Abstract
Objectives
Appropriate assessment of community‐acquired pneumonia (CAP) allows accurate severity scoring and hence optimal management, leading to reduced morbidity and mortality. British Thoracic Society (BTS) guidelines provide an appropriate score. Adherence to BTS guidelines was assessed in our medical assessment unit (MAU) in 2001/2 and again in 2005/6, 3 years after introducing an educational programme.
Methods
A retrospective case‐note study, comparing diagnosis, documentation of severity, management and outcome of CAP during admission to MAU during 3 months of each winter in 2001/2 and 2005/6.
Results
In 2001/2, 65/165 patients were wrongly coded as CAP and 100 were included in the study. In 2005/6 43/130 were excluded and 87 enrolled. In 2005/6, 87% did not receive a severity score, a significant increase from 48% in 2001/2 (p<0.0001). Parenteral antibiotics were given to 79% of patients in 2001/2 and 77% in 2005/6, and third generation cephalosporins were given to 63% in 2001/2 and 54% in 2005/6 (p = NS). In 2001, 15 different antibiotic regimens were prescribed, increasing to 19 in 2005/6.
Conclusions
Coding remains poor. Adherence to CAP management guidelines was poor and has significantly worsened. Educational programmes, alone, do not improve adherence. Restriction of antibiotic prescribing should be considered.
Community‐acquired pneumonia (CAP) accounted for almost 100 000 (2%) of acute hospital admissions in the UK in the financial year 2004/5,1,2 and is associated with significant morbidity, mortality and expenditure.3 The appropriate assessment of patients with CAP allows accurate classification of severity of disease and optimal management.4 Early identification of severity significantly improves prognosis. Furthermore, CAP patients can avoid unnecessary admission and inappropriate antibiotic prescribing.5
National guidelines for the assessment of severity of CAP and its management have been produced in many countries. In the UK, the British Thoracic Society (BTS) initially described guidelines for the management of CAP in 19936 and updated these in 20013 and again in 2004,7 with particular reference to severity scoring. Lim et al validated a prognostic score for mortality in CAP patients in 2003.8 This included the previous “CURB” score of Confusion, raised Urea, increased Respiratory rate and hypotension (BP) to which they added age over 65 to produce the CURB‐65 score. Scoring of 0 or 1 for each of the 5 points produced a prognostic index of outcome with a score of 0 suggesting a 30 day mortality risk of 0.7% and a score of 5 predicting a 57% mortality risk. Severe CAP was classified as a score of ⩾3. This score is simpler to use and more clinically useful than the more complex scoring system proposed by the Infectious Diseases Society of America.9,10,11 Implementation and maintained adherence to these guidelines is necessary to realise the benefits in morbidity, mortality and cost reduction.12,13
Historically, adherence to guidelines has been poor, resulting in inappropriate management which may affect both morbidity and mortality.14,15 Misuse or over‐use of antibiotics can result in antibiotic‐associated diarrhoea or colonisation with antibiotic resistant organisms, increased hospital stay and increased costs.16,17,18
In 2001/2, a retrospective study showed that adherence to the guidelines was poor in the acute medical assessment unit (MAU) of the Royal Liverpool University Hospital.19 We present the further findings of 2005/6 alongside these, after the introduction of an educational programme.
AIMS AND METHODS
The primary aim of the study is to compare adherence to BTS CAP guidelines before and after an educational programme. Secondary observations included patient outcome, which antibiotics or combinations of antibiotics were used, and the route of antibiotic delivery.
A retrospective study of all patients with an ICD‐10 discharge diagnosis code of pneumonia over the winters of 2001/2 and 2005/6 was undertaken. Winter was defined as November, December and January. Case‐notes for all these patients were retrieved and reviewed by two of us (PC and MB), extracting details of demography, severity scoring of CAP and antibiotic prescriptions on a standard proforma. This information was taken from the initial patient clerking and documented drug prescriptions that had been performed by the admitting physician (usually the pre‐registration house officer or senior house officer, less commonly the medical registrar).
In accordance with prevailing BTS guidelines, severity was assessed in 2001/2 using a CURB score as “core” and the presence of hypoxaemia and/or bilateral or multilobar involvement on chest x ray as “additional” adverse prognostic features, along with assessment of pre‐existing disease or age >50 years. In 2005/6, the simpler CURB‐65 score was used as a one step assessment. Appropriate assessment of severity was assumed if either a CURB‐65, or equivalent in 2001/2, score was recorded, or if the various parts of the score were included in any part of the medical admission notes and a specific comment on severity was then made.
