Abstract
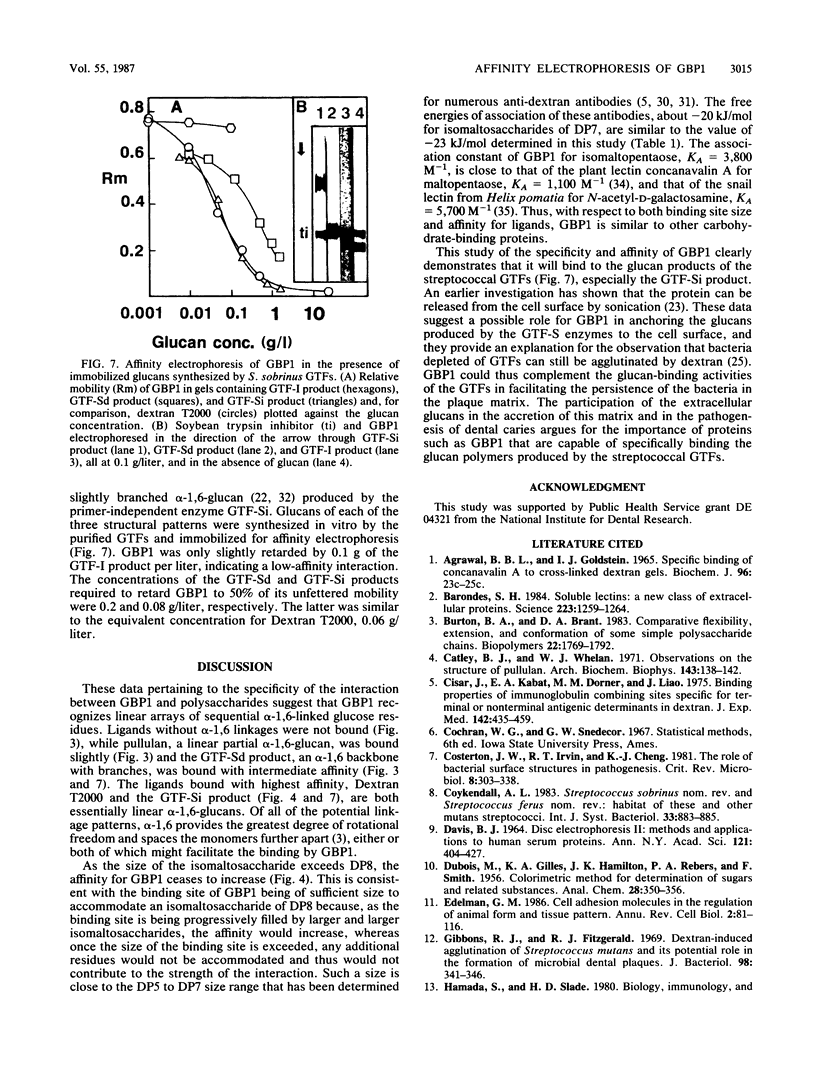
Glucan-binding protein 1 (GBP1), the most abundant glucan-binding protein isolated from culture supernatants of Streptococcus sobrinus 6715-49, has been purified by affinity chromatography on Sephadex G-50 followed by gel permeation chromatography with Bio-Gel P-10. The specificity and affinity of GBP1 for glucans were assessed by affinity electrophoresis. GBP1 did not detectably bind to glucans lacking linear arrays of alpha-1,6 linkages. The association constant for the linear alpha-1,6-glucan Dextran T2000 was 3 x 10(7) M-1. Providing small isomaltosaccharide ligands to compete with this dextran indicated that the binding site maximally accommodated isomaltosaccharides with a degree of polymerization of 8. When glucans produced by purified S. sobrinus glucosyltransferases were tested, GBP1 displayed the highest affinity for the glucan from the soluble-product, primer-independent glucosyltransferase.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barondes S. H. Soluble lectins: a new class of extracellular proteins. Science. 1984 Mar 23;223(4642):1259–1264. doi: 10.1126/science.6367039. [DOI] [PubMed] [Google Scholar]
- Catley B. J., Whelan W. J. Observations on the structure of pullulan. Arch Biochem Biophys. 1971 Mar;143(1):138–142. doi: 10.1016/0003-9861(71)90193-7. [DOI] [PubMed] [Google Scholar]
- Cisar J., Kabat E. A., Dorner M. M., Liao J. Binding properties of immunoglobulin combining sites specific for terminal or nonterminal antigenic determinants in dextran. J Exp Med. 1975 Aug 1;142(2):435–459. doi: 10.1084/jem.142.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Irvin R. T., Cheng K. J. The role of bacterial surface structures in pathogenesis. Crit Rev Microbiol. 1981;8(4):303–338. doi: 10.3109/10408418109085082. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Cell adhesion molecules in the regulation of animal form and tissue pattern. Annu Rev Cell Biol. 1986;2:81–116. doi: 10.1146/annurev.cb.02.110186.000501. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Fitzgerald R. J. Dextran-induced agglutination of Streptococcus mutans, and its potential role in the formation of microbial dental plaques. J Bacteriol. 1969 May;98(2):341–346. doi: 10.1128/jb.98.2.341-346.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horejsí V. Affinity electrophoresis. Methods Enzymol. 1984;104:275–281. [PubMed] [Google Scholar]
- Jones G. W., Isaacson R. E. Proteinaceous bacterial adhesins and their receptors. Crit Rev Microbiol. 1983;10(3):229–260. doi: 10.3109/10408418209113564. [DOI] [PubMed] [Google Scholar]
