Abstract
A retrospective observational study in Taiwan, 1998–2004, identified 92 patients with group G streptococcal bacteremia; 86 had Streptococcus dysgalactiae subspecies equisimilis. The most common diagnosis was cellulitis (48 cases), followed by primary bacteremia (34 cases). Infection recurred in 9 patients. Mortality rate was low (3.3%); resistance to quinupristin-dalfopristin was high.
Keywords: Group G streptococcus, bacteremia, Taiwan, dispatch
Group G streptococci (GGS) are part of the normal microbial flora of the gastrointestinal tract, vagina, and skin and cause a variety of infections (1). Major underlying illnesses in patients with GGS bacteremia are malignancy, cardiovascular disease, diabetes mellitus, bone and joint diseases, and cirrhosis (1,2). Reported mortality rates for patients with GGS bacteremia also vary, ranging from 5% to 30% (1–3). Recent studies of β-hemolytic streptococci isolates carrying Lancefield group G antigen showed that they consist of Streptococcus dysgalactiae subspecies equisimilis, S. anginosus, and S. canis (2,4–6). To supplement the limited clinical information about bacteremia caused by GGS strains identified to the species level (2–4), we conducted a retrospective observational study.
The Study
We included all patients with GGS-positive blood cultures who had been treated from April 1998 through August 2004 at National Taiwan University Hospital, a 2,000-bed teaching hospital in northern Taiwan. We recorded demographic parameters, underlying illness, clinical diagnosis, and outcome for each patient. Clinical diagnosis was based on the attending physician’s judgment and examination results. Recurrence of bacteremia was defined as repeated positive blood culture after complete treatment (at least 14 days) of previous bacteremia.
Differentiation of GGS was based on colony size, hemolytic reaction, Voges-Proskauer reaction, and β-glucuronidase activity. All β-hemolytic streptococci, whether large or small colonies, were tested for Lancefield group by using an agglutination kit (Streptex; Murex Biotech Ltd., Dartford, UK). PCR to differentiate between S. anginosus and S. dysgalactiae subsp. equisimilis was performed for all GGS isolates as described (7). For identification of S. canis, a probable isolate was identified by a negative β-glucuronidase result and further confirmed with the 16sRNA method as described (8). Susceptibilities of these isolates were tested by using the broth microdilution method as defined by the Clinical and Laboratory Standards Institute (formerly National Committee for Clinical Laboratory Standards) (9).
To determine the similarity of isolates in cases of recurrence, we used pulsed-field gel electrophoresis (PFGE) as described (10). The emm typing of isolates in cases of recurrence were also determined as described (11). The first 160 bases sequenced by emmseq2 that had >95% identity were defined as having the same genotype (11).
During the study period, 106 episodes of GGS bacteremia in 92 patients had been recorded; 56 episodes occurred during the first half of the study period (before June 2001) and 50 episodes during the second half. The causative agent was S. dysgalactiae subsp. equisimilis for 99 episodes, S. anginosus for 5, and S. canis for 2. Bacteremia recurred for 9 patients (1 had 4 episodes, and 3 had 3 episodes); bacteremia was nosocomial for 7 patients and polymicrobial for 5. The clinical characteristics of the patients are summarized in Table 1. All 3 patients who died had a diagnosis of the primary bacteremia caused by S. dysgalactiae subsp. equisimilis.
Table 1. Clinical characteristic of 92 patients with group G streptococcal bacteremia, April 1998–August 2004, Taiwan.
| Characteristic | No. (%) patients |
|---|---|
| Age, y | |
| <10 | 1 (1.1) |
| 10–50 | 12 (13.0) |
| 51–75 | 68 (73.9) |
| >75 | 11 (12.0) |
| Median (range) |
72 (10–93) |
| Sex | |
| Male | 58 (63.0) |
| Female |
34 (37.0) |
| Underlying diseases | |
| Malignancy | 35 (38.0) |
| Genital | 10 (10.9) |
| Head and neck | 8 (8.7) |
| Gastrointestinal | 6 (6.5) |
| Hematologic | 3 (3.3) |
| Tissue edema | 25 (27.2) |
| Heart disease | 20 (21.7) |
| Post–coronary artery bypass graft | 6 (6.5) |
| Diabetes mellitus | 16 (17.4) |
| Central nervous system disease | 15 (16.3) |
| Liver cirrhosis | 9 (9.8) |
| Chronic renal disease | 8 (8.7) |
| Chronic lung disease | 6 (6.5) |
| Bone disease | 5 (5.4) |
| Deep venous thrombosis |
2 (2.2) |
| Type of infection | |
| Cellulitis | 48 (52.1)* |
| Primary bacteremia | 34 (36.9) |
| Deep-seated abscess | 4 (4.2)† |
| Neutropenia and fever | 3 (3.3) |
| Septic arthritis | 2 (2.2) |
| Urinary tract infection | 1 (1.1) |
| Infective endocarditis | 1 (1.1) |
| Pneumonia |
1 (1.1) |
| Initial findings | |
| Fever | 86 (93.5) |
| Leukocytosis (>10,000 cells/μL) | 34 (37.0) |
| Septic shock |
4 (5.4) |
| Outcome | |
| Death | 3 (3.3) |
| Recurrence of bacteremia | 9 (9.8) |
*Includes 2 patients who also had septic arthritis. †Includes 2 patients with psoas muscle abscess, 1 with epidural abscess, and 1 with deep neck infection.