Hospital policy advised the use of intravenous co‐amoxiclav or ceftriaxone together with intravenous clarithromycin for empirical treatment of severe cases. Non‐severe cases were treated with oral amoxicillin and/or a macrolide, in accordance with BTS guidelines.
After the first audit, a programme of lectures was instituted which took place during one of each of the F1 (PRHO, n = 21) and F2 (SHO, n = 35) “bleep free” timetabled weekly teaching sessions. A minimum attendance of at least 70% is mandatory for these sessions. (However, we could not confirm attendance nor had we requested feedback from those attending) These sessions were repeated for each new F1 (6 month) and F2 (4 month) attachment. Presentations were also made at the medical department's weekly meeting, attended by all junior and middle grades. Thus at least two opportunities were provided for the junior grades to attend guideline education lectures—mitigating against absence due to on‐call commitments and annual leave. The lectures were carried out by PD and included an overview of the BTS CAP guidelines. The lectures discussed background, and the reasons for and the importance of implementation of these guidelines. These were followed by the distribution of small laminated handouts with the guidelines on and posters in the doctors' rooms and walls of the MAU. (A hospital formulary, including a section on antibiotic prescribing, is also made freely available. Antibiotic recommendations are made by the antibiotic action group, which comprises a pharmacist, a microbiologist and both infectious diseases and general physicians. There is no current restriction to prescribing for the treatment of CAP. An electronic formulary is currently being planned.)
Entry exclusion criteria
Immunosuppressed patients or current injecting drug users were excluded. Cases presenting within 2 weeks of discharge from hospital were presumed to represent hospital acquired pneumonia and were also excluded. Finally any case that was judged to have been incorrectly coded as CAP was excluded from further analysis.
CAP was accepted as a diagnosis if there was documented evidence of clinical or radiological findings consistent with new pulmonary consolidation.
Data were double‐entered in Excel 2000. Statistical analysis was undertaken using EPI Info 6.04 statistical package; χ2 test was used for comparing proportions and Student's t test was used for continuous data. A significance level of 5% was accepted.
RESULTS
During 3 months in 2001/2, 65/165 patients who were discharged with a diagnosis of CAP were miscoded, leaving 100 who were enrolled. In 2005/6, 43/130 were excluded and 87 enrolled (p = NS). Patients who were miscoded included those with infective exacerbations of chronic obstructive pulmonary diseases, congestive cardiac failure and various other non‐cardiorespiratory ailments. These were similar in the two cohorts. CAP made up 3.5% of 2853 total admissions to the MAU during the winter of 2001/2 and 3.01% of 2890 admissions in the winter of 2005/6.
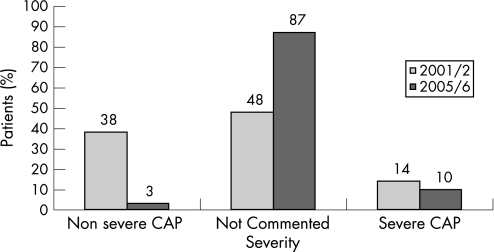
Demographic data in the two periods of time were similar (table 1). Forty‐eight per cent of patients did not have a documented severity assessment in 2001/2, and in 2005/6 this proportion had substantially increased to 87% (p<0.0001). Of those who did have a severity assessment, significantly fewer (3/13) were noted to have non‐severe CAP in 2005/6 compared to 38/52 in 2001/2 (p<0.003 χ2 with Yates' correction) (fig 1).
Table 1 Demographic details for 2001/2 and 2005/6.
| 2001/2002 | 2005/2006 | ||
|---|---|---|---|
| Patients discharged with diagnosis of CAP | 165 | 130 | No significant differences between cohorts |
| Excluded | 65 | 43 | |
| Total number enrolled (n) | 100 | 87 | |
| Male (%) | 43 (43) | 37 (43) | |
| Median (range) age | 74 (17–93) | 75 (26–96) | |
| Month of admission | |||
| November (%) | 28 (28) | 17 (20) | |
| December (%) | 46 (46) | 37(43) | |
| January (%) | 26 (26) | 33 (38) | |
| Mortality within 30 days of admission (95% CI) | 20 (20%)12.7–29.2% | 28 (32%)22.6–43.1% | p = 0.06 |
CAP, community acquired pneumonia; CI, confidence interval.
