Abstract
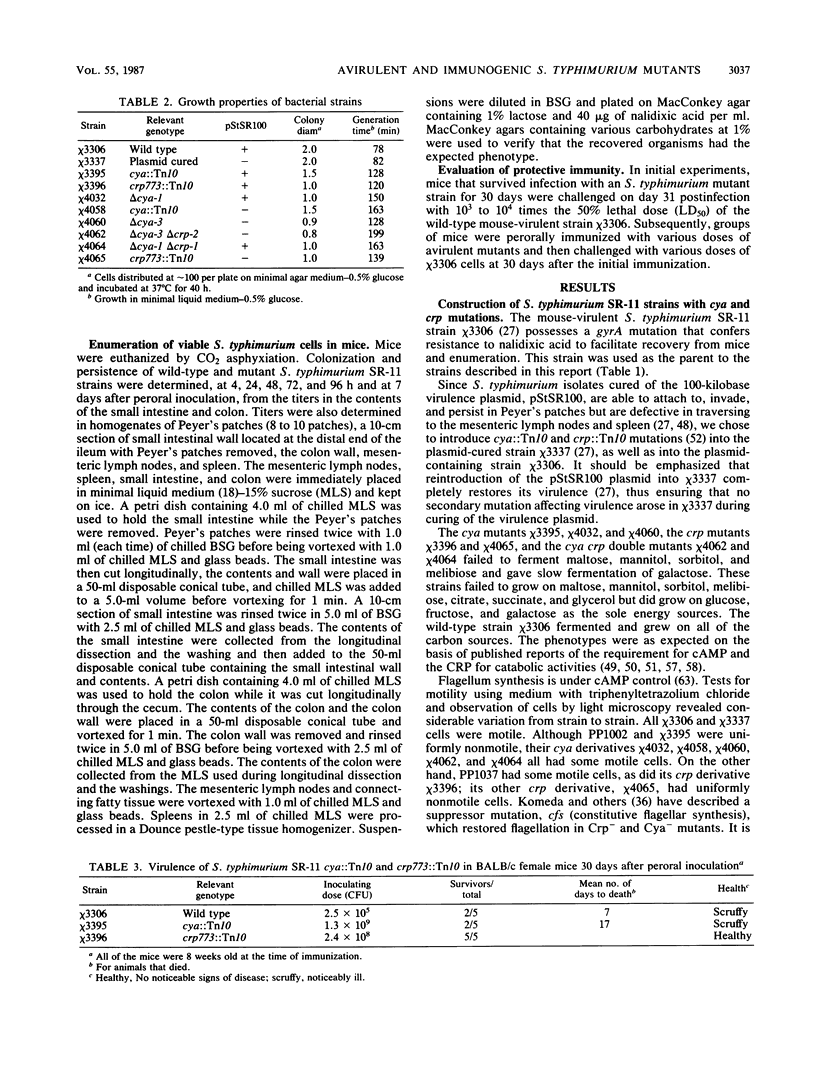
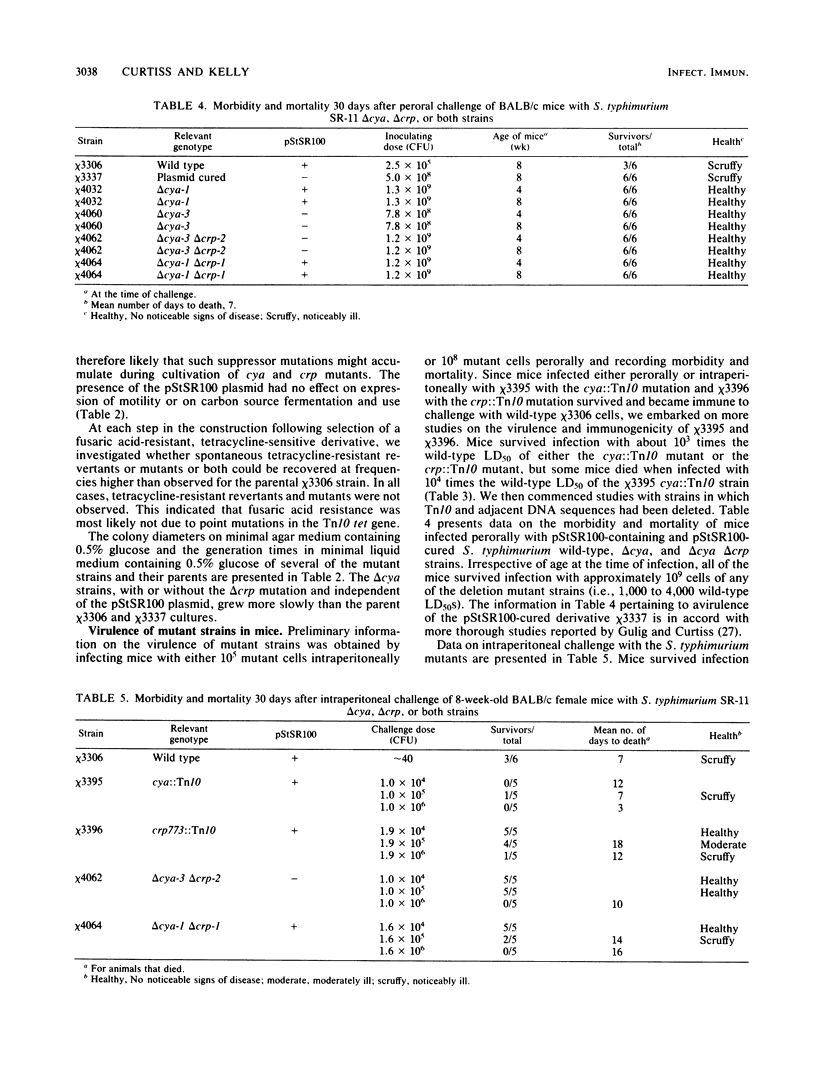
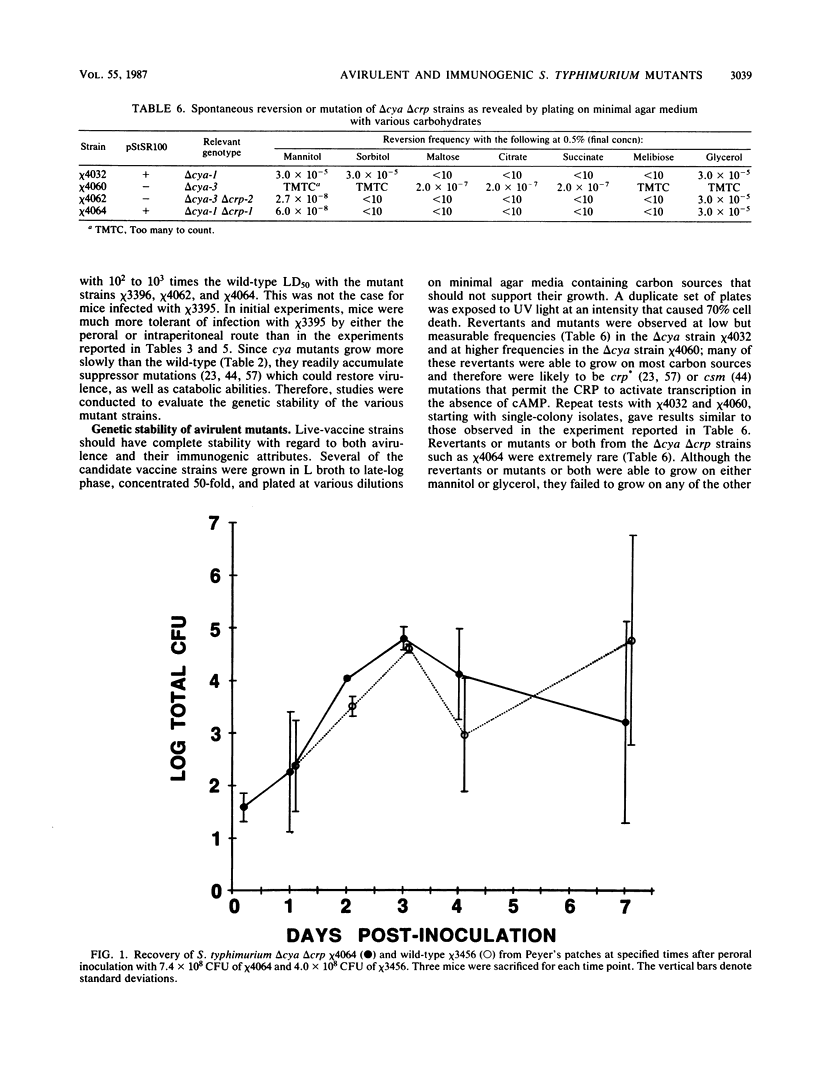
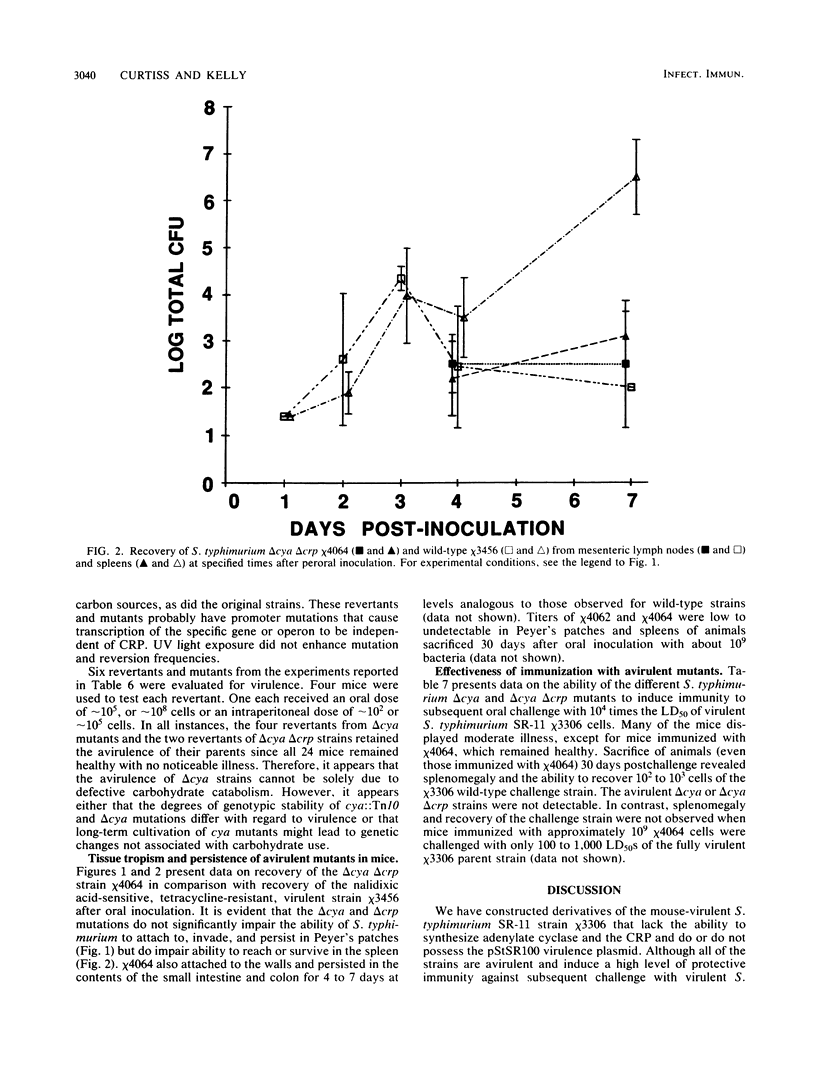
Salmonella typhimurium SR-11 mutants with cya::Tn10 or crp::Tn10 mutations were found to be avirulent and immunogenic for BALB/c mice. Fusaric acid-resistant derivatives with deletions of the Tn10 and adjacent DNA sequences were constructed in S. typhimurium SR-11 strains with or without the virulence plasmid pStSR100. These delta cya delta crp strains grew more slowly than wild-type strains. They possessed wild-type ability to attach to, invade, and persist in gut-associated lymphoid tissue for up to a week but exhibited a diminished ability to reach mesenteric lymph nodes and the spleen. Mice 4 to 8 weeks old were resistant to oral infection with 10(9) cells of several different delta cya and delta cya delta crp strains (the equivalent to 10(4) 50% lethal doses of wild-type S. typhimurium SR-11) and 30 days after immunization became resistant to oral challenge with 10(3) to 10(4) 50% lethal doses of wild-type S. typhimurium SR-11.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper M. D., Ames B. N. Transport of antibiotics and metabolite analogs by systems under cyclic AMP control: positive selection of Salmonella typhimurium cya and crp mutants. J Bacteriol. 1978 Jan;133(1):149–157. doi: 10.1128/jb.133.1.149-157.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asherson G. L., Zembala M., Perera M. A., Mayhew B., Thomas W. R. Production of immunity and unresponsiveness in the mouse by feeding contact sensitizing agents and the role of suppressor cells in the peyer's patches, mesenteric lymph nodes and other lymphoid tissues. Cell Immunol. 1977 Sep;33(1):145–155. doi: 10.1016/0008-8749(77)90142-3. [DOI] [PubMed] [Google Scholar]
- BACON G. A., BURROWS T. W., YATES M. The effects of biochemical mutation on the virulence of Bacterium typhosum: the virulence of mutants. Br J Exp Pathol. 1950 Dec;31(6):714–724. [PMC free article] [PubMed] [Google Scholar]
