H.et al Dysplasia‐associated lesion or mass (DALM) detected by colonoscopy in long‐standing ulcerative colitis: an indication for colectomy. Gastroenterology 198180366–374. [PubMed] [Google Scholar]
- 5.Woolrich A J, DaSilva M D, Korelitz B I. Surveillance in the routine management of ulcerative colitis: The predictive value of low grade dysplasia. Gastroenterology 1992103431–438. [DOI] [PubMed] [Google Scholar]
- 6.Fuson J A, Farmer R G, Hawk W A.et al Endoscopic surveillance for cancer in chronic ulcerative colitis. Am J Gastroenterol 198073120–126. [PubMed] [Google Scholar]
- 7.Hendriksen C, Kreiner S, Binder V. Long term prognosis in ulcerative colitis–based on results from a regional patient group from the county of Copenhagen. Gut 198526158–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lashner B A, Silverstein M D, Hanauer S B. Hazard rates for dysplasia and cancer in ulcerative colitis. Results from a surveillance program. Dig Dis Sci 1989341536–1541. [DOI] [PubMed] [Google Scholar]
- 9.Leidenius M, Kellokumpu I, Husa A.et al Dysplasia and carcinoma in longstanding ulcerative colitis: An endoscopic and histological surveillance programme. Gut 1991321521–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lofberg R, Brostrom O, Karlen P.et al Colonoscopic surveillance in long‐standing total ulcerative colitis‐A 15 year follow‐up study. Gastroenterology 1991991021–1031. [DOI] [PubMed] [Google Scholar]
- 11.Lynch D A, Lobo A J, Sobala G M.et al Failure of colonoscopic surveillance in ulcerative colitis. Gut 1993341075–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nugent F W, Haggitt R C, Gilpin P A. Cancer surveillance in ulcerative colitis. Gastroenterology 19911001241–1248. [PubMed] [Google Scholar]
- 13.Gyde S N, Prior P, Allan R N.et al Colorectal cancer in ulcerative colitis: A cohort study of primary referrals from three centres. Gut 198829206–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brostrom M O, Lofberg R, Ost A. Cancer surveillance of patients with longstanding ulcerative colitis: A clinical and endoscopical and histological study. Gut 1986271408–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins R H, Feldman M, Fordtran J S. Colon cancer, dysplasia and surveillance in patients with ulcerative colitis: a critical review. N Engl J Med 19873161654–1658. [DOI] [PubMed] [Google Scholar]
- 16.Goldmann H. Significance and detection of dysplasia in chronic colitis. Cancer 1996782261–2263. [DOI] [PubMed] [Google Scholar]
- 17.Riddell R H, Goldman H, Ransohoff D F.et al Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical implications. Hum Pathol 198314931–968. [DOI] [PubMed] [Google Scholar]
- 18.Shelton A A, Lehman R E, Schrosck T R.et al Retrospective review of colorectal cancer in ulcerative colitis at a tertiary centre. Arch Surg 1996131806–810. [DOI] [PubMed] [Google Scholar]
- 19.Eaden J A, Mayberry J F. Colorectal cancer complicating ulcerative colitis: a review. Am J Gastroenterol 2000952710–2719. [DOI] [PubMed] [Google Scholar]
- 20.Ekbom A, Helmick C, Zack M.et al Ulcerative colitis and colorectal cancer. A population‐based study. N Engl J Med 19903231228–1233. [DOI] [PubMed] [Google Scholar]
- 21.Rutegard J, Ahsgren L, Stenling R.et al Cancer surveillance in an unselected population. Scand J Gastroenterology 198823139–145. [DOI] [PubMed] [Google Scholar]
- 22.Gyde S. Screening for colorectal cancer in ulcerative colitis: dubious benefits and high costs. Gut 1990311089–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Axon A T R. Cancer surveillance in ulcerative colitis – a time for reappraisal. Gut 199435587–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi P M. Colonoscopic surveillance reduces mortality from colorectal cancer in ulcerative colitis. Gastroenterology 1993105418–424. [DOI] [PubMed] [Google Scholar]
