Abstract
Testis cancer is an increasing problem, especially in northern European male populations. However, survival has improved dramatically over one generation. Environmental factors may have a role in the aetiology with high oestrogen concentrations implicated. Testis cancer is subdivided between seminoma and non‐seminoma. At presentation, a testicular lump is the most common finding and radical inguinal orchidectomy is recommended for most. Further multidisciplinary management is determined by histological subtype and stage and involves chemotherapy, radiotherapy and surgery, with many patients only undergoing surveillance. There is increasing emphasis on reducing toxicity of treatments in long term survivors. Treatment refractory testis cancer remains a significant challenge.
Keywords: testis cancer, epidemiology, aetiology, treatment, long‐term toxicity
Testis cancer is the most common solid malignancy of young men, but only accounts for about 1% of all cancers in men.1 The incidence has been increasing worldwide. Germ cell tumours (GCT) represent over 95% of these cancers and for the purpose of this review; references to testis cancer will imply GCTs.
Advances in the last 40 years in treatments of this group of patients have led to dramatic improvements in disease‐free survival. Better treatments are, however, still needed for high risk patients. There are many long term survivors and late toxicities from treatment are more prominent than in other solid malignancies. It is important to balance the risk of long term toxicities with effective treatment without reducing efficacy.
Epidemiology
The incidence of testis cancer has been steadily increasing over the last 40 years.2 It appears to be most common in northern European populations with age standardised incidence rates between 4 and 10 per 100 000, whereas in Asian, African and African American men, incidence rates are much lower, ranging between 0.2 to 1 per 100 000.3 The peak incidence is between the ages of 15 to 35 years. Five year survival rates have increased significantly over the last 30 years from about 63% to more than 90%.3
Aetiology
The aetiology of testis cancer is not clearly understood. Rising incidence suggests a role for environmental factors, which is supported by an increasing frequency of other testicular problems such as declining sperm counts and increasing incidence of testicular maldescent. High oestrogen concentrations in utero have been suggested as a potential common factor. Sons of women who received the synthetic oestrogen, diethylstilboestrol, had an increased incidence of testicular abnormalities. Certain environmental oestrogens have been implicated in feminisation of male animals. To date there has been no direct link between oestrogen exposure and the risk of testis cancer. There are, however, several known risk factors (box 1).3
Box 1: Risk factors for testis cancer
Cryptorchidism (testicular maldescent)—2–4 fold increase in risk
Carcinoma in situ (intratubular germ cell neoplasia)
Prior history of testis cancer or extragonadal germ cell tumour
Family history—relative risk increased 6–10 fold in brothers or sons of affected man.
HIV infection—slightly increased risk of seminoma
Down syndrome
Testicular trauma
Approximately 2% of individuals with testis cancer report an affected first degree relative, and concordant twin studies have also demonstrated a higher incidence of testis cancer in monozygotic compared to dizygotic twins.4 Analyses of affected families have so far failed to identify a specific gene although linkage to the Xq27 region has been reported.5 Duplication or amplification of the short arm of chromosome 12 (12p) is seen in almost all cases of testis cancer, implying that a key gene is present in this area.6
Pathology
GCTs can be subdivided into three main groups: infantile/prepubertal, adolescent/young adult, and spermatocytic seminoma. They originate from germ cells at different stages of development. By far the most common is the adolescent/young adult type and this is the type discussed in this review.
Intratubular germ cell neoplasia, unclassified type (ITGCN) is considered to be the precursor to invasive GCTs. It is also often referred to as carcinoma in situ. It is thought that primordial germ cells undergo abnormal cell division in response to environmental factors in utero giving rise to ITGCN.7 This is followed by the duplication of 12p, as well as several other chromosomal abnormalities, rendering these cells susceptible to stimulation by gonadotrophins and subsequent development of invasive tumours. Activation to pluripotency of neoplastic germ cells of these tumours gives rise to NSGCTs (non‐seminomatous germ cell tumours—that is, yolk sac tumour, embryonal carcinoma and choriocarcinoma) in a way similar to the reprogramming of a primordial germ cell to an embryonic germ cell. Factors causing reprogramming are unknown.
There are two main subtypes of GCTs arising in young men:
Pure seminoma: 50% of all testicular GCTs
Non‐seminomas: consist of a heterogeneous group with varying patterns including the combination of non‐seminoma and seminoma.
There are two main classifications used in the histopathological examination of testicular tumours, the British Testicular Tumour Panel classification and the World Health Organization system (table 1). The former is widely used in the UK and Australia, and the latter is commonly used in North America and Europe.
Table 1 Pathological classification for testis cancer.
| British Testicular Tumour Panel and Registry | World Health Organization |
|---|---|
| Seminoma | Seminoma |
| Spermatocytic seminoma | Spermatocytic seminoma |
| Teratoma | Non‐seminomatous germ cell tumour |
| • Teratoma differentiated | Mature teratoma |
| • Malignant teratoma intermediate | Embryonal carcinoma with teratoma |
| • Malignant teratoma undifferentiated | Embryonal carcinoma |
| • Yolk sac tumour | Yolk sac tumour |
| • Malignant teratoma trophoblastic | Choriocarcinoma |
It is common practice for pathologists to describe all cell types within the specimen as well as commenting on the presence or absence of lymphovascular invasion, involvement of tunica albuginea, tunica vaginalis, rete testis and spermatic cord. However, in practical terms, further clinical management depends on whether the tumour is seminoma or NSGCT and the presence or absence of lymphovascular invasion.
Clinical presentation
Testis cancers commonly present as a unilateral lump or painless swelling noticed incidentally.8 Pain is less common, with a third of patients presenting with a dull ache, and acute pain is uncommon occurring in 10% of patients at presentation. Testis cancers uncommonly present with symptoms attributable to metastatic disease. These are summarised in box 2.