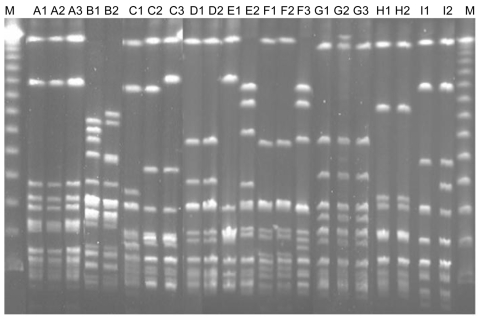
Among the 9 patients with recurrent bacteremia, the causative agent was S. dysgalactiae subsp. equisimilis for 8 and S. canis for 1. PFGE performed with all 13 available isolates from recurrent cases showed that 10 were identical to that of the initial episode, including 1 in a patient with recurrence of S. canis bacteremia. Sequence typing showed emm type stG485 for 4 patients. The clinical characteristics of the patients and emm typing results are shown in Table 2; PFGE results are shown in the Figure. The underlying diseases of patients with recurrent episodes included genital cancer (4 [44.4%] patients) and history of cellulitis (6 [66.7%]), each of which was significantly correlated with the likelihood of recurrence (p<0.01 for each). Further analysis showed that a previous history of cellulitis was significantly correlated with female sex (p = 0.01), genital cancer (p<0.01), tissue edema (p = 0.02), heart disease (p = 0.04), and post–coronary artery bypass graft (p = 0.03).
Table 2. Summary of characteristics of patients with recurrence of group G streptococcus bacteremia, April 1998–August 2004, Taiwan*.
| Patient no. | Age, y/ sex | Isolate | Date of isolation | Underlying disease | Clinical diagnosis | emm type | PFGE pattern |
|---|---|---|---|---|---|---|---|
| 1 | 67/F | A1 | 2001 May 28 | Coronary heart disease, post–coronary artery bypass graft |
Cellulitis | stG166b | – |
| A2 | 2002 Jul 18 | Cellulitis | stG166b | Identical | |||
|
|
|
A3 |
2003 Oct 14 |
Cellulitis |
stG166b |
Identical |
|
| 2 | 33/M | B1† | 2002 Nov 13 | Alcoholic liver cirrhosis, child C |
Primary bacteremia | STL1929.1 | – |
|
|
|
B2† |
2002 Oct 15 |
Primary bacteremia |
STL1929.1 |
Identical |
|
| 3 | 47/F | C1 | 1998 May 15 | Vulvar cancer after surgery and radiotherapy |
Cellulitis | stG166b | – |
| C2 | 2002 Jan 18 | Cellulitis | stG6.1 | Related | |||
|
|
|
C3 |
2002 Dec 19 |
Cellulitis |
stG6.1 |
Identical |
|
| 4 | 49/M | D1 | 2000 May 24 | Nasopharyngeal carcinoma after chemotherapy and radiotherapy |
Cellulitis | stG485 | – |
|
|
|
D2 |
2000 Aug 9 |
Cellulitis |
stG485 |
Identical |
|
| 5 | 28/M | E1 | 1998 Dec 26 | von Willebrand disease, type I |
Cellulitis | stG485 | – |
|
|
|
E2 |
1999 Aug 28 |
Cellulitis |
stG840 |
Different |
|
| 6 | 72/F | F1 | 1998 Aug 24 | Cervical cancer after surgery and radiotherapy, diabetes mellitus |
Cellulitis | stG485 | – |
| F2 | 1998 Oct 23 | Cellulitis | stG485 | Identical | |||
|
|
|
F3 |
1999 Dec 3 |
Cellulitis |
stG840 |
Different |
|
| 7 | 55/F | G1 | 1999 Oct 9 | Cervical cancer after surgery and radiotherapy |
Cellulitis | stG485 | – |
| G2 | 2000 Apr 18 | Cellulitis | stG485 | Identical | |||
| G3 | 2001 Sep 24 | Cellulitis | stG485 | Identical | |||
|
|
|
NA |
2000 Jul 19 |
Cellulitis |
NA |
NA |
|
| 8 | 46/M | H1 | 2001 Aug 21 | Acute myeloid leukemia (M4) |
Primary bacteremia | stGLP 1.0 | – |
|
|
|
H2 |
2001 Sep 6 |
Primary bacteremia |
stGLP 1.0 |
Identical |
|
| 9 | 80/F | I1 | 2003 May 5 | Cervical cancer with lung metastasis and obstructive uropathy | Primary bacteremia | stG245.0 | – |
| I2 | 2003 Nov 17 | Primary bacteremia | stG245.0 | Identical |
*PFGE, pulsed-field gel electrophoresis; NA, not available. †Streptoccus canis.