- Jovin T. M. Multiphasic zone electrophoresis. 3. Further analysis and new forms of discontinuous buffer systems. Biochemistry. 1973 Feb 27;12(5):890–898. doi: 10.1021/bi00729a016. [DOI] [PubMed] [Google Scholar]
- Klemm P. Fimbrial adhesions of Escherichia coli. Rev Infect Dis. 1985 May-Jun;7(3):321–340. doi: 10.1093/clinids/7.3.321. [DOI] [PubMed] [Google Scholar]
- McCabe M. M., Hamelik R. M., Smith E. E. Purification of dextran-binding protein from cariogenic Streptococcus mutans. Biochem Biophys Res Commun. 1977 Sep 9;78(1):273–278. doi: 10.1016/0006-291x(77)91250-5. [DOI] [PubMed] [Google Scholar]
- McCabe M. M. Purification and characterization of a primer-independent glucosyltransferase from Streptococcus mutans 6715-13 mutant 27. Infect Immun. 1985 Dec;50(3):771–777. doi: 10.1128/iai.50.3.771-777.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe M. M., Smith E. E. Relationship between cell-bound dextransucrase and the agglutination of Streptococcus mutans. Infect Immun. 1975 Sep;12(3):512–520. doi: 10.1128/iai.12.3.512-520.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W. E., Kurelec B., Zahn R. K., Müller I., Vaith P., Uhlenbruck G. Aggregation of sponge cells. Function of a lectin in its homologous biological system. J Biol Chem. 1979 Aug 25;254(16):7479–7481. [PubMed] [Google Scholar]
- Pricer W. E., Jr, Ashwell G. The binding of desialylated glycoproteins by plasma membranes of rat liver. J Biol Chem. 1971 Aug 10;246(15):4825–4833. [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Sharon J., Kabat E. A., Morrison S. L. Association constants of hybridoma antibodies specific for alpha (1 leads to 6) linked dextran determined by affinity electrophoresis. Mol Immunol. 1982 Mar;19(3):389–397. doi: 10.1016/0161-5890(82)90204-8. [DOI] [PubMed] [Google Scholar]
- Sharon J., Kabat E. A., Morrison S. L. Immunochemical characterization of binding sites of hybridoma antibodies specific for alpha (1 leads to 6) linked dextran. Mol Immunol. 1982 Mar;19(3):375–388. doi: 10.1016/0161-5890(82)90203-6. [DOI] [PubMed] [Google Scholar]
- Shimamura A., Tsumori H., Mukasa H. Three kinds of extracellular glucosyltransferases from Streptococcus mutans 6715 (serotype g). FEBS Lett. 1983 Jun 27;157(1):79–84. doi: 10.1016/0014-5793(83)81120-x. [DOI] [PubMed] [Google Scholar]
- Sutherland I. W. Microbial exopolysaccharides -- their role in microbial adhesion in aqueous systems. Crit Rev Microbiol. 1983;10(2):173–201. doi: 10.3109/10408418209113562. [DOI] [PubMed] [Google Scholar]
- Yoshii T., Ishiyama I. Thermodynamics of binding between saccharides and Helix pomatia A hemagglutinin. Biochim Biophys Acta. 1983 Jan 12;742(1):235–242. doi: 10.1016/0167-4838(83)90381-3. [DOI] [PubMed] [Google Scholar]