Box 2: Clinical manifestations of testis cancer from metastatic disease
Systemic symptoms: anorexia, malaise, weight loss
Cough or shortness of breath due to pulmonary metastases
Neck mass due to lymph node metastases
Lower back pain from bulky retroperitoneal disease
Lower extremity swelling due to iliac or caval obstruction or thrombosis (unilateral or bilateral)
Nausea, vomiting or gastrointestinal haemorrhage from retroduodenal metastases
Bony pain
Central or peripheral nervous system symptoms from cerebral, spinal cord or peripheral nerve root involvement
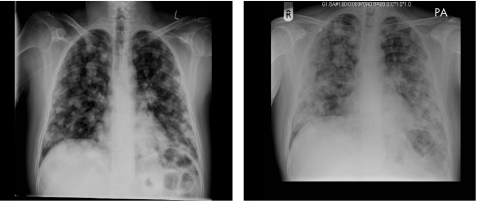
In any man with a solid firm mass within the testis, the diagnosis is testis cancer until proven otherwise. Prompt diagnosis and treatment offers the patient the best chance of a cure. Occasionally, metastatic disease can be rapidly progressive, warranting emergency treatment. An example of this is demonstrated by the chest radiograph appearances taken 4 days apart of a 32‐year‐old man with poor prognosis advanced germ cell cancer (fig 1). The differential diagnosis of testis cancer is summarised in box 3.
Figure 1 Chest radiographs taken 4 days apart of a 32‐year‐old man with poor prognosis metastatic germ cell cancer indicating rapid progression of disease.
Investigations
Initial diagnostic evaluation of men with suspected testis cancer is with scrotal ultrasound. This is effective in distinguishing intrinsic from extrinsic testicular lesions.9 A plain film is carried out before surgery to exclude overt metastatic disease. The only other radiological investigation that is routinely performed is high resolution computed tomography (CT) scanning of the chest, abdomen and pelvis.
Box 3: Differential diagnosis of testis cancer
Testicular torsion: acute, severe pain is a common presenting feature
Epididymitis/epididymo‐orchitis: associated with fever, pain not as acute
Hydrocoele
Varicocoele
Hernia
Haematoma
Spermatocoele
Three serum tumour markers must be measured in any man with suspected testis cancer: α‐fetoprotein (AFP), the β unit of human chorionic gonadotrophin (β‐HCG) and lactate dehydrogenase (LDH). In NSGCT the serum concentrations of AFP and β‐HCG are elevated in over 80% of men. In pure seminoma, AFP is not elevated and fewer than 20% of men have elevated β‐HCG. LDH is less specific but has independent prognostic value in patients with advanced GCTs. It is increased in 60% of NSGCTs and 80% of seminomas. Serum tumour markers alone are not diagnostic of testis cancer but very high values in men rarely occur outside of testis cancer.
Management
Semen cryopreservation should be offered to all men diagnosed with testis cancer before starting treatment if they wish to preserve fertility. Ideally, a baseline sperm count and sperm banking should be performed before radiological diagnostic evaluation to avoid radiation exposure of sperm, but this is not always feasible. All cases of testis cancer should be discussed by a multidisciplinary team comprising specialist surgeons, oncologists, histopathologists, radiologists and specialist nurses.
Surgery
Radical inguinal orchidectomy is recommended for all patients with suspected testis cancer to allow accurate histological evaluation as well as local tumour control. The procedure involves isolating and clamping the spermatic cord at the external inguinal ring, exteriorising the testis with its tunics, opening the tunica vaginalis, and inspecting and palpating the testis carefully.10 If the diagnosis is unclear, a biopsy is taken and examined under frozen section. Once the diagnosis is established, the inguinal canal is opened, the spermatic cord is divided at the level of the internal inguinal ring, and the testis is removed. The inguinal approach is preferable to the scrotal approach as there is a theoretical risk of lymphatic spread of testicular cancer cells to the scrotal skin and its lymphatic drainage. Complications include retroperitoneal haemorrhage, wound infection, seroma formation, local hypoaesthesia and persistent inguinal and scrotal neuralgia.
Partial orchidectomy may have a role in selected patients in whom the likelihood of a testicular neoplasm is low, based on ultrasonographic findings, age, physical examination and tumour markers.11 In men with bilateral tumours, consideration should be given to this approach, especially if maintaining fertility is important. However, in the majority of men undergoing surgery the radical approach is currently advisable, although interest is increasing in testis preservation techniques through clinical trials.
Patients should be offered the option of a testicular prosthesis and consideration should be given to contralateral testicular biopsy. About 5% of patients with testis cancer have ITGCN in their contralateral testis and in the majority of cases this will proceed to an invasive GCT.12 Biopsy is usually considered in high risk patients as defined by a small testis volume (<12 ml), history of cryptorchidism, and young age (<30 years).13 If ITGCN is found then the options available are low dose radiotherapy to prevent tumour progression, or surveillance and surgery once the need arises. Radiotherapy is not recommended for men who wish to preserve their fertility.
Staging of testis cancer
A clinical staging system that is widely used is the Royal Marsden Hospital classification (table 2).14 After surgery it is essential to monitor tumour markers and assess their rate of decline. If markers do not decline as predicted or start to rise postoperatively the contralateral testis should be examined for a metachronous primary. If this is not found, the patient should be treated as having metastatic disease, even if imaging studies do not corroborate this.
Table 2 Royal Marsden Hospital staging of testis cancer.
| Stage | |
|---|---|
| I | No evidence of metastasis |
| IM | Rising concentrations of serum markers with no other evidence of metastasis |
| II | Abdominal node metastasis |
| • A | <2 cm in diameter |
| • B | 2–5 cm in diameter |
| • C | >5 cm in diameter |
| III | Supra‐diaphragmatic nodal metastasis |
| • M | Mediastinal |
| • N | Supraclavicular, cervical or axillary |
| • O | No abdominal node metastasis |
| • ABC | Node stage as defined in stage II |
| IV | Extra lymphatic metastasis |
| Lung | |
| • L1 | <3 metastases |
| • L2 | 3 metastases or more, <2 cm in diameter |
| • L3 | 3 metastases or more, one or more of which >2 cm in diameter |
| H+: liver metastases; Br+: brain metastases; Bo+: bone metastases | |
The International Germ Cell Cancer Collaborative Group (IGCCG) prognostic classification is the generally accepted prognostic tool used for treatment decisions and eligibility for clinical trials in metastatic disease (table 3).15 It relies on the extent of disease and tumour markers together with the primary site. This was in part developed because of the variations in classifications and staging systems used throughout the world, making it difficult to compare trial data. Supplementary prognostic information is gained by observing the rate of decline of tumour markers after starting chemotherapy. Further management of GCTs depends on their clinical stage and the pathological classification of the tumour.