- BACON G. A., BURROWS T. W., YATES M. The effects of biochemical mutation on the virulence of Bacterium typhosum; the loss of virulence of certain mutants. Br J Exp Pathol. 1951 Apr;32(2):85–96. [PMC free article] [PubMed] [Google Scholar]
- Baird G. D., Manning E. J., Jones P. W. Evidence for related virulence sequences in plasmids of Salmonella dublin and Salmonella typhimurium. J Gen Microbiol. 1985 Jul;131(7):1815–1823. doi: 10.1099/00221287-131-7-1815. [DOI] [PubMed] [Google Scholar]
- Barton J. W., Melton T. Generation of deletions in the 3'-flanking sequences of the Escherichia coli crp gene that induce cyclic AMP suppressor functions. J Bacteriol. 1987 Feb;169(2):654–659. doi: 10.1128/jb.169.2.654-659.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin W. H., Jr, Turnbough C. L., Jr, Posey B. S., Briles D. E. The ability of Salmonella typhimurium to produce the siderophore enterobactin is not a virulence factor in mouse typhoid. Infect Immun. 1985 Nov;50(2):392–397. doi: 10.1128/iai.50.2.392-397.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienenstock J., McDermott M., Befus D., O'Neill M. A common mucosal immunologic system involving the bronchus, breast and bowel. Adv Exp Med Biol. 1978;107:53–59. doi: 10.1007/978-1-4684-3369-2_7. [DOI] [PubMed] [Google Scholar]
- Bochner B. R., Huang H. C., Schieven G. L., Ames B. N. Positive selection for loss of tetracycline resistance. J Bacteriol. 1980 Aug;143(2):926–933. doi: 10.1128/jb.143.2.926-933.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A., Hormaeche C. E., Demarco de Hormaeche R., Winther M., Dougan G., Maskell D. J., Stocker B. A. An attenuated aroA Salmonella typhimurium vaccine elicits humoral and cellular immunity to cloned beta-galactosidase in mice. J Infect Dis. 1987 Jan;155(1):86–92. doi: 10.1093/infdis/155.1.86. [DOI] [PubMed] [Google Scholar]
- CURTIS S. R., 3rd CHROMOSOMAL ABERRATIONS ASSOCIATED WITH MUTATIONS TO BACTERIOPHAGE RESISTANCE IN ESCHERICHIA COLI. J Bacteriol. 1965 Jan;89:28–40. doi: 10.1128/jb.89.1.28-40.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P. B., Collins F. M. The route of enteric infection in normal mice. J Exp Med. 1974 May 1;139(5):1189–1203. doi: 10.1084/jem.139.