Figure 1 Documented severity of pneumonia: a comparison of 2001/2 and 2005/6.
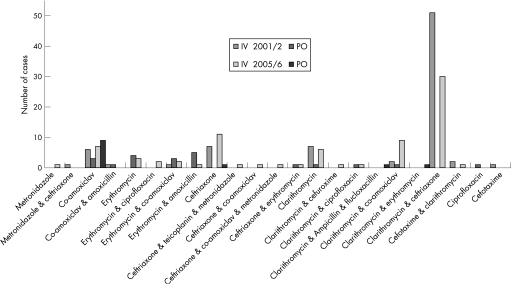
Fifteen different antibiotic regimens were used in 2001/2 and 19 in 2005/6 (fig 2). Antibiotics were given parenterally to 79% of patients in 2001/2 and 77% in 2005/6 (p = 0.74). Third generation cephalosporins were given to 63% in 2001/2 and 54% in 2005/6 (p = 0.21). In 2005/6 the antibiotics used for those cases given a severity score were as follows: of eight cases with severe CAP, six received a third generation cephalosporin, one received co‐amoxiclav and one received neither. Of the three diagnosed with non‐severe CAP, none received third generation cephalosporins.
Figure 2 Range and frequency of antibiotics used in 2001/2 and 2005/6.
In 2001/2, 81% (51/63) of instances of third generation cephalosporin use and 81% (50/62) of instances of IV clarithromycin use were inappropriate, or not justified by severity scoring according to BTS guidelines. In 2005/6 in 82% (41/50) of instances of third generation cephalosporin use and 85% (35/41) instances of IV clarithromycin use had not been justified. Furthermore, 29% (25/77) unclassified cases received IV clarithromycin and a third generation cephalosporin in combination.
DISCUSSION
In both reviews of this busy MAU there was consistently poor documentation of the severity of CAP when patients were first assessed and diagnosed. The frequency with which severity assessment was documented significantly decreased between 2001/2 and 2005/6, in spite of apparently appropriate educational initiatives. More than half of the patients with CAP received third generation cephalosporins, which are only recommended for use in severe cases by the BTS guidelines.7 Similarly, there continued to be a preference for other parenteral antibiotics, particularly macrolides.
Others have described similar poor practice. Barlow et al recently reported a trend for increasing broad‐spectrum antibiotic use, which accelerated after the introduction of the BTS guidelines.20 This was attributed to a “just in case” approach to antibiotic choice for less severe patients. The apparent lack of severity assessment noted in our study suggests that cephalosporin use occurred in spite of, not because of, the BTS guidelines, although there is clearly some awareness of the guidelines as indicated by the occasional recording of the CURB‐65 mnemonic in the notes. It may be that aspects of the guidelines, such as the recommendation for cephalosporin and macrolide use, have filtered through to the front line physicians, but insufficient understanding has led to inappropriate prescribing. Barlow et al also used education and audit based interventions before implementation of the guidelines, but unlike us, they showed a significant increase in appropriate antibiotic prescribing after the introduction of a “multifaceted education programme” after annual review.21
Failure of doctors to follow guidelines is not news,14 and continues despite attempts to disseminate knowledge of guidelines through educational activities, audit and feedback. A systematic review of studies evaluating guideline implementation strategies found, at best, modest to moderate effects, and noted that British healthcare organisations' resources for guideline implementation were usually insufficient to allow much more than dissemination of educational materials or lunchtime educational meetings—interventions whose effects were usually only short‐lived.22 Interventions specific to improving hospital antibiotic prescribing practices have also been reviewed.23 Both persuasive (for example, educational approaches) and restrictive interventions (for example, formulary restriction) were found to be successful in improving the appropriateness of prescribing and reducing antimicrobial resistance or hospital‐acquired infections. However, the authors found restrictive interventions more effective when studies' outcomes were compared over the same time frame.23
On the other hand, Zillich et al, who assessed different antibiotic control mechanisms, found the use of a restrictive formulary alone was associated with increased antimicrobial resistance (AMR).24 They supported the hypothesis “that restrictive formularies may increase AMR rates if they decrease diversity (or mixing) in antibiotic use”.