Table 3 IGCCG prognostic classification of germ cell tumours of the testis.
| NSGCT | Seminoma | |
|---|---|---|
| Good prognosis | All of: | Any primary site |
| Testis or retroperitoneal primary tumours, no non‐pulmonary visceral metastases (ie, lung metastases only) | No non‐pulmonary visceral metastases (ie, lung metastases only) | |
| AFP <1000 ng/ml | Normal AFP | |
| β‐HCG <5000 mIU/ml | Any β‐HCG, any LDH | |
| LDH <1.5×ULN | ||
| Intermediate prognosis | Testis or retroperitoneal primary | Any primary site |
| No non‐pulmonary visceral metastases and any of: | Non‐pulmonary visceral metastases, normal AFP | |
| AFP >1000–<10000 ng/ml | Any β‐HCG, any LDH | |
| β‐HCG >5000–<50000 mIU/ml | ||
| LDH >1.5–<10×ULN | ||
| Poor prognosis | Any of: | No patients in this group |
| Mediastinal primary site | ||
| Non‐pulmonary visceral metastases | ||
| AFP >10000 ng/ml | ||
| β‐HCG >500000 mIU/l, LDH >10×ULN |
AFP, α‐fetoprotein, β‐HCG, β‐human chorionic gonadotrophin; IGCCG, International Germ Cell Cancer Collaborative Group; LDH, lactate dehydrogenase; NSGCT, non‐seminomatous germ cell tumour; ULN, upper limit of normal.
Stage I seminoma
About 85% of men present with stage I disease and a further 10% present with stage II disease. The options post‐orchidectomy involve active surveillance, radiotherapy or single agent chemotherapy.
Surveillance
About 15% of men with stage I disease will relapse within 4 years.16 Salvage rates are high so active surveillance has the advantage of avoiding unnecessary treatment and adverse effects. However, men undergoing surveillance may need multi‐agent chemotherapy on relapse. Regular and reliable attendance is also necessary. In one pooled analysis of over 600 men undergoing surveillance for stage I seminoma for an average of 7 years, the only significant risk factors for risk of relapse were tumour size ⩾4 cm and rete testis invasion.17
Radiotherapy
Traditionally radiotherapy was administered to the ipsilateral renal hilum and pelvic lymph nodes and the bilateral para‐aortic nodes as well as the regional lymph nodes of the involved testis. This so called “dog‐leg” field yielded excellent results with 5 year relapse‐free survival rates in excess of 94%.18 However, significant gastrointestinal toxicity and increased risk of second malignancies have led to strategies to reduce the radiation field without compromising on efficacy.
More recently, the radiation field has been limited to the para‐aortic nodes (“PA strip”). The evidence for this was provided by a number of trials including a prospective trial by the Medical Research Council (MRC) Testicular Tumour Working Group.19 Three year survival was similar as were the total number of relapses, with a slightly higher pelvic relapse rate in the smaller field group. There was a significant reduction in short term morbidity in the smaller field group.
A recent MRC trial comparing two different doses of irradiation (30 Gy in 15 fractions compared to 20 Gy in 10 fractions) in stage I seminoma demonstrated no difference in disease‐free survival with a median follow up of 5 years.20 Quality of life data indicated better tolerance for lower dose radiotherapy suggesting that the dose of radiation can be safely reduced.
Chemotherapy
Single agent carboplatin has recently become established as an alternative to adjuvant radiotherapy in stage I seminoma. A pooled analysis of phase II trials using two cycles of adjuvant carboplatin demonstrated a relapse rate of 2.9%.21 A joint MRC and European Organisation for Research and Treatment of Cancer (EORTC) trial recently reported results of a randomised phase III trial comparing adjuvant radiotherapy to a single course of carboplatin dosed at an area under the concentration × time curve (AUC) of 7.22 Almost 1500 men with stage I seminoma were randomised and with a median follow up of 4 years there was no significant difference in relapse‐free survival. There was a trend of relapse in the para‐aortic nodes in the carboplatin arm. Longer term follow up is needed to determine whether carboplatin can safely replace radiotherapy, but many centres are now offering it as an alternative. Whether patients should be given one or two cycles remains controversial as most phase II trials have shown lower relapse rates when two cycles are given.
All options should be considered for patients with stage I disease, although less radiotherapy is being given and increasing numbers of patients are undergoing surveillance or chemotherapy.
Stage II seminoma
Stage II disease is defined as metastatic disease that is confined to the infradiaphragmatic lymphatics. Treatment usually consists of radiotherapy or platinum based chemotherapy post‐orchidectomy. The optimal treatment depends on the volume of nodal disease, with chemotherapy being offered to patients with bulkier disease due to the increased risk of renal damage and out of field recurrences with radiotherapy. The definition of bulky disease varies, but in general this is nodal disease >5 cm in greatest dimension.
Non‐bulky disease is usually treated with radiotherapy alone to the para‐aortic and high ipsilateral iliac lymph nodes.23 Five year survival rates average about 90% and, with the benefit of effective salvage treatments, overall cure rate is also about 90%.
There is some evidence supporting the use of chemotherapy in this setting. A recent Spanish study demonstrated a progression‐free survival rate of 91% and overall survival rate of 98%, with no late toxicities using bleomycin, etoposide and cisplatin (BEP) or etoposide and cisplatin (EP).24 Randomised trials are needed comparing this approach to radiotherapy before any definitive conclusions can be made.
Patients with bulky stage II seminoma either at presentation or at relapse tend not to respond to radiotherapy as well, with 5 year disease‐free survival rates of about 65%; with the benefit of salvage treatment, 5 year overall survival is about 77%.25 Chemotherapy has been investigated as first line treatment following orchidectomy. The optimal chemotherapy regimen has not been defined but four courses of EP or three courses of BEP are usually recommended.
Management of post‐treatment residual masses tends to be determined by their size. If <3 cm then surveillance alone is adequate; if ⩾3 cm then management is not as simple, with some recommending surgical resection.26 There is little evidence supporting adjuvant treatment after combination chemotherapy for advanced seminomas and some of these masses show fibrosis alone.27 Positron emission tomography (PET) scanning is often utilised to determine whether there is any viable tumour in such cases. In the multicentre SEMPET study 51 patients who had CT confirmed residual masses post‐chemotherapy for bulky seminoma underwent PET scanning.28 All 19 cases with residual lesions >3 cm and 35 of 37 cases with lesions <3 cm were correctly predicted by PET scanning. Specificity and sensitivity for FDG (fluoro‐deoxy‐D‐glucose) PET were 100% and 80% compared with 74% and 70% for CT scans, respectively.