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebra J. J., Gearhart P. J., Kamat R., Robertson S. M., Tseng J. Origin and differentiation of lymphocytes involved in the secretory IgA responses. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):201–215. doi: 10.1101/sqb.1977.041.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. D., Lyon F. L., Lowe K. L., Farrand A. L., el-Morshidy S. Oral immunization of mice with attenuated Salmonella enteritidis containing a recombinant plasmid which codes for production of the B subunit of heat-labile Escherichia coli enterotoxin. Infect Immun. 1986 Sep;53(3):685–692. doi: 10.1128/iai.53.3.685-692.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd 1984 Kreshover lecture. Genetic analysis of Streptococcus mutans virulence and prospects for an anticaries vaccine. J Dent Res. 1986 Aug;65(8):1034–1045. doi: 10.1177/00220345860650080101. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd Biological containment and cloning vector transmissibility. J Infect Dis. 1978 May;137(5):668–675. doi: 10.1093/infdis/137.5.668. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Charamella L. J., Stallions D. R., Mays J. A. Parental functions during conjugation in Escherichia coli K-12. Bacteriol Rev. 1968 Dec;32(4 Pt 1):320–348. [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd Ultraviolet-induced genetic recombination in a partially diploid strain of Escherichia coli. Genetics. 1968 Jan;58(1):9–54. doi: 10.1093/genetics/58.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson C. O., Ealding W. Generalized systemic and mucosal immunity in mice after mucosal stimulation with cholera toxin. J Immunol. 1984 Jun;132(6):2736–2741. [PubMed] [Google Scholar]
- Formal S. B., Baron L. S., Kopecko D. J., Washington O., Powell C., Life C. A. Construction of a potential bivalent vaccine strain: introduction of Shigella sonnei form I antigen genes into the galE Salmonella typhi Ty21a typhoid vaccine strain. Infect Immun. 1981 Dec;34(3):746–750. doi: 10.1128/iai.34.3.746-750.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garges S., Adhya S. Sites of allosteric shift in the structure of the cyclic AMP receptor protein. Cell. 1985 Jul;41(3):745–751. doi: 10.1016/s0092-8674(85)80055-6. [DOI] [PubMed] [Google Scholar]
