Abstract
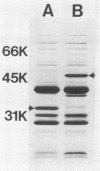
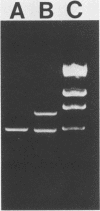
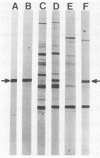
One major outer membrane protein (P1) of Haemophilus influenzae type b (Hib), with an apparent molecular weight of 34,000 (34K) as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), has been shown to be heat modifiable. After heating at 100 degrees C for 5 min in 2% SDS, the P1 protein exhibits an apparent molecular weight of 49,000 (49K) in SDS-PAGE. Monoclonal antibodies (MAbs) reactive with P1 bound to the surface of Hib, and one of these MAbs had a protective effect against the development of Hib bacteremia in an animal model for invasive Hib disease. A 6-kilobase Hib DNA insert containing the gene encoding this P1 protein was cloned into Escherichia coli by using the gamma gt11 expression vector. Recombinant phage expressing P1 were identified by screening phage plaques with a MAb directed against the P1 protein. Expression of the P1 protein by an E. coli lysogen carrying the recombinant phage was independent of both vegetative phage growth and induction of lacZ gene-directed transcription of the Hib DNA insert. The Hib DNA insert encoding the P1 protein was subcloned into the plasmid vector pBR322, and a transformant containing the recombinant plasmid pFRG100 was identified with the P1 protein-directed MAb in a colony blot-radioimmunoassay. Western blot (immunoblot) analysis determined that the recombinant P1 protein possessed heat-modifiability characteristics identical to those of the native Hib protein. The P1 protein was expressed on the surface of both the E. coli lysogen containing the recombinant phage and the E. coli transformant containing pFRG100. Western blot analysis of acute- and convalescent-phase sera from infants with Hib meningitis showed that antibodies in the convalescent-phase sera recognized the P1 protein expressed by the E. coli transformant containing pFRG100. The availability of this cloned Hib DNA insert encoding the Hib P1 protein and the expression of this protein on the surface of recombinant E. coli should facilitate the investigation of P1 for both its vaccinogenic potential and its functional role in the outer membrane of Hib.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baxter-Gabbard K. L. A simple method for the large-scale preparation of sucrose gradients. FEBS Lett. 1972 Jan 15;20(1):117–119. doi: 10.1016/0014-5793(72)80031-0. [DOI] [PubMed] [Google Scholar]
- Bricker J., Mulks M. H., Plaut A. G., Moxon E. R., Wright A. IgA1 proteases of Haemophilus influenzae: cloning and characterization in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1983 May;80(9):2681–2685. doi: 10.1073/pnas.80.9.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochi S. L., Broome C. V. Vaccine prevention of Haemophilus influenzae type b disease: past, present and future. Pediatr Infect Dis. 1986 Jan-Feb;5(1):12–19. doi: 10.1097/00006454-198601000-00003. [DOI] [PubMed] [Google Scholar]
- Coulton J. W., Wan D. T. The outer membrane of haemophilus influenzae type b: cell envelope associations of major proteins. Can J Microbiol. 1983 Feb;29(2):280–287. doi: 10.1139/m83-046. [DOI] [PubMed] [Google Scholar]
- Engleberg N. C., Pearlman E., Eisenstein B. I. Legionella pneumophila surface antigens cloned and expressed in Escherichia coli are translocated to the host cell surface and interact with specific anti-Legionella antibodies. J Bacteriol. 1984 Oct;160(1):199–203. doi: 10.1128/jb.160.1.199-203.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin A. L., Kenny G. E. Haemophilus influenzae type b isolates show antigenic variation in a major outer membrane protein. Infect Immun. 1984 Nov;46(2):570–577. doi: 10.1128/iai.46.2.570-577.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C., Blake M. S., Koomey J. M., Seiff M., Derman A. Cloning of the structural genes of three H8 antigens and of protein III of Neisseria gonorrhoeae. J Exp Med. 1986 Sep 1;164(3):868–881. doi: 10.1084/jem.164.3.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granoff D. M., Munson R. S., Jr Prospects for prevention of Haemophilus influenzae type b disease by immunization. J Infect Dis. 1986 Mar;153(3):448–461. doi: 10.1093/infdis/153.3.448. [DOI] [PubMed] [Google Scholar]