Stage I NSGCT
The options for men with stage I NSGCT in the UK consist of surveillance with treatment given at relapse or chemotherapy. In the USA and parts of Europe, primary retroperitoneal lymph node dissection (RPLND) is also considered. Surveillance tends to be reserved for highly motivated men with low risk disease. Patients at high risk of relapse can be identified by certain histological factors that are summarised in box 4.29 In clinical practice, the presence of vascular invasion alone is widely accepted as the parameter on which to base the decision to administer adjuvant chemotherapy. In patients with none of these risk factors, relapse rates are between 10–15%, while if several risk factors are present then risk of relapse approaches 50%.30
Box 4: Risk factors for recurrence in patients with stage I NSGCT
Lymphovascular invasion
Embryonal component
Absence of yolk sac tumour component
Tumour stage above T2
The main advantage of surveillance is the avoidance of unnecessary treatment for the 70% of patients with stage I disease who do not relapse after orchidectomy. However, there are disadvantages to this approach: intensive follow up requiring additional radiation exposure and uncertainty for the patient, and an additional chemotherapy cycle if the patients do relapse compared to two cycles in the adjuvant setting.
Adjuvant chemotherapy consists of two cycles of BEP with an etoposide dose of 360 mg/m2. Recurrence rates are reduced from 50% to <5%. The Anglian Germ Cell Cancer Group published a retrospective review of 382 patients with stage I NSGCT treated between 1978 and 2000; all men before 1986 were followed by surveillance, and from 1986 onwards those with >30% risk of relapse were offered adjuvant chemotherapy.31 Relapse occurred in 30% on surveillance and mortality rate was 2.6%. Only 4% of men who received adjuvant treatment relapsed and 1.4% died from progressive disease.
This approach has the advantages of reducing the anxiety of recurrence and intensive surveillance for the patient as well as reducing the risk of recurrence at all sites. Short term and long term toxicities are of concern and are addressed later in this review.
In the USA, RPLND is the preferred treatment option in this patient group. This approach is considered to be safe especially with nerve sparing surgery, and additional staging information is obtained as current radiological techniques are inadequate in evaluation of retroperitoneal nodal disease in almost a third of patients.32 Over‐treatment with chemotherapy is also avoided. There is a risk of retrograde ejaculation and hence infertility, but this is <5% with nerve sparing techniques in specialist centres.33 Criticisms of the surgical approach include the increased morbidity of surgery and the fact that 10% of patients relapse outside of the retroperitoneum.10 After RPLND the options available are either surveillance with chemotherapy at relapse or adjuvant chemotherapy.
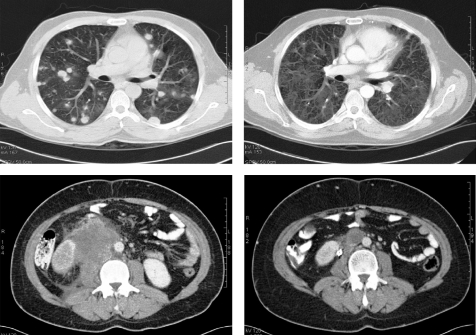
Advanced germ cell tumours
Five year survival rates for GCTs now exceed 95% and even patients with disseminated disease are very curable—a remarkable statistic for a solid malignancy. Figure 2 demonstrates complete response in a patient with an advanced, widespread metastatic GCT. The main reason for this improvement has been treatment advances since the early 1970s. The pivotal study, published in 1981, combined cisplatin with bleomycin and vinblastine resulting in cure rates exceeding 50%.34 Vinblastine was replaced with etoposide, improving efficacy and limiting toxicities, and the three‐drug combination of BEP has become the mainstay of treatment for men with advanced seminoma and NSGCTs.35 Cure rates are around 80% and no regimen to date has been shown to be superior; the scheduling and number of cycles varies according to prognosis. Wherever possible, men should be referred to centres with expertise in management of GCTs.
Figure 2 A 32‐year‐old man with poor prognosis advanced germ cell tumour. CT scans show complete response to chemotherapy.
Men with testicular cancer are separated into prognostic groups derived from the IGCCC as previously discussed. The distribution of patients in each category is summarised in table 4.
Table 4 Percentage of patients with testis cancer in each prognostic category.
| Seminoma | NSGCT | |
|---|---|---|
| Good prognosis | 90% | 60% |
| Intermediate prognosis | 10% | 25% |
| Poor prognosis | – | 15% |
NSGCT, non‐seminomatous germ cell tumour.
Good prognosis advanced disease
Long term outcome of chemotherapy in this risk group is excellent with long term relapse‐free survival in excess of 90%. Modifications to the use of four cycles of BEP have been made by decreasing the number of cycles from four to three. In 2001 the EORTC published data confirming that for good prognostic disease three cycles of 3 day BEP (500 mg/m2) was sufficient with a progression‐free survival at 2 years of 90.4%.36 An earlier US study also demonstrated similar results.37
Studies have also been performed omitting bleomycin to avoid the serious pulmonary toxicity associated with this drug. A US study compared three cycles of BEP with three cycles of EP in good prognosis patients with metastatic disease,38 and a European study compared four cycles of EP with four of BEP.39 The consensus based on these trials is that the omission of bleomycin leads to an inferior outcome. A more recent trial by the same European group demonstrated equivalence of three versus four cycles of BEP and of 5 days versus 3 days per cycle in good prognosis germ cell cancer.40 The dose of etoposide should be 500 mg/m2 per cycle. In general, most cancer centres recommend three cycles of 3 day BEP in this patient group, with four cycles of EP an alternative for patients with compromised lung function.
Intermediate and poor prognosis disease
For this group of patients four cycles of 5 day BEP is the standard. Five year survival for intermediate and poor risk patients is 80% and 48%, respectively.15 There is significant interest in improving the outcome using different regimens of chemotherapy, but as this group of patients comprises a minority of all patients with metastatic disease, generating adequate randomised data can be challenging. The MRC are currently investigating the CBOP/BEP (carboplatin, bleomycin, vincristine and cisplatin followed by BEP) regimen. A phase II study demonstrated an 87.6% 5 year survival which has led to the phase III study comparing with BEP currently recruiting.41
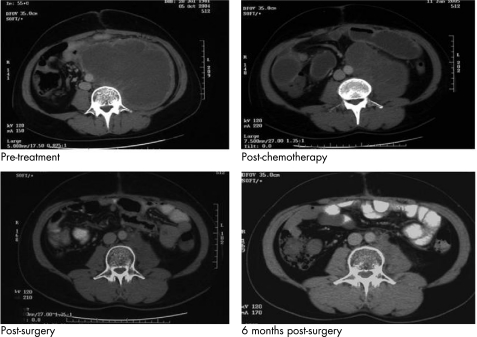
Men in these risk groups often have residual tumours seen on imaging studies at the completion of chemotherapy. These patients with residual disease, particularly in the retroperitoneum but also mediastinum and neck, should be considered for further surgery to render them disease‐free. Figure 3 demonstrates an example of multi‐modality treatment of a patient who underwent initial chemotherapy followed by retroperitoneal surgery. The resected specimen revealed mainly necrotic tissue, but some cells stained positive for AFP so he underwent retroperitoneal radiotherapy. This case is an excellent example of the multidisciplinary management of testis cancer.