- 25.Connell W R, Talbot I C, Harpaz N.et al Clinicopathological characteristics of colorectal carcinoma complicating ulcerative colitis. Gut 1994351419–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karlen P, Kornfeld D, Brostrom O.et al Is colonoscopic surveillance reducing colorectal cancer mortality in ulcerative colitis? Gut 199842711–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koobatian G J, Choi P M. Safety of surveillance colonoscopy in long‐standing ulcerative colitis. Am J Gastroenterol 1994891472–1475. [PubMed] [Google Scholar]
- 28.Ransohoff D F, Riddell R H, Levin B. Ulcerative colitis and colonic cancer: problems in assessing the diagnostic usefulness of mucosal dysplasia. Dis Colon Rectum 198528383–388. [DOI] [PubMed] [Google Scholar]
- 29.Bernstein C N. ALMs versus DALMs in ulcerative colitis: polypectomy or colectomy? Gastroenterology 19991171488–1492. [DOI] [PubMed] [Google Scholar]
- 30.Schlemper R J, Riddell R H, Kato Y.et al The Vienna classification of gastrointestinal neoplasia. Gut 200047251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engelsgjerd M, Farraye F A, Odze R D. Polypectomy may be adequate treatment for adenoma‐like dysplastic lesions in chronic ulcerative colitis. Gastroenterology 19991171288–1294. [DOI] [PubMed] [Google Scholar]
- 32.Fogt F, Urbanski S J, Sanders M E.et al Distinction between dysplasia‐associated lesion or mass (DALM) and adenoma in patinets with ulcerative colitis. Hum Pathol 200031288–291. [DOI] [PubMed] [Google Scholar]
- 33.Tytgat G N, Dhir V, Gopinath N. Endoscopic appearances of dysplasia and cancer in inflammatory bowel disease. Eur J Cancer 1995311174–1177. [DOI] [PubMed] [Google Scholar]
- 34.Bernstein C N, Shanahan F, Weinstein W M. Are we telling the truth about surveillance colonoscopy in ulcerative colitis? Lancet 199434371–74. [DOI] [PubMed] [Google Scholar]
- 35.Bernstein C N, Weinstein W M, Levine D S.et al Physicians' perceptions of dysplasia and approaches to surveillance colonoscopy in ulcerative colitis. Am J Gastroenterol 1995902106–2114. [PubMed] [Google Scholar]
- 36.Rubin P H, Friedman S, Harpaz N.et al Colonoscopic polypectomy in chronic colitis: conservative management after endoscopic resection of dysplastic polyps. Gastroenterology 19991171295–1300. [DOI] [PubMed] [Google Scholar]
- 37.Odze R D, Brown C A, Hartmann C J.et al Genetic alterations in chronic ulcerative colitis‐associated adenoma‐like DALMS are similar to non‐colitic sporadic adenomas. Am J Surg Pathgol 2000241209–1216. [DOI] [PubMed] [Google Scholar]
- 38.Rubin C E, Haggit R C, Burmer G C.et al DNA aneuploidy in colonic biopsies predicts future development of dysplasia in ulcerative colitis. Gastroenterology 19921031611–1620. [DOI] [PubMed] [Google Scholar]
- 39.Dixon M F, Brown L J R, Gilmour H M.et al Observer variation in the assessment of dysplasia in ulcerative colitis. Histopathology 198813385–397. [DOI] [PubMed] [Google Scholar]
- 40.Hurlstone D P, Sanders D S, Hunter M D.et al Endoscopic mucosal resection for flat neoplasia in chronic ulcerative colitis: can we change the endoscopic management paradigm? Gut 200656838–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hurlstone D P, Sanders D S, Lobo A J.et al Indigo carmine‐assisted high‐magnification chromoscopic colonoscopy for the detection and characterisation of intraepithelial neoplasia in ulcerative colitis: a prospective evaluation. Endoscopy 2005371186–1192. [DOI] [PubMed] [Google Scholar]