Figure.
Pulsed-field gel electrophoresis profiles of all isolates from patients with recurrent group G streptococcal bacteremia. Isolates B1 and B2, Streptococcus canis; other isolates, S. dysgalactiae subsp. equisimilis (see designation of the isolates in Table 2). Lane M, molecular mass marker.
Bacteremia caused by β-hemolytic S. anginosus with group G antigen was identified for 5 patients, none of whom had cellulitis, compared with 48 (55.8%) of the 86 patients with S. dysgalactiae subsp. equisimilis who did have cellulitis (p = 0.03). Polymicrobial bacteremia and nosocomial bacteremia were found in a higher percentage of patients with S. anginosus (60% and 40.0%, respectively) than of patients with S. dysgalactiae subsp. equisimilis bacteremia (4.7% and 5.8%, respectively); p<0.01 and p = 0.02, respectively. The 1 patient with S. canis bacteremia was a 33-year-old man with no history of dog bite. He had alcohol-associated liver cirrhosis of Child C (severe) classification and leg edema. He had 2 episodes of S. canis bacteremia 1 month apart. Echocardiogram results showed no evidence of valvular vegetation. For the first episode, the patient received a 14-day course of cefotaxime.
Antimicrobial drug–susceptibility testing showed decreased susceptibility to only macrolides (susceptibity rates: azithromycin 67.4%, clarithromycin 73.9%), clindamycin (87.0%), and quinupristin-dalfopristin (33.7%) (Appendix Table). No clinical factor correlated with macrolide resistance. All isolates of recurrent bacteremia were susceptible to macrolides.
Conclusions
We documented 5 cases of primary bacteremia caused by β-hemolytic group G S. anginosus and unintentionally documented recurrence of S. canis bacteremia. S. canis bacteremia in humans was first clearly described in 1997 (12).
Our finding of 5 β-hemolytic S. anginosus isolates and 1 S. canis isolate in patients with GGS bacteremia in this study differs from findings of previous studies (2,3). Factors that may have contributed to this discrepancy include serotype determination and PCR method. Serotype determination was performed for all β-hemolytic streptococci isolated in our hospital, whether colonies were large or small, which might have led to the detection of more streptococcal isolates with G antigen. The PCR method developed in our hospital and used in this study could effectively differentiate S. anginosus from S. dysgalactiae subsp. equisimilis (7).
Information about clinical infection with S. milleri with group G antigen is limited (4). In a previous study of GGS bacteremia, Cohen-Poradosu et al. reported that 6 of 84 patients had recurrence of bacteremia (3). We found recurrence in 9 of the 92 patients. Risk factors were similar to those previously reported for non–group A streptococcal cellulitis (13). PFGE of these isolates showed that a high percentage of recurrence was caused by identical strains. Although Cohen-Poradosu et al. reported that emm type stG840 was the most common strain (3), we found emm type stG485 to be most common.
For years in Taiwan, macrolide resistance of streptococci has been a major health problem (14,15). A previous study found erythromycin resistance in 23.5% of GGS strains (14). Although we did not test for erythromycin resistance, we found some resistance even to new macrolides. Since restriction of macrolide use in Taiwan, a linear relationship has been noted between the decline in erythromycin use and the decline in erythromycin resistance in S. pyogenes (15). Our study, however, found no decline in macrolide resistance from first half of the study period (27.1%) to the second half (37.0%).
In summary, in our study, infection with S. dysgalactiae subsp. equisimilis was the most common cause of GGS bacteremia. Infection recurred for ≈10%. The mortality rate for patients with GGS bacteremia was relatively low (<10%), but resistance to quinupristin-dalfopristin was extremely high.