Figure 3 A 25‐year‐old man with poor prognosis advanced germ cell tumour. CT images indicating response to chemotherapy and surgery.
Furthermore, pathological review of the resected specimen confirming viable tumour would suggest a significant risk of relapse.42 These patients can be offered further chemotherapy or radiotherapy; however, there is some debate over how beneficial this approach actually is.
Salvage treatment for relapsed and refractory testicular germ cell tumours
Following first line chemotherapy, up to 50% of men with intermediate or poor risk germ cell cancer will require salvage treatment for relapsed disease. Men with stage I NSGCT or seminoma who relapse while undergoing surveillance can usually be salvaged with standard dose cisplatin based chemotherapy or RPLND followed by chemotherapy, and are largely cured by their subsequent treatment.
Relapsed germ cell cancer is still a chemosensitive disease and potentially curable in approximately 30% of cases. Several different relapse regimens have been investigated using cytotoxics that have demonstrated activity in the relapsed setting. The optimum salvage regimen still needs to be defined, but most patients retain platinum sensitivity at relapse. Vinblastine, etoposide and cisplatin (VIP) used as a salvage regimen has a complete response rate of 50% and long term survival of 30%.43 More recently paclitaxel has been added to other active drugs, notably ifosfamide and cisplatin, with a 19–77% complete response rate44,45 and an 85% 2 year survival rate.45 Suggested prognostic factors at relapse are inadequate response to initial treatment, progression‐free interval <2 years, and non‐testicular primary.
Websites for doctors and patients, self‐help groups and sources of good quality patient information
http://www.cancerhelp.org.uk/ CancerHelp UK is a free information service from Cancer Research UK about cancer and cancer care for people with cancer and their families.
http://www.orchid‐cancer.org.uk/ Aims to raise funds to increase public awareness and improve and simplify treatment of men's cancer (testicular and prostate cancers) and to help people understand these cancers and their treatment.
http://www.dipex.org/testicularcancer DIPEx (Database of Individual Patient Experiences) is an Oxford based registered charity. It is a database of audio, video and transcript of interviews with patients experiencing a particular illness or health problem. Includes a module on testicular cancer.
http://www.cancer.gov/cancerinfo/types/testicular/ US cancer information website with excellent patient and health professional sections.
http://www.cancerbackup.org.uk/Cancertype/Testes UK organisation aiming to help people live with cancer by providing information and emotional support for patients, their families and health professionals.
http://www.icr.ac.uk/ Provides links to many cancer support and information resources for patients and carers. Includes Everyman—action against male cancer—the Institute of Cancer Research's campaign to raise awareness of testicular and prostate cancer.
http://www.cancerindex.org/ An extremely comprehensive and well‐resourced site with a wealth of information on cancers and links to a wide range of other sources of information.
High dose chemotherapy with autologous stem cell rescue has been investigated as a second or third line treatment. Several phase II studies or retrospective analyses have demonstrated efficacy with acceptable toxicity. Longstanding complete remissions have been reported in 15–25% of patients. A retrospective series from Einhorn's group reported a 57% disease‐free rate with a median follow up of 39 months.46
However, a recent study suggested no difference in outcome between conventional standard treatment (cisplatin, ifosfamide and etoposide or vinblastine) and high dose treatment (carboplatin, etoposide and cyclophospamide).47 Similar complete and partial response rates were seen in each arm and there were no significant differences seen in overall survival.
Long term toxicities of treatment
Many patients with testis tumours are young and a proportion receive significant doses of chemotherapy and radiotherapy. As cure rates tend to be high, many are expected to achieve a normal life expectancy. Consequently, toxicities from treatments are increasingly important and have a bearing on optimising management. The most common long term toxicities are cardiovascular disease, second cancers, and reduction in fertility.
Cardiovascular disease
In a Royal Marsden cohort study of almost 1000 patients, there was a significantly increased risk of a cardiac event in all patients who had received treatment for testis cancer.48 The relative risk was 2.74 for radiotherapy and 2.59 for chemotherapy. In a retrospective analysis of 453 men with stage I or II seminoma treated with post‐orchidectomy radiotherapy, the standardised mortality ratio (SMR) for deaths from all causes was only significant beyond 15 years of follow up (SMR 1.89).49 For cardiac deaths the SMR was also significant beyond 15 years (1.95). The mechanism for cardiac mortality has been postulated to be related to irradiation either to the mediastinum or para‐aortic nodes.
Second malignancies
The same study also reported a cancer SMR of 1.9 but the study was based on a cohort treated between 1951 and 1999, so whether the results can be translated to modern radiotherapy techniques has been questioned.48 The risk of second malignancy with chemotherapy is also a concern. In an international study, the relative risk of second malignancy at 10–15 years was 2.42.50 In the Royal Marsden study the relative risk of second malignancy was 1.6, which was not statistically significant.48
Fertility issues
Approximately 50% of men with testis cancer have some degree of underlying impairment of spermatogenesis.51 It is not known why this occurs but it has been suggested that common aetiological factors are responsible for both low semen quality and testis cancer. All patients should be checked for hypogonadism (luteinising hormone and β‐HCG detected on assay) before initiation of treatment.
It is known that after chemotherapy, concentrations of follicle stimulating hormone and luteinising hormone rise and testosterone concentrations fall. It also commonly causes azoospermia, but most patients recover. In a recently published series from the Royal Marsden, 13% of patients had hypogonadism, and in patients who were normospermic before treatment, 80% had spermatogenesis 5 years after treatment.52 Another multicentre study reported a reduction in fertility rates of about 30% after cancer treatment, with radiation likely to have the biggest impact.53 Patients are usually advised not to attempt conception for 6 months after adjuvant treatment, but the evidence to support this is lacking. It is likely that irradiated sperm creates a damaged zygote that is miscarried before the woman detects pregnancy.