- 42.Hurlstone D P, McAlindon M E, Sanders D S.et al Further validation of high‐magnification chromoscopic‐colonoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology 2004126376–378. [DOI] [PubMed] [Google Scholar]
- 43.Kiesslich R, Fritsch J, Holtmann M.et al Methylene blue‐aided chromoendoscopy for the detection of intraepitheial neoplasia and colon cancer in ulcerative colitis. Gastroenterology 2003124880–888. [DOI] [PubMed] [Google Scholar]
- 44.Kiesslich R, Hoffman A, Neurath M F. Colonoscopy, tumors, and inflammatory bowel disease ‐ new diagnostic methods. Endoscopy 2006385–10. [DOI] [PubMed] [Google Scholar]
- 45.Paris Workshop Participants The Paris endoscopic classification of superficial neoplastic lesions: Esophagus, stomach and colon. Gastrointest Endosc 200258S3–43. [DOI] [PubMed] [Google Scholar]
- 46.Hurlstone D P, Fujii T. Practical uses of chromoendoscopy and magnification at colonoscopy. Gastrointest Endosc Clin N Am 200515687–702. [DOI] [PubMed] [Google Scholar]
- 47.Hurlstone D P, Cross S S, Adam I.et al Endoscopic morphological anticipation of submucosal invasion in flat and depressed colorectal lesions: clinical implications and subtype analysis of the Kudo type V pit pattern using high‐magnification‐chromoscopic colonoscopy. Colorectal Disease 20046369–375. [DOI] [PubMed] [Google Scholar]
- 48.Kosaka T. Clinico‐pathological study of the minute elevated lesion of the colorectal mucosa. Journal of the Japanese Society of Coloproctology 197528218–226. [Google Scholar]
- 49.Nishizawa Mea Pertaining to histopathogenesis, growth and progression of early cancer of the colon and rectum in terms of dissecting microscopy and clinical aspects. Stomach and Intestine 1985201036–1041. [Google Scholar]
- 50.Kudo S, Rubio C A, Teixeira C R.et al Pit pattern in colorectal neoplasia: endoscopic magnifying view. Endoscopy 200133367–373. [DOI] [PubMed] [Google Scholar]
- 51.Hurlstone D P, Cross S S, Adam I.et al Efficacy of high magnification chromoscopic colonoscopy for the diagnosis of neoplasia in flat and depressed lesions of the colorectum: a prospective analysis. Gut 200453284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hurlstone D P, Cross S S. Role of aberrant crypt foci detected using high‐magnification chromoscopic colonoscopy in human colorectal carcinogenesis. J Gastroenterol Hepatol 200520173–191. [DOI] [PubMed] [Google Scholar]
- 53.Rutter M D, Saunders B P, Schofield G.et al Pancolonic indigo carmine dye spraying for the detection of dysplasia in ulcerative colitis. Gut 200453256–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hurlstone D P, Fujii T, Lobo A J. Early detection of colorectal cancer using high‐magnification chromoscopic colonoscopy. Br J Surg 200289272–282. [DOI] [PubMed] [Google Scholar]
- 55.Odze R D, Farraye F A, Hecht J L.et al Long‐trem follow‐up after polypectomy treatment for adenoma‐like dysplastic lesions in ulcerative colitis. Clin Gastroenterol Hepatol 20042534–541. [DOI] [PubMed] [Google Scholar]
- 56.Connell W R, Lennard‐Jones J E, Williams C B.et al Factors affecting the outcome of endoscopic surveillance for cancer in ulcerative colitis. Gastroenterology 1994107934–944. [DOI] [PubMed] [Google Scholar]
- 57.Negent F W, Haggitt R C, Gilpin P A. Cancer surveillance in ulcerative colitis. Gastroenterology 19911001241–1248. [PubMed] [Google Scholar]
- 58.Hurlstone D P, Cross S S, Adam I.et al A prospective clinicopathological and endoscopic evaluation of flat and depressed colorectal lesions in the UK. Am J Gastroenterol 2003982543–2549. [DOI] [PubMed] [Google Scholar]