Supplementary Material
In vitro susceptibilities of 92 isolates of group G Streptococcus, April 1998-August 2004, Taiwan
Biography
Dr Liao is an infectious diseases specialist at the Department of Internal Medicine, Far-Eastern Memorial Hospital. His major research interests are clinical and epidemiologic studies and pathogenesis of gram-positive bacterial infections, particularly streptococcal and methicillin-resistant Staphylococcus aureus infections.
Footnotes
Suggested citation for this article: Liao C-H, Liu L-C, Huang Y-T, Teng L-J, Hsueh P-R. Bacteremia caused by group G streptococci, Taiwan. Emerg Infect Dis [serial on the Internet]. 2008 May [date cited]. Available from http://www.cdc.gov/EID/content/14/5/837.htm
References
- 1.Auckenthaler R, Hermans PE, Washington JA II. Group G streptococcal bacteremia: clinical study and review of the literature. Rev Infect Dis. 1983;5:196–204. [DOI] [PubMed] [Google Scholar]
- 2.Woo PC, Fung AM, Lau SK, Wong SS, Yuen KY. Group G beta-hemolytic streptococcal bacteremia characterized by 16S ribosomal RNA gene sequencing. J Clin Microbiol. 2001;39:3147–55. 10.1128/JCM.39.9.3147-3155.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen-Poradosu R, Jaffe J, Lavi D, Grisariu-Greenzaid S, Nir-Paz R, Valinsky L, et al. Group G streptococcal bacteremia in Jerusalem. Emerg Infect Dis. 2004;10:1455–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Facklam R. What happened to the streptococci: overview of taxonomic and nomenclature changes. Clin Microbiol Rev. 2002;15:613–30. 10.1128/CMR.15.4.613-630.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vandamme P, Pot B, Falsen E, Kersters K, Devriese LA. Taxonomic study of Lancefield streptococcal groups C, G, and L (Streptococcus dysgalactiae) and proposal of S. dysgalactiae subsp. equisimilis subsp. nov. Int J Syst Bacteriol. 1996;46:774–81. [DOI] [PubMed] [Google Scholar]
- 6.Lawrence J, Yajko DM, Hadley WK. Incidence and characterization of beta-hemolytic Streptococcus milleri and differentiation from S. pyogenes (group A), S. equisimilis (group C), and large-colony group G streptococci. J Clin Microbiol. 1985;22:772–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu LC, Tsai JC, Hsueh PR, Teng LJ. Rapid differentiation between members of the anginosus group and Streptococcus dysgalactiae subsp. equisimilis within beta-hemolytic group C and G streptococci by PCR. J Clin Microbiol. 2006;44:1836–8. 10.1128/JCM.44.5.1836-1838.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whatmore AM, Engler KH, Gudmundsdottir G, Efstratiou A. Identification of isolates of Streptococcus canis infecting humans. J Clin Microbiol. 2001;39:4196–9. 10.1128/JCM.39.1.4196-4199.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A6. 5th ed. Wayne (PA): The Committee, 2004. [Google Scholar]
- 10.Hsueh PR, Teng LJ, Lee LN, Yang PC, Ho SW, Luh KT. Dissemination of high-level penicillin–, extended-spectrum cephalosporin–, and erythromycin–resistant Streptococcus pneumoniae clones in Taiwan. J Clin Microbiol. 1999;37:221–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Facklam R, Beall B, Efstratiou A, Fischetti V, Johnson D, Kaplan E, et al. emm typing and validation of provisional M types for group A streptococci. Emerg Infect Dis. 1999;5:247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bert F, Lambert-Zechovsky N. Septicemia caused by Streptococcus canis in a human. J Clin Microbiol. 1997;35:777–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baddour LM, Bisno AL. Non-group A beta-hemolytic streptococcal cellulitis. Association with venous and lymphatic compromise. Am J Med. 1985;79:155–9. 10.1016/0002-9343(85)90003-8 [DOI] [PubMed] [Google Scholar]
- 14.Wu JJ, Lin KY, Hsueh PR, Liu JW, Pan HI, Sheu SM. High incidence of erythromycin-resistant streptococci in Taiwan. Antimicrob Agents Chemother. 1997;41:844–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsueh PR, Shyr JM, Wu JJ. Changes in macrolide resistance among respiratory pathogens after decreased erythromycin consumption in Taiwan. Clin Microbiol Infect. 2006;12:296–8. 10.1111/j.1469-0691.2005.01348.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In vitro susceptibilities of 92 isolates of group G Streptococcus, April 1998-August 2004, Taiwan