Recent developments and future directions
Testis cancer patients are more likely to be cured than those with most other solid cancers so there is a trend towards minimising exposure to toxic treatments. Recent trials using less radiotherapy and carboplatin in early stage seminoma have demonstrated this, as well as increasing use of surveillance strategies in all types of testis cancer. More accurate staging and assessment of response to treatments using PET scanning is evolving.
Perhaps the biggest challenge for oncologists treating testis cancer is the treatment of cisplatin refractory germ cell cancer. Recent trials have demonstrated promising results with combination chemotherapy, with response rates of over 30%.54,55,56 The best results have been seen with gemcitabine and oxaliplatin.54,55 Three drug combinations such as gemcitabine–oxaliplatin–paclitaxel or paclitaxel–gemcitabine–cisplatin are currently being developed in clinical trials, but significant toxicity in these heavily pre‐treated patients is a major limiting factor. It is hoped that successful development of new regimens in this patient group will allow new options for earlier stages of the disease. Patients should be strongly encouraged to participate in clinical trials. As our understanding of the molecular mechanisms of testis cancer development increases it is hoped that novel targeted treatments will be further explored.
Key references
Einhorn LH. Curing metastatic testicular cancer. Proc Nat Acad Sci 2002;99:4592–5.
Williams SD, Birch R, Einhorn LH, et al. Treatment of disseminated germ‐cell tumors with cisplatin, bleomycin, and either vinblastine or etoposide. N Eng J Med 1987;316:1435–40.
de Wit R, Roberts JT, Wilkinson PM, et al. Equivalence of three or four cycles of bleomycin, etoposide, and cisplatin chemotherapy and of a 3‐ or 5‐day schedule in good‐prognosis germ cell cancer: a randomized study of the European Organization for Research and Treatment of Cancer Genitourinary Tract Cancer Cooperative Group and the Medical Research Council. J Clin Oncol 2001;19:1629–40.
Oliver RT, Mason MD, Mead GM, et al. Radiotherapy versus single‐dose carboplatin in adjuvant treatment of stage I seminoma: a randomised trial. Lancet 2005;366:293–300.
Jones WG, Fossa SD, Mead GM, et al. Randomized trial of 30 versus 20 Gy in the adjuvant treatment of stage I Testicular Seminoma: a report on Medical Research Council Trial TE18, European Organization for the Research and Treatment of Cancer Trial 30942 [ISRCTN 18525328]. J Clin Oncol 2005;23:1200–08.
Multiple choice questions (true (T)/false (F); answers after the references)
1. Epidemiology:
The incidence of testes cancer has remained steady over the last 40 years
It is much more common in African men
It has a peak incidence between the ages of 15 and 35 years
The 5 year survival is currently about 65%
2. Clinical presentation
It commonly presents as an acutely painful mass
Epididyo‐orchitis and hydrocoele are among the differential diagnoses
Ultrasound and magnetic resonance imaging scan of the chest and abdomen are essential investigations
AFP, β‐HCG and LDH are the tumour markers that need to be measured
3. Seminoma:
It is much less common than non‐small germ cell cancers
The majority of patients present with stage I disease
Significant risk factors for risk of relapse are tumour size ⩾2 cm and lymphovascular invasion
Adjuvant treatment options include radiotherapy or single agent carboplatin chemotherapy
Retroperitoneal lymph node dissection is recommended for the majority of patients with residual masses after treatment for advanced seminoma
4. Non‐seminomatous germ cell tumours:
Lymphovascular invasion is considered to be the most important prognostic risk factor in early stage disease
All patients with metastatic disease should be treated with four cycles of BEP chemotherapy, assuming there is no contraindication to bleomycin
Patients with residual disease in the retroperitoneum, mediastinum and neck should be considered for further surgery to render them disease‐free
For patients with relapsed disease the salvage treatment of choice is high dose chemotherapy with autologous stem cell rescue
Five year survival for intermediate and poor risk patients is 80% and 30%, respectively
5. Long term toxicities of treatment:
Cardiovascular disease, lymphoedema and lung fibrosis are the main long term toxicities of testis cancer treatment
Concerns about long term toxicities have led to less treatment being given to testis cancer patients as a whole
Radiotherapy is contraindicated in patients with ICGNU in the contralateral testis if they wish to preserve their fertility
Most men have normal fertility at diagnosis
Semen cryopreservation is recommended for all men undergoing treatment
Acknowledgements
We thank Dr Paul Rogers, consultant medical oncologist at the Royal Berkshire Cancer Centre, for providing the images for fig 1 and 2.
Abbreviations
AFP - α‐fetoprotein, AUC, area under the concentration × time curve
β‐HCG - β‐human chorionic gonadotrophin
BEP - bleomycin, etoposide and cisplatin
CT - computed tomography
EORTC - European Organisation for Research and Treatment of Cancer
EP - etoposide and cisplatin
GCT - germ cell tumour
IGCCG - International Germ Cell Cancer Collaborative Group
ITGCN - intratubular germ cell neoplasia
LDH - lactate dehydrogenase
MRC - Medical Research Council
NSGCT - non‐seminomatous germ cell tumour
PET - positron emission tomography
RPLND - primary retroperitoneal lymph node dissection
SMR - standardised mortality ratio
VIP - vinblastine, etoposide and cisplatin
ANSWERS
(A) F (B) F (C) T (D) F
(A) F (B) T (C) F (D) T
(A) F (B) T (C) F (D) T (E) F
(A) T (B) F (C) T (D) F (E) F
(A) F (B) T (C) T (D) F (E) T
Footnotes
Competing interest statements: None declared
References
- 1.Office for National Statistics Cancer statistics registrations: registrations of cancer diagnosed in 2002 England. Series MB1 no.33. London: Office for National Statistics, 2005
- 2.Huyghe E, Matsuda T, Thonneau P. Increasing incidence of testicular cancer worldwide: a review. J Urol 20031705–11. [DOI] [PubMed] [Google Scholar]
- 3.Garner M J, Turner M C, Ghadirian P.et al Epidemiology of testicular cancer: an overview. Int J Cancer 2005116331–339. [DOI] [PubMed] [Google Scholar]
- 4.Lutke Holzik M F, Rapley E A, Crockford G P.et al Genetic predisposition of to testicular germ‐cell tumours. Lancet Oncol 20045363–371. [DOI] [PubMed] [Google Scholar]
- 5.Rapley E A, Crockford G P, Teare D.et al Localization to Xq27 of a susceptibility gene for testicular germ cell tumours. Nat Genet 200024197–200. [DOI] [PubMed] [Google Scholar]
- 6.Bosl G J, Ilson D H, Rodriguez E.et al Clinical relevance of the i (12p) marker chromosome in germ cell tumors. J Natl Cancer Inst 199486349–355. [DOI] [PubMed] [Google Scholar]
- 7.Looijenga L H, de Munnik H, Oosterhuis J W. A molecular model for the development of germ‐cell cancer. Int J Cancer 199983809–814. [DOI] [PubMed] [Google Scholar]
- 8.Bosl G J, Motzer R J. Testicular germ‐cell cancer. N Engl J Med 1997337242–253. [DOI] [PubMed] [Google Scholar]
- 9.Benson C B. The role of ultrasound in diagnosis and staging of testicular tumors. Semin Urol 19886189–202. [PubMed] [Google Scholar]
- 10.Skinner E C, Skinner D G. Surgery of testicular neoplasms. In: Walsh PC, Retik AB, Vaughan ED, Wein AJ, eds. Campbell's urology, 7th ed. WB Saunders 19983410–3432.