It would seem reasonable to consider a multifaceted approach as proposed by both Barlow and Zillich. This could include the dissemination of education packs and lectures as well as a restrictive element to improve the justification of antibiotic prescribing.
Our implementation strategy was an educational programme with no restrictive element and it was conducted over a discrete period of time. Turnover of staff is such that few who received the intervention were still practising in the department at the time of the re‐audit. The period between assessments has also seen a change in working patterns away from the old “firm” system where juniors worked closely with the same middle grade and consultant staff, to a shift system where they work more independently. Thus, different groups managing CAP need to be targeted.
It is possible that the physicians made severity assessments that guided their antibiotic choice but simply did not document them. Others have pointed out that the doctor's assessment is paramount and “no prediction rule should supersede clinical judgment”.25 However, in so far as note taking is considered a record of a physician's decision‐making process, it is fair to conclude that the lack of severity documentation represents a failure to follow the BTS guidelines.
Learning points
Adherence to guidelines for management of community acquired pneumonia is poor
Educational programmes, alone, do not appear to improve adherence to guidelines
Restrictive antimicrobial programmes, in addition, should also be considered
Limitations
Although the two cohorts appear comparable, we accept the potential for both under and over diagnosis using National Health Service ICD coding. With ICD‐10 coding on discharge it is likely that patients with CAP were missed; however, it is reassuring that our figures are similar to national statistics for CAP (2% of acute admissions). The slightly higher prevalence of 3.5% and 3.01% in the two periods at the Royal Liverpool University Hospital would be expected during the peak months for pneumonia, in an inner city hospital providing services to a poor socioeconomic group. Thus it seems reasonable to compare these two cohorts. Although the education programme appeared to be robust, we failed to record attendance or glean feedback as to its relevance. Finally, we have not commented on over or under treatment of the unclassified group. The aim was to assess adherence to guidelines.
Conclusion
A multifaceted approach is needed to improve awareness of and adherence to the BTS guidelines for management of CAP. Although the guidelines hold the promise of improved outcomes, their implementation has been inadequate. Severity of illness is not well documented or assessed and there is confusion about choice of antibiotics, with a tendency to over prescribe parenteral cephalosporins and macrolides. More successful implementation will require regular re‐sensitisation of physicians with educational measures, in a cycle that matches the pattern of staff turnover, and the use of restrictive measures to control antibiotic prescribing. There are increasing numbers of guideline documents, each requiring a time commitment to study. However, given the important consequences of inappropriate antibiotic prescribing, decision makers in the NHS will need to consider allocating due priority and resources to this endeavour.
Abbreviations
ARM - antimicrobial resistance
BTS - British Thoracic Society
CAP - community‐acquired pneumonia
CURB - Confusion, raised Urea, increased Respiratory rate and hypotension (BP)
ICD‐10 - International classification of diseases, 10th ed
MAU - medical assessment unit
PRHO - pre‐registration house officer
SHO - senior house officer
Footnotes
Conflict of interest: none stated
References
- 1.National Health Service Headline data, hospital episode statistics. NHS. http://wwwhesonlinenhsuk/Ease/servlet/AttachmentRetriever? site_id = 1937&file_name = d \efmfiles\1937\Accessing\DataTables\ Headline\Headlinepdf&short_name = Headlinepdf&u_id = 5394 2006
- 2.National Health Service Primary diagnosis, hospital episode statistics service. NHS. http://wwwhesonlinenhsuk/Ease/servlet/AttachmentRetriever? site_id = 1937&file_name = d \efmfiles\1937\Accessing\DataTables\Diagnosis \summary\PrimaryDiagnosispdf&short_name = PrimaryDiagnosispdf&u_id = 5388 2006