- 59.Hurlstone D P, Cross S S, Slater R.et al Detecting diminutive colorectal lesions at colonoscopy: a randomised controlled trial of pan‐colonic versus targeted chromoscopy. Gut 200453376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brooker J C, Saunders B P, Shah S G.et al Total colonic dye‐spray increases the detection of diminutive adenomas during routine colonoscopy: a randomized controlled trial. Gastrointest Endosc 200256333–338. [DOI] [PubMed] [Google Scholar]
- 61.Rex D K, Cutler C S, Lemmel G T.et al Colonoscopic miss rates of adenomas determined by back‐to‐back colonoscopies. Gastroenterology 199711224–28. [DOI] [PubMed] [Google Scholar]
- 62.Hixson L J, Fennerty M B, Sampliner R E.et al Prospective study of the frequency and size distribution of polyps missed by colonoscopy. J Natl Cancer Inst 1990821769–1772. [DOI] [PubMed] [Google Scholar]
- 63.Brooker J C, Saunders B P, Shah S G.et al Treatment with argon plasma coagulation reduces recurrence after piecmeal resection of large sessile colonic polyps: a randomised trial and recommendations. Gastrointest Endosc 200255371–375. [DOI] [PubMed] [Google Scholar]
- 64.Ahmad N A, Kochman M L, Long W B.et al Efficacy, safety, and clinical outcomes of endoscopic mucosal resection: a study of 101 cases. Gastrointest Endosc 200255390–396. [DOI] [PubMed] [Google Scholar]
- 65.Hurlstone D P, Cross S S, Brown S.et al A prospective evaluation of high‐magnification chromoscopic colonoscopy in predicting completeness of EMR. Gastrointest Endosc 200459642–650. [DOI] [PubMed] [Google Scholar]
- 66.Hurlstone D P, Sanders D S, Cross S S.et al Colonoscopic resection of lateral spreading tumours: a prospective analysis of endoscopic mucosal resection. Gut 2004531334–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kiesslich R, Burg J, Vieth M.et al Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology 2004127706–713. [DOI] [PubMed] [Google Scholar]
- 68.Kiesslich R, Goetz M, Lammersdorf K. Chromoscopy guided endomicroscopy increases the diagnostic yield of intraepithelial neoplasia in ulcerative colitis with reduced number of biopsies. Gastroenterology 2007132874–882. [DOI] [PubMed] [Google Scholar]
- 69.Wagnieres G A, Star W M, Wilson B C. In vivo fluorescence spectroscopy and imaging for oncological applications. Photochem Photobiol 199868603–632. [PubMed] [Google Scholar]
- 70.Kapadia C R, Cutruzzola F W, O'Brien K M.et al Laser‐induced fluorescence spectroscopy of human colonic mucosa. Detection of adenomatous transformation. Gastroenterology 199099150–157. [DOI] [PubMed] [Google Scholar]
- 71.Haringsma J, Tytgat G N J, Yano H.et al Autofluorescence endoscopy: feasibility of detection of GI neoplasms unapparent to white light endoscopy with an evolving technology. Gastrointest Endosc 200153642–650. [DOI] [PubMed] [Google Scholar]
- 72.Cothren R M, Richards‐Kortum R, Sivak M V.et al Gastrointestinal tissue diagnosis by laser‐induced fluorescence spectroscopy at endoscopy. Gastrointest Endosc 199036105–111. [DOI] [PubMed] [Google Scholar]
- 73.Cothren R M, Sivak M V, Van Dam J.et al Detection of dysplasia at colonoscopy using laser‐induced fluorescence: a blinded study. Gastrointest Endosc 199644168–176. [DOI] [PubMed] [Google Scholar]
- 74.Chwirot B W, Kowalska M, Sypniewska N.et al Spectrally resolved fluorescence imaging of colonic adenomas. J Photochem Photobiol 199950174–183. [DOI] [PubMed] [Google Scholar]
- 75.Wang T D, Van Dam J, Crawford J M.et al Fluorescence endoscopic imaging of human colonic adenomas. Gastroenterology 19961111182–1191. [DOI] [PubMed] [Google Scholar]
- 76.Wang T D, Crawford J M, Feld M S.et al In vivo identification of colonic dysplasia using fluorescence endoscopic imaging. Gastrointest Endosc 199949447–455. [DOI] [PubMed] [Google Scholar]