To the Editor: Mediterranean spotted fever (MSF) is a Rickettsia conorii infection endemic to the Mediterranean. In this case, a 55-year-old man was referred to the Necker-Enfants Malades Hospital, Paris, France, for fever, myalgia, and hypotensive shock. The patient had been in Southern France (Montpellier) 6 days before symptom onset and had been bitten by a tick on the left hand. Four days later, he reported fatigue, fever (39°C), and myalgia. His medical history showed polycystic kidney disease, which had necessitated hemodialysis and a kidney transplant. He was receiving ongoing treatment with an immunosuppressive regime of cyclosporine, prednisolone, and tacrolimus; his baseline hemoglobin level was 15 g/dL, and creatinine level was 230 μmol/L.
At admission, the patient’s temperature was 39.5°C, blood pressure 55/40 mm Hg, and heart rate 104 beats/min. Physical examination showed a diffusely tender abdomen with guarding, no hepatosplenomegaly, a nontender renal transplant, and no lymphadenopathy. Results of cardiovascular, respiratory, and neurologic examinations were unremarkable. A diffuse maculopapular cutaneous eruption was noted on the lower limbs; no eschar was detected.
Laboratory analyses showed the following values: hemoglobin 7.9 g/dL, platelet count 115 × 109/L, leukocyte count 6.7 × 109/L (neutrophils 5.2 × 109/L, lymphocytes 1.4 × 109/L); serum creatinine 466 μmol/L, and C-reactive protein 156 mg/L. Blood cultures were negative. Serologic study results were negative for HIV, hepatitis viruses, Epstein-Barr virus, cytomegalovirus, Legionella, Mycoplasma, Coxiella, Bartonella, Leishmania, and Toxoplasma spp. Serologic testing obtained at day 1 was negative for spotted fever group (SFG) rickettsiosis.
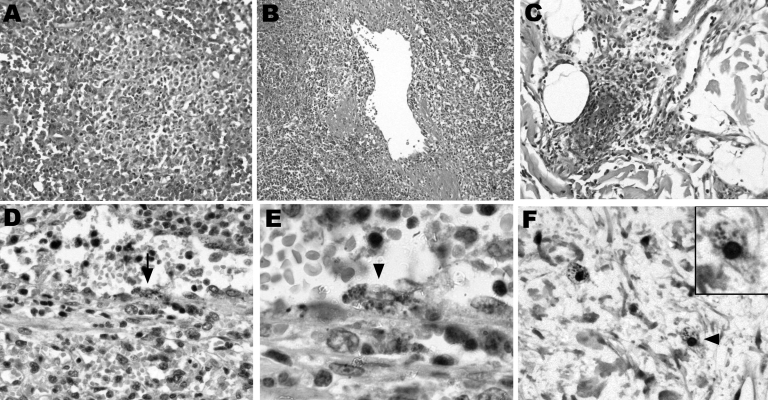
A computed tomographic scan showed hemoperitoneum secondary to a ruptured subcapsular splenic hematoma (Appendix Figure), and an emergency splenectomy was performed. Histopathologic evaluation of the spleen showed white pulp atrophy; the red pulp indicated congestion and ill-defined nodules, varying in size and comprising macrophages, polymorphonuclear neutrophils, and necrotic cells (Figure, panels A, B). Skin biopsy of the macular eruption on day 2 demonstrated a leukocytoclastic vasculitis with nonocclusive luminal thrombi in the dermal capillaries (Figure, panel C).
Figure.
Histopathologic and immunohistochemical labelings of spleen and skin tissue samples. Tissue samples were fixed in 10% formalin, paraffin-embedded, and examined after hematoxylin-eosin staining, Gimenez staining, or immunostaining with the R47 anti-Rickettsia conorii polyclonal rabbit antibody. The spleen red pulp indicated congestion and ill-defined nodules varying in size and comprising macrophages, polymorphonuclear neutrophils, and necrotic cells (A, magnification ×100). A diffuse macrophage infiltration with abundant hemophagocytosis (not shown) and venulitis (B, magnification ×50) was also observed. In the skin, leukocytoclastic vasculitis with focal vascular necrosis and nonocclusive luminal thrombi were noted in dermal capillaries (C, magnification ×100). Intracellular images evocative of rickettsiae were observed in the splenic arteriolar endothelium upon immunohistochemical staining (D, arrow, magnification ×200; magnified view shown in E, arrowhead, magnification ×500). No infected cells were observed in nodular inflammatory splenic lesions. Immunohistochemical staining also disclosed intracellular immunolabeled dots in cells that could correspond to infected dermal macrophages (F, arrowhead, magnification ×300; magnified view shown in inset, magnification ×600), at a distance from the vascular alterations. Endothelial cells of dermal capillaries were also immunolabeled (Appendix Figure).
Universal 16S rRNA gene PCR amplification on spleen and skin tissue samples and direct sequencing identified an R. conorii–specific 16S rRNA sequence match. We confirmed this by using primers for gltA and ompA specific for R. conorii. Immunohistochemical staining demonstrated Rickettsia in endothelial cells and macrophages in the spleen and skin (Figure, panels D–F). Blood culture, skin biopsy specimens, and splenic tissue cultures were subsequently R. conorii positive. Doxycycline therapy (100 mg intravenously twice a day) was instituted at day 2 because rickettsiosis was suspected. The patient dramatically improved within 72 hours and remained well 36 months after diagnosis.