- 11.Passarella M, Usta M F, Bivalacqua T J.et al Testicular‐sparing surgery: a reasonable option in selected patients with testicular lesions. BJU International 200391337–340. [DOI] [PubMed] [Google Scholar]
- 12.Hoei‐Hansen C E, Rajpert‐De Meyts E, Daugaard G.et al Carcinoma in situ testis, the progenitor of testicular germ cell tumours: a clinical review. Ann Oncol 200516863–868. [DOI] [PubMed] [Google Scholar]
- 13.Krege S, Souchon R, Schmoll H J. German Testicular Cancer Study Group. Interdisciplinary consensus on diagnosis and treatment of testicular germ cell tumors: result of an update conference on evidence‐based medicine (EBM). Eur J Urol 2001401962–1968. [DOI] [PubMed] [Google Scholar]
- 14.Horwich A. Testicular cancer. In: Horwich A, ed. Oncology – a multidisciplinary textbook. London: Chapman and Hall, 1995485–498.
- 15.International Germ Cell Cancer Collaborative Group International germ cell consensus classification: a prognostic factor based staging system for metastatic germ cell cancers. J Clin Oncol 199715594–603. [DOI] [PubMed] [Google Scholar]
- 16.Warde P R, Chung P, Sturgeon J.et al Should surveillance be considered the standard of care in stage I seminoma? J Clin Oncol 200523382s [abstract] [Google Scholar]
- 17.Warde P, Specht L, Horwich A.et al Prognostic factors for relapse in stage I seminoma managed by surveillance: a pooled analysis. J Clin Oncol 2002204448–4452. [DOI] [PubMed] [Google Scholar]
- 18.Bamberg M, Schmidberger H, Meisner C.et al Radiotherapy for stages I and IIA/B testicular seminoma. Int J Cancer 199983823–827. [DOI] [PubMed] [Google Scholar]
- 19.Fossa S D, Horwich A, Russell J M.et al Optimal planning target volume for stage I testicular seminoma: a Medical Research Council randomized trial. J Clin Oncol 1999171146–1153. [DOI] [PubMed] [Google Scholar]
- 20.Jones W G, Fossa S D, Mead G M.et al Randomized trial of 30 versus 20 Gy in the adjuvant treatment of stage I testicular seminoma: a report on Medical Research Council Trial TE18, European Organization for the Research and Treatment of Cancer Trial 30942 [ISRCTN 18525328]. J Clin Oncol 2005231200–1208. [DOI] [PubMed] [Google Scholar]
- 21.Oliver T, Dieckmann K P, Steiner H.et al Pooled analysis of phase 2 reports of 2 v 1 course of carboplatin as adjuvant for stage I seminoma. J Clin Oncol 200523395s [abstract] [Google Scholar]
- 22.Oliver R T, Mason M D, Mead G M.et al Radiotherapy versus single‐dose carboplatin in adjuvant treatment of stage I seminoma: a randomised trial. Lancet 2005366293–300. [DOI] [PubMed] [Google Scholar]
- 23.Classen J, Schmidberger H, Meisner C.et al Radiotherapy for stages IIA/B testicular seminoma: final report of a prospective multicenter clinical trial. J Clin Oncol 2003211101–1106. [DOI] [PubMed] [Google Scholar]
- 24.Garcia Del Muro X, Maroto P, Guma J.et al Chemotherapy as an alternative to radiotherapy in the treatment of stages IIA/IIB testicular seminoma: a Spanish Germ Cell Cancer Group study. Proc Am Soc Clin Oncol 200423388a [abstract]. [DOI] [PubMed] [Google Scholar]
- 25.Bosl G J, Bajorin D F, Sheinfeld J.et al Cancer of the testis. In: De Vita VT, Hellman S, Rosenberg SA, eds. Cancer: principles and practice of oncology. Philadelphia: Lippincott‐Raven, 20051491–1518.