- Gulig P. A., Hansen E. J. Coprecipitation of lipopolysaccharide and the 39,000-molecular-weight major outer membrane protein of Haemophilus influenzae type b by lipopolysaccharide-directed monoclonal antibody. Infect Immun. 1985 Sep;49(3):819–827. doi: 10.1128/iai.49.3.819-827.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A., McCracken G. H., Jr, Frisch C. F., Johnston K. H., Hansen E. J. Antibody response of infants to cell surface-exposed outer membrane proteins of Haemophilus influenzae type b after systemic Haemophilus disease. Infect Immun. 1982 Jul;37(1):82–88. doi: 10.1128/iai.37.1.82-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A., McCracken G. H., Jr, Holmans P. L., Hansen E. J. Immunogenic proteins in cell-free culture supernatants of Haemophilus influenzae type b. Infect Immun. 1984 Apr;44(1):41–48. doi: 10.1128/iai.44.1.41-48.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmans P. L., Loftus T. A., Hansen E. J. Cloning and surface expression in Escherichia coli of a structural gene encoding a surface protein of Haemophilus influenzae type b. Infect Immun. 1985 Oct;50(1):236–242. doi: 10.1128/iai.50.1.236-242.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., Warren K. A., Tam M., Frank M. M. Monoclonal antibodies directed against gonococcal protein I vary in bactericidal activity. J Immunol. 1985 May;134(5):3411–3419. [PubMed] [Google Scholar]
- Kimura A., Gulig P. A., McCracken G. H., Jr, Loftus T. A., Hansen E. J. A minor high-molecular-weight outer membrane protein of Haemophilus influenzae type b is a protective antigen. Infect Immun. 1985 Jan;47(1):253–259. doi: 10.1128/iai.47.1.253-259.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A., Hansen E. J. Antigenic and phenotypic variations of Haemophilus influenzae type b lipopolysaccharide and their relationship to virulence. Infect Immun. 1986 Jan;51(1):69–79. doi: 10.1128/iai.51.1.69-79.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb M. R., Smith D. H. Human antibody response to individual outer membrane proteins of Haemophilus influenzae type b. Infect Immun. 1982 Sep;37(3):1032–1036. doi: 10.1128/iai.37.3.1032-1036.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxon E. R., Vaughn K. A. The type b capsular polysaccharide as a virulence determinant of Haemophilus influenzae: studies using clinical isolates and laboratory transformants. J Infect Dis. 1981 Apr;143(4):517–524. doi: 10.1093/infdis/143.4.517. [DOI] [PubMed] [Google Scholar]
- Munson R. S., Jr, Granoff D. M. Purification and partial characterization of outer membrane proteins P5 and P6 from Haemophilus influenzae type b. Infect Immun. 1985 Sep;49(3):544–549. doi: 10.1128/iai.49.3.544-549.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson R. S., Jr, Shenep J. L., Barenkamp S. J., Granoff D. M. Purification and comparison of outer membrane protein P2 from Haemophilus influenzae type b isolates. J Clin Invest. 1983 Aug;72(2):677–684. doi: 10.1172/JCI111017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T. F., Bartos L. C., Rice P. A., Nelson M. B., Dudas K. C., Apicella M. A. Identification of a 16,600-dalton outer membrane protein on nontypeable Haemophilus influenzae as a target for human serum bactericidal antibody. J Clin Invest. 1986 Oct;78(4):1020–1027. doi: 10.1172/JCI112656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T. F., Dudas K. C., Mylotte J. M., Apicella M. A. A subtyping system for nontypable Haemophilus influenzae based on outer-membrane proteins. J Infect Dis. 1983 May;147(5):838–846. doi: 10.1093/infdis/147.5.838. [DOI] [PubMed] [Google Scholar]
- Roberts M., Stull T. L., Smith A. L. Comparative virulence of Haemophilus influenzae with a type b or type d capsule. Infect Immun. 1981 May;32(2):518–524. doi: 10.1128/iai.32.2.518-524.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson S. M., Frisch C. F., Gulig P. A., Kettman J. R., Johnston K. H., Hansen E. J. Monoclonal antibodies directed against a cell surface-exposed outer membrane protein of Haemophilus influenzae type b. Infect Immun. 1982 Apr;36(1):80–88. doi: 10.1128/iai.36.1.80-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas W. R., Rossi A. A. Molecular cloning of DNA coding for outer membrane proteins of Haemophilus influenzae type b. Infect Immun. 1986 Jun;52(3):812–817. doi: 10.1128/iai.52.3.812-817.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vachon V., Lyew D. J., Coulton J. W. Transmembrane permeability channels across the outer membrane of Haemophilus influenzae type b. J Bacteriol. 1985 Jun;162(3):918–924. doi: 10.1128/jb.162.3.918-924.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Alphen L., Riemens T., Poolman J., Zanen H. C. Characteristics of major outer membrane proteins of Haemophilus influenzae. J Bacteriol. 1983 Aug;155(2):878–885. doi: 10.1128/jb.155.2.878-885.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]