MSF is a rickettsiosis belonging to the tick-borne SFG caused by R. conorii, an obligate intracellular bacteria transmitted by the dog tick Rhipicephalus sanguineus. Endemic to Mediterranean countries, MSF generally results in a benign febrile illness accompanied by a maculopapular rash, myalgia, and local black eschar at a tick bite inoculation site. A minority of persons seeking treatment display a malignant form, which results from disseminated vasculitis associated with increased vascular permeability, thrombus-mediated vascular occlusion, and visceral perivascular lymphohistiocytic infiltrates (1). Focal thrombi have been identified in almost all organs of patients with fatal cases. Manifestations of MSF include neurologic involvement, multi-organ failure, gastric hemorrhage, and acute respiratory distress syndrome; the case-fatality rate is 1.4%–5.6%.
Splenic rupture has been reported in the course of infection with several microbial agents, including Epstein-Barr virus (2), HIV, rubella virus, Bartonella spp. (3), Salmonella spp., mycobacteria (4), and Plasmodium spp. (5). Splenomegaly as a result of MSF has also been documented previously (6); however, splenic rupture in the context of tick-borne illness has only previously been reported for R. typhi (7) and Coxiella burnetii infections (8).
SFG rickettsioses have rarely been described in transplant recipients. Barrio et al. reported a case of MSF in a liver transplant recipient with clinical resolution of infection (9), and a case of Rocky Mountain spotted fever after heart transplantation has been described (10).
Seroconversion remains the principal diagnostic tool for the rickettsioses, but often no detectable antibody is found in the early phase of the disease. Spleen and skin tissue samples allowed rapid 16S rRNA gene PCR and sequencing before the results of other diagnostic procedures were obtained. Immunostaining allowed detection of R. conorii in spleen and skin tissue samples and illustrated the cell tropism of this intracellular bacterium for cells morphologically similar to endothelial cells and possibly macrophages. Although R. conorii infection of postmortem human splenic samples from patients with fatal cases has been documented by immunohistochemical testing, R. conorii has not been described previously in spleen tissue of those who have survived malignant MSF.
This case expands the spectrum of infectious agents associated with spontaneous splenic rupture and solid organ transplantation. Rickettsioses are a significant risk both for those living in disease-endemic regions and for international travelers. To facilitate early detection and treatment, physicians must be vigilant for atypical symptoms, especially in immunocompromised persons.
Supplementary Material
Coronal view of unenhanced abdominal computed tomography demonstrating splenic enlargement with endocapsular hematoma and intraperitoneal hemorrhage (arrows).
Acknowledgments
We thank Pascale Cossart for her support, Véronique Villiers for help with immunohistochemical labeling, and Jean-Marc Rolain for culture of R. conorii from blood and tissue specimens.
Footnotes
Suggested citation for this article: Schmulewitz L, Moumile K, Patey-Mariaud de Serre N, Poirée S, Gouin E, Mechaï F, et al. Splenic rupture and malignant Mediterranean spotted fever [letter]. Emerg Infect Dis [serial on the Internet]. 2008 Jun [date cited]. Available from http://www.cdc.gov/EID/content/14/6/995.htm
References
- 1.Walker DH, Herrero-Herrero JI, Ruiz-Beltran R, Bullon-Sopelana A, Ramos-Hidalgo A. The pathology of fatal Mediterranean spotted fever. Am J Clin Pathol. 1987;87:669–72. [DOI] [PubMed] [Google Scholar]
- 2.Konvolinka CW, Wyatt DB. Splenic rupture and infectious mononucleosis. J Emerg Med. 1989;7:471–5. 10.1016/0736-4679(89)90148-0 [DOI] [PubMed] [Google Scholar]
- 3.Daybell D, Paddock CD, Zaki SR, Comer JA, Woodruff D, Hansen JK, et al. Disseminated infection with Bartonella henselae as a cause of spontaneous splenic rupture. Clin Infect Dis. 2004;39:e21–4. 10.1086/422001 [DOI] [PubMed] [Google Scholar]
- 4.Safioleas MC, Stamatakos MC, Safioleas CM, Diab AI, Agapitos EB. Co-existence of spontaneous splenic rupture and tuberculosis of the spleen. Saudi Med J. 2006;27:1588–90. [PubMed] [Google Scholar]
- 5.Gockel HR, Heidemann J, Lorenz D, Gockel I. Spontaneous splenic rupture in tertian malaria. Infection. 2006;34:43–5. 10.1007/s15010-006-4126-8 [DOI] [PubMed] [Google Scholar]
- 6.Colomba C, Saporito L, Frasca Polara V, Rubino R, Titone L. Mediterranean spotted fever: clinical and laboratory characteristics of 415 Sicilian children. BMC Infect Dis. 2006;6:60. 10.1186/1471-2334-6-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fergie J, Purcell K. Spontaneous splenic rupture in a child with murine typhus. Pediatr Infect Dis J. 2004;23:1171–2. [PubMed] [Google Scholar]
- 8.Wade AJ, Walker T, Athan E, Hughes AJ. Spontaneous splenic rupture: a rare complication of Q fever in Australia. Med J Aust. 2006;184:364. [DOI] [PubMed] [Google Scholar]
- 9.Barrio J, de Diego A, Ripoll C, Perez-Calle JL, Núñez O, Salcedo M, et al. Mediterranean spotted fever in liver transplantation: a case report. Transplant Proc. 2002;34:1255–6. 10.1016/S0041-1345(02)02807-5 [DOI] [PubMed] [Google Scholar]
- 10.Rallis TM, Kriesel JD, Dumler JS, Wagoner LE, Wright ED, Spruance SL. Rocky Mountain spotted fever following cardiac transplantation. West J Med. 1993;158:625–8. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Coronal view of unenhanced abdominal computed tomography demonstrating splenic enlargement with endocapsular hematoma and intraperitoneal hemorrhage (arrows).