- Germanier R., Füer E. Isolation and characterization of Gal E mutant Ty 21a of Salmonella typhi: a candidate strain for a live, oral typhoid vaccine. J Infect Dis. 1975 May;131(5):553–558. doi: 10.1093/infdis/131.5.553. [DOI] [PubMed] [Google Scholar]
- Germanier R., Fürer E. Immunity in experimental salmonellosis. II. Basis for the avirulence and protective capacity of gal E mutants of Salmonella typhimurium. Infect Immun. 1971 Dec;4(6):663–673. doi: 10.1128/iai.4.6.663-673.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman R. H., Hornick R. B., Woodard W. E., DuPont H. L., Snyder M. J., Levine M. M., Libonati J. P. Evaluation of a UDP-glucose-4-epimeraseless mutant of Salmonella typhi as a liver oral vaccine. J Infect Dis. 1977 Dec;136(6):717–723. doi: 10.1093/infdis/136.6.717. [DOI] [PubMed] [Google Scholar]
- Gulig P. A., Curtiss R., 3rd Plasmid-associated virulence of Salmonella typhimurium. Infect Immun. 1987 Dec;55(12):2891–2901. doi: 10.1128/iai.55.12.2891-2901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett J., Kotlarski I., Mathan V., Francki K., Rowley D. The colonization of Peyer's patches by a strain of Salmonella typhimurium cured of the cryptic plasmid. J Infect Dis. 1986 Jun;153(6):1119–1125. doi: 10.1093/infdis/153.6.1119. [DOI] [PubMed] [Google Scholar]
- Hahn P., Cannon de Rodriguez W., Skala J. P. Effect of age and diet on cyclic nucleotide concentrations in the intestinal mucosa of developing rats. J Nutr. 1986 May;116(5):887–891. doi: 10.1093/jn/116.5.887. [DOI] [PubMed] [Google Scholar]
- Helmuth R., Stephan R., Bunge C., Hoog B., Steinbeck A., Bulling E. Epidemiology of virulence-associated plasmids and outer membrane protein patterns within seven common Salmonella serotypes. Infect Immun. 1985 Apr;48(1):175–182. doi: 10.1128/iai.48.1.175-182.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoiseth S. K., Stocker B. A. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981 May 21;291(5812):238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- Hong J. S., Smith G. R., Ames B. N. Adenosine 3':5'-cyclic monophosphate concentration in the bacterial host regulates the viral decision between lysogeny and lysis. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2258–2262. doi: 10.1073/pnas.68.9.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Rabert D. K., Svinarich D. M., Whitfield H. J. Association of adhesive, invasive, and virulent phenotypes of Salmonella typhimurium with autonomous 60-megadalton plasmids. Infect Immun. 1982 Nov;38(2):476–486. doi: 10.1128/iai.38.2.476-486.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Roth J., Botstein D. Genetic engineering in vivo using translocatable drug-resistance elements. New methods in bacterial genetics. J Mol Biol. 1977 Oct 15;116(1):125–159. doi: 10.1016/0022-2836(77)90123-1. [DOI] [PubMed] [Google Scholar]
- Komeda Y., Suzuki H., Ishidsu J. I., Iino T. The role of cAMP in flagellation of Salmonella typhimurium. Mol Gen Genet. 1976 Dec 31;142(4):289–298. doi: 10.1007/BF00271253. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LURIA S. E., BURROUS J. W. Hybridization between Escherichia coli and Shigella. J Bacteriol. 1957 Oct;74(4):461–476. doi: 10.1128/jb.74.4.461-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Herrington D., Murphy J. R., Morris J. G., Losonsky G., Tall B., Lindberg A. A., Svenson S., Baqar S., Edwards M. F. Safety, infectivity, immunogenicity, and in vivo stability of two attenuated auxotrophic mutant strains of Salmonella typhi, 541Ty and 543Ty, as live oral vaccines in humans. J Clin Invest. 1987 Mar;79(3):888–902. doi: 10.1172/JCI112899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloy S. R., Nunn W. D. Selection for loss of tetracycline resistance by Escherichia coli. J Bacteriol. 1981 Feb;145(2):1110–1111. doi: 10.1128/jb.145.2.1110-1111.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskell D. J., Sweeney K. J., O'Callaghan D., Hormaeche C. E., Liew F. Y., Dougan G. Salmonella typhimurium aroA mutants as carriers of the Escherichia coli heat-labile enterotoxin B subunit to the murine secretory and systemic immune systems. Microb Pathog. 1987 Mar;2(3):211–221. doi: 10.1016/0882-4010(87)90022-2. [DOI] [PubMed] [Google Scholar]