- 26.Flechon A, Bompas E, Biron P.et al Management of post‐chemotherapy residual masses in advanced seminoma. J Urol 20021681975–1979. [DOI] [PubMed] [Google Scholar]
- 27.Horwich A, Paluchowska A, Norman R.et al Residual mass following chemotherapy of seminoma. Ann Oncol 1997837–40. [DOI] [PubMed] [Google Scholar]
- 28.De Santis M, Becherer A, Bokemeyer C.et al 2–18fluoro‐deoxy‐D‐glucose positron emission tomography is a reliable predictor for viable tumor in postchemotherapy seminoma; an update of the prospective multicenter SEMPET trial. J Clin Oncol 2004221034–1039. [DOI] [PubMed] [Google Scholar]
- 29.Albers P, Siener R, Kliesch S. Risk factors for relapse in clinical stage I nonseminomatous testicular germ cell tumors: results of the german testicular cancer study group trial. J Clin Oncol 2003211505–1512. [DOI] [PubMed] [Google Scholar]
- 30.Heidenreich A, Sesterhenn I A, Mostofi F K.et al Prognostic risk factors that identify patients with clinical stage I nonseminomatous germ cell tumors at low risk and high risk for metastasis. Cancer 1998831002–1011. [PubMed] [Google Scholar]
- 31.Oliver R T, Ong J, Shamash J.et al Long‐term follow up of Anglian Germ Cell Cancer Group surveillance versus patients with stage I nonseminoma treated with adjuvant chemotherapy. Urology 200463556–561. [DOI] [PubMed] [Google Scholar]
- 32.Foster R S, Donohue J P. Retroperitoneal lymph node dissection for the management of clinical stage I nonseminoma. J Urol 20001631788–1792. [PubMed] [Google Scholar]
- 33.Stephenson A J, Sheinfeld J. The role of retroperitoneal lymph node dissection in the management of testicular cancer. Urologic Oncology: Seminars and Original Investigations 200422225–235. [DOI] [PubMed] [Google Scholar]
- 34.Einhorn L H, Williams S D, Troner M.et al The role of maintenance therapy in disseminated testicular cancer. N Engl J Med 1981305727–731. [DOI] [PubMed] [Google Scholar]
- 35.Williams S D, Birch R, Einhorn L H.et al Treatment of disseminated germ‐cell tumors with cisplatin, bleomycin, and either vinblastine or etoposide. N Eng J Med 19873161435–1440. [DOI] [PubMed] [Google Scholar]
- 36.De Wit R, Roberts J T, Wilkinson P M.et al Equivalence of three or for cycles of bleomycin, etoposide, and ciplatinum chemotherapy and of a 3 or 5‐day schedule in good‐prognosis germ cell cancer: a randomized study of the European Organization for Research and Treatment of Cancer Genitourinary Tract Cancer Cooperative Group and the Medical Research Council. J Clin Oncol 20011991–96. [DOI] [PubMed] [Google Scholar]
- 37.Saxman S B, Finch D, Gonin R.et al Long‐term follow up of a phase III study of three versus four cycles of bleomycin, etoposide, and cisplatin in favorable‐prognosis germ‐cell tumors: the Indian University experience. J Clin Oncol 199816702–706. [DOI] [PubMed] [Google Scholar]
- 38.Loehrer P J, Johnson D, Elison P.et al Importance of bleomycin in favorable prognosis disseminated germ‐cell tumors: an Eastern Cooperative Oncology Group trial. J Clin Oncol 199513470–476. [DOI] [PubMed] [Google Scholar]
- 39.de Wit R, Stoter G, Kaye S B.et al Importance of bleomycin in combination chemotherapy for good prognosis testicular nonseminom: a randomized study of the European Organization for Research and Treatment of Cancer Genitourinary Tract Cancer Cooperative Group. J Clin Oncol 1997151837–1843. [DOI] [PubMed] [Google Scholar]
- 40.de Wit R, Roberts J T, Wilkinson P M.et al Equivalence of three or four cycles of bleomycin, etoposide, and cisplatin chemotherapy and of a 3‐ or 5‐day schedule in good‐prognosis germ cell cancer: a randomized study of the European Organization for Research and Treatment of Cancer Genitourinary Tract Cancer Cooperative Group and the Medical Research Council. J Clin Oncol 2001191629–1640. [DOI] [PubMed] [Google Scholar]
- 41.Christian J, Huddart R, Norman A.et al Intensive induction chemotherapy with CBOP/BEP in patients with poor prognosis germ cell tumors. J Clin Oncol 200321871–877. [DOI] [PubMed] [Google Scholar]
- 42.Berney D M, Shamash J, Hendry W F.et al Prediction of relapse after lymph node dissection for germ cell tumours: can salvage chemotherapy be avoided? Br J Cancer 200184340–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loehrer P, Gonin R, Nicols C.et al Vinblastine plus ifosfamide plus cisplatin as initial salvage therapy in recurrent germ cell tumor. J Clin Oncol 1998162500–2504. [DOI] [PubMed] [Google Scholar]
- 44.Mead G M, Cullen M H, Huddart R.et al A phase II trial of TIP (paclitaxel, ifosfamide and cisplatin) given as second‐line (post BEP) salvage chemotherapy for patients with metastatic germ cell cancer: a Medical Research Council trial. Br J Cancer 200593178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Motzer R J, Sheinfeld J, Mazumdar M.et al Paclitaxel, ifosfamide, and cisplatin second‐line therapy for patients with relapsed testicular germ cell cancer. J Clin Oncol 2000182413–2418. [DOI] [PubMed] [Google Scholar]
- 46.Bhatia S, Abonour R, Porcu P.et al High dose chemotherapy as initial salvage chemotherapy in patients with relapsed testicular cancer. J Clin Oncol 2000183346–3351. [DOI] [PubMed] [Google Scholar]
- 47.Pico J, Rosti G, Kramar A.et al A randomized trial of high dose chemotherapy in the salvage treatment of patients failing first line platinum chemotherapy for advanced germ cell tumors. Ann Oncol 2005161152–1159. [DOI] [PubMed] [Google Scholar]
- 48.Huddart R, Norman A, Shahidi M.et al Cardiovascular disease as a long‐term complication of treatment of testicular cancer. J Clin Oncol 2003211513–1523. [DOI] [PubMed] [Google Scholar]
- 49.Zagars G K, Ballo M T, Lee A K.et al Mortality after cure of testicular seminoma. J Clin Oncol 200422640–647. [DOI] [PubMed] [Google Scholar]
- 50.Travis L, Curtis R, Storm H.et al Risk of second malignant neoplasms among long‐term survivors of testicular cancer. J Natl Cancer Inst 1997891429–1439. [DOI] [PubMed] [Google Scholar]
- 51.Fossa S D, Kravdal O. Fertility in Norwegian testicular cancer patients. Br J Cancer 200082737–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huddart R A, Norman A, Moynihan C.et al Fertility, gonadal and sexual function in survivors of testicular cancer. Br J Cancer 200593200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huyghe E, Matsuda T, Daudin M.et al Fertility after testicular cancer treatments: results of a large multicenter study. Cancer 2004100732–737. [DOI] [PubMed] [Google Scholar]
- 54.Kollmansberger C, Beyer J, Liersch R.et al Combination chemotherapy with gemcitabine plus oxaliplatin in patients with intensively pretreated or refractory germ cell cancer: a study of the German Testicular Cancer Study Group. J Clin Oncol 200422108–114. [DOI] [PubMed] [Google Scholar]
- 55.Pectasides D, Pectasides M, Farmakis D.et al Gemcitabine and oxaliplatin (GEMOX) in patients with cisplatin‐refractory germ cell tumors: a phase II study. Ann Oncol 200415493–497. [DOI] [PubMed] [Google Scholar]
- 56.Farmakis D, Pectasides D, Pectasides M.et al Oxaliplatin and irinotecan plus granulocyte‐colony stimulating factor (G‐CSF) as third‐line treatment in relapsed or cisplatin‐refractory germ‐cell tumor (GCT) patients: a phase II study. Proc Am Soc Clin Oncol. 2004;23:389 Abstract 4545 [DOI] [PubMed]