- 3.British Thoracic Society Guidelines for the management of community acquired pneumonia in adults. Thorax 200156(Suppl IV)iv1–NaN64.11713364 [Google Scholar]
- 4.Barlow G D, Lamping D L, Davey P G.et al Evaluation of outcomes in community‐acquired pneumonia: a guide for patients, physicians, and policy‐makers. Lancet Infect Dis 20033476–488. [DOI] [PubMed] [Google Scholar]
- 5.el Moussaoui R, de Borgie C A, van den Broek P.et al Effectiveness of discontinuing antibiotic treatment after three days versus eight days in mild to moderate‐severe community acquired pneumonia: randomised, double blind study. BMJ 20063321355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.British Thoracic Society Guidelines for the management of community‐acquired pneumonia in adults admitted to hospital. Br J Hosp Med 199349346–350. [PubMed] [Google Scholar]
- 7.British Thoracic Society Guidelines for the management of community acquired pneumonia in adults 2004 update. http://wwwbrit‐thoracicorguk/c2/uploads/MACAPrevisedApr04pdf 2004
- 8.Lim W S, van der Eerden M M, Laing R.et al Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 200358377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mandell L A, Bartlett J G, Dowell S F.et al Update of practice guidelines for the management of community‐acquired pneumonia in immunocompetent adults. Clin Infect Dis 2003371405–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Capelastegui A, Espana P P, Quintana J M.et al Validation of a predictive rule for the management of community‐acquired pneumonia. Eur Respir J 200627151–157. [DOI] [PubMed] [Google Scholar]
- 11.Ewig S, Torres A, Woodhead M. Assessment of pneumonia severity: a European perspective. Eur Respir J 2006276–8. [DOI] [PubMed] [Google Scholar]
- 12.Dean N C, Silver M P, Bateman K A.et al Decreased mortality after implementation of a treatment guideline for community‐acquired pneumonia. Am J Med 2001110451–457. [DOI] [PubMed] [Google Scholar]
- 13.Marrie T J, Lau C Y, Wheeler S L.et al A controlled trial of a critical pathway for treatment of community‐acquired pneumonia. CAPITAL Study Investigators. Community‐Acquired Pneumonia Intervention Trial Assessing Levofloxacin. JAMA 2000283749–755. [DOI] [PubMed] [Google Scholar]
- 14.Cabana M D, Rand C S, Powe N R.et al Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA 19992821458–1465. [DOI] [PubMed] [Google Scholar]
- 15.Nathwani D, Williams F, Winter J.et al Use of indicators to evaluate the quality of community‐acquired pneumonia management. Clin Infect Dis 200234318–323. [DOI] [PubMed] [Google Scholar]
- 16.Shek F W, Stacey B S, Rendell J.et al The rise of Clostridium difficile: the effect of length of stay, patient age and antibiotic use. J Hosp Infect 200045235–237. [DOI] [PubMed] [Google Scholar]
- 17.Spencer R C. Clinical impact and associated costs of Clostridium difficile‐associated disease. J Antimicrob Chemother 199841(Suppl C)5–12. [DOI] [PubMed] [Google Scholar]
- 18.Thomas C, Stevenson M, Riley T V. Antibiotics and hospital‐acquired Clostridium difficile‐associated diarrhoea: a systematic review. J Antimicrob Chemother 2003511339–1350. [DOI] [PubMed] [Google Scholar]
- 19.Beadsworth M, Nye F J, Beeching N J. Implementation of new BTS guidelines in acute medical assessment units. J Infect 20034788–89. [DOI] [PubMed] [Google Scholar]
- 20.Barlow G, Nathwani D, Davey P. The effect of implementing the British Thoracic Society community‐acquired pneumonia guidelines on antibiotic prescribing and costs in a UK teaching hospital. Clin Microbiol Infect 200612498–500. [DOI] [PubMed] [Google Scholar]
- 21.Barlow G, Nathwani D, Williams F.et al Reducing door to antibiotic time in community‐acquired pneumonia: controlled before and after evaluation and cost‐effectiveness analysis. Thorax 20066267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grimshaw J M, Thomas R E, MacLennan G.et al Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess. 2004;8: iii–iv, 1–72, (6) [DOI] [PubMed]
- 23.Davey P, Brown E, Fenelon L.et al Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev 2005(4)CD003543. [DOI] [PubMed]
- 24.Zillich A. Antimicrobial use control measures to prevent and control antimicrobial resistance in US hospitals. Infect Control Hosp Epidemiol 2006271088–1095. [DOI] [PubMed] [Google Scholar]
- 25.Lim W S, Macfarlane J T. Importance of severity of illness assessment in management of lower respiratory infections. Curr Opin Infect Dis 200417121–125. [DOI] [PubMed] [Google Scholar]