- McCaughan G., Basten A. Immune system of the gastrointestinal tract. Int Rev Physiol. 1983;28:131–157. [PubMed] [Google Scholar]
- Melton T., Snow L. L., Freitag C. S., Dobrogosz W. J. Isolation and characterization of cAMP suppressor mutants of Escherichia coli K12. Mol Gen Genet. 1981;182(3):480–489. doi: 10.1007/BF00293939. [DOI] [PubMed] [Google Scholar]
- Movva R. N., Green P., Nakamura K., Inouye M. Interaction of cAMP receptor protein with the ompA gene, a gene for a major outer membrane protein of Escherichia coli. FEBS Lett. 1981 Jun 15;128(2):186–190. doi: 10.1016/0014-5793(81)80077-4. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Sato S., Ohya T., Suzuki S., Ikeda S., Koeda T. Plasmid-cured Salmonella enteritidis AL1192 as a candidate for a live vaccine. Infect Immun. 1985 Nov;50(2):586–587. doi: 10.1128/iai.50.2.586-587.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old D. C., Corneil I., Gibson L. F., Thomson A. D., Duguid J. P. Fimbriation, pellicle formation and the amount of growth of salmonellas in broth. J Gen Microbiol. 1968 Apr;51(1):1–16. doi: 10.1099/00221287-51-1-1. [DOI] [PubMed] [Google Scholar]
- Pardon P., Popoff M. Y., Coynault C., Marly J., Miras I. Virulence-associated plasmids of Salmonella serotype Typhimurium in experimental murine infection. Ann Inst Pasteur Microbiol. 1986 Jul-Aug;137B(1):47–60. doi: 10.1016/s0769-2609(86)80093-x. [DOI] [PubMed] [Google Scholar]
- Pastan I., Adhya S. Cyclic adenosine 5'-monophosphate in Escherichia coli. Bacteriol Rev. 1976 Sep;40(3):527–551. doi: 10.1128/br.40.3.527-551.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I., Perlman R. Cyclic adenosine monophosphate in bacteria. Science. 1970 Jul 24;169(3943):339–344. doi: 10.1126/science.169.3943.339. [DOI] [PubMed] [Google Scholar]
- Perlman R. L., Pastan I. Pleiotropic deficiency of carbohydrate utilization in an adenyl cyclase deficient mutant of Escherichia coli. Biochem Biophys Res Commun. 1969 Sep 24;37(1):151–157. doi: 10.1016/0006-291x(69)90893-6. [DOI] [PubMed] [Google Scholar]
- Postma P. W., Keizer H. G., Koolwijk P. Transport of trehalose in Salmonella typhimurium. J Bacteriol. 1986 Dec;168(3):1107–1111. doi: 10.1128/jb.168.3.1107-1111.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R. N., Raj C. V. Salmonella typhimurium mutants affecting establishment of lysogeny. Mol Gen Genet. 1973 Sep 5;125(2):119–123. doi: 10.1007/BF00268864. [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr, Schmidt M. R., Leibowitz M. Cyclic AMP-dependent synthesis of fimbriae in Salmonella typhimurium: effects of cya and pts mutations. J Bacteriol. 1978 Apr;134(1):356–358. doi: 10.1128/jb.134.1.356-358.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E., Roth J. R. Linkage map of Salmonella typhimurium, Edition VI. Microbiol Rev. 1983 Sep;47(3):410–453. doi: 10.1128/mr.47.3.410-453.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieger H. Phage P22-mutants with increased or decreased transduction abilities. Mol Gen Genet. 1972;119(1):75–88. doi: 10.1007/BF00270447. [DOI] [PubMed] [Google Scholar]
- Scholte B. J., Postma P. W. Mutation in the crp gene of Salmonella typhimurium which interferes with inducer exclusion. J Bacteriol. 1980 Feb;141(2):751–757. doi: 10.1128/jb.141.2.751-757.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzel E. H., Jr, Bachman S., Levine R. A. Cyclic nucleotide activity in gastrointestinal tissues and fluids. Anal Biochem. 1977 Apr;78(2):395–405. doi: 10.1016/0003-2697(77)90100-2. [DOI] [PubMed] [Google Scholar]
- Tramont E. C., Chung R., Berman S., Keren D., Kapfer C., Formal S. B. Safety and antigenicity of typhoid-Shigella sonnei vaccine (strain 5076-1C). J Infect Dis. 1984 Feb;149(2):133–136. doi: 10.1093/infdis/149.2.133. [DOI] [PubMed] [Google Scholar]
- Yancey R. J., Breeding S. A., Lankford C. E. Enterochelin (enterobactin): virulence factor for Salmonella typhimurium. Infect Immun. 1979 Apr;24(1):174–180. doi: 10.1128/iai.24.1.174-180.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T., Gots J. S. Requirement of adenosine 3', 5'-cyclic phosphate for flagella formation in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1970 Aug;103(2):513–516. doi: 10.1128/jb.103.2.513-516.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]


