Abstract
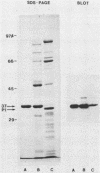
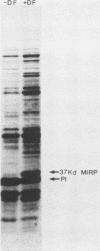
In humans, gonococcal infection occurs in environments limited with respect to free iron. Neisseria gonorrhoeae produces increased quantities of iron-regulated membrane proteins when grown under in vitro conditions which restrict the availability of free iron. Using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot (immunoblot) techniques, we studied the reactivity of specific antibodies to the 37-kilodalton (kDa) major iron-regulated protein (MIRP) of gonococci grown under iron-limiting conditions. Antibodies reactive with the 37-kDa MIRP were distinguished from those reactive with protein I by using purified 37-kDa MIRP or gonococcal protein preparations. Acute-phase sera from patients with disseminated gonococcal infection (DGI) reacted strongly to both the 37-kDa MIRP and protein I. Acute sera from nine patients with uncomplicated gonorrhea did not exhibit strong reactivity with the 37-kDa MIRP and were indistinguishable from five control sera. When compared with acute-phase sera, convalescent-phase sera from patients with DGI failed to demonstrate increased reactivity, whereas convalescent-phase sera from one of nine patients with uncomplicated gonorrhea developed reactivity to the 37-kDa MIRP. These data indicate that (i) the 37-kDa MIRP is expressed and antigenic in vivo and (ii) humans with DGI consistently develop a systemic antibody response to the 37-kDa MIRP.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker-Zander S. A., Hook E. W., 3rd, Bonin P., Handsfield H. H., Lukehart S. A. Antigens of Treponema pallidum recognized by IgG and IgM antibodies during syphilis in humans. J Infect Dis. 1985 Feb;151(2):264–272. doi: 10.1093/infdis/151.2.264. [DOI] [PubMed] [Google Scholar]
- Black J. R., Black W. J., Cannon J. G. Neisserial antigen H.8 is immunogenic in patients with disseminated gonococcal and meningococcal infections. J Infect Dis. 1985 Apr;151(4):650–657. doi: 10.1093/infdis/151.4.650. [DOI] [PubMed] [Google Scholar]
- Blake M. S., Gotschlich E. C. Purification and partial characterization of the opacity-associated proteins of Neisseria gonorrhoeae. J Exp Med. 1984 Feb 1;159(2):452–462. doi: 10.1084/jem.159.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon J. G., Black W. J., Nachamkin I., Stewart P. W. Monoclonal antibody that recognizes an outer membrane antigen common to the pathogenic Neisseria species but not to most nonpathogenic Neisseria species. Infect Immun. 1984 Mar;43(3):994–999. doi: 10.1128/iai.43.3.994-999.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckels J. E. The surface of Neisseria gonorrhoeae: isolation of the major components of the outer membrane. J Gen Microbiol. 1977 Apr;99(2):333–341. doi: 10.1099/00221287-99-2-333. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. J., Hayes S. F., Mayer L. W., Shafer W. M., Tessier S. L. Analyses of gonococcal H8 antigen. Surface location, inter- and intrastrain electrophoretic heterogeneity, and unusual two-dimensional electrophoretic characteristics. J Exp Med. 1985 Dec 1;162(6):2017–2034. doi: 10.1084/jem.162.6.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook E. W., 3rd, Olsen D. A., Buchanan T. M. Analysis of the antigen specificity of the human serum immunoglobulin G immune response to complicated gonococcal infection. Infect Immun. 1984 Feb;43(2):706–709. doi: 10.1128/iai.43.2.706-709.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K. H., Gotschlich E. C. Isolation and characterization of the outer membrane of Neisseria gonorrhoeae. J Bacteriol. 1974 Jul;119(1):250–257. doi: 10.1128/jb.119.1.250-257.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lammel C. J., Sweet R. L., Rice P. A., Knapp J. S., Schoolnik G. K., Heilbron D. C., Brooks G. F. Antibody-antigen specificity in the immune response to infection with Neisseria gonorrhoeae. J Infect Dis. 1985 Nov;152(5):990–1001. doi: 10.1093/infdis/152.5.990. [DOI] [PubMed] [Google Scholar]
- Mickelsen P. A., Blackman E., Sparling P. F. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from lactoferrin. Infect Immun. 1982 Mar;35(3):915–920. doi: 10.1128/iai.35.3.915-920.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietzner T. A., Barnes R. C., JeanLouis Y. A., Shafer W. M., Morse S. A. Distribution of an antigenically related iron-regulated protein among the Neisseria spp. Infect Immun. 1986 Jan;51(1):60–68. doi: 10.1128/iai.51.1.60-68.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietzner T. A., Luginbuhl G. H., Sandstrom E., Morse S. A. Identification of an iron-regulated 37,000-dalton protein in the cell envelope of Neisseria gonorrhoeae. Infect Immun. 1984 Aug;45(2):410–416. doi: 10.1128/iai.45.2.410-416.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice P. A., Kasper D. L. Characterization of gonococcal antigens responsible for induction of bactericidal antibody in disseminated infection. J Clin Invest. 1977 Nov;60(5):1149–1158. doi: 10.1172/JCI108867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XIV. Cell wall protein differences among color/opacity colony variants of Neisseria gonorrhoeae. Infect Immun. 1978 Jul;21(1):292–302. doi: 10.1128/iai.21.1.292-302.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanstrom R., Shank P. R. X-Ray Intensifying Screens Greatly Enhance the Detection by Autoradiography of the Radioactive Isotopes 32P and 125I. Anal Biochem. 1978 May;86(1):184–192. doi: 10.1016/0003-2697(78)90333-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weinberg E. D. Iron and infection. Microbiol Rev. 1978 Mar;42(1):45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S. E., Sparling P. F. Response of Neisseria gonorrhoeae to iron limitation: alterations in expression of membrane proteins without apparent siderophore production. Infect Immun. 1985 Feb;47(2):388–394. doi: 10.1128/iai.47.2.388-394.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey R. J., Finkelstein R. A. Assmilation of iron by pathogenic Neisseria spp. Infect Immun. 1981 May;32(2):592–599. doi: 10.1128/iai.32.2.592-599.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak K., Diaz J. L., Jackson D., Heckels J. E. Antigenic variation during infection with Neisseria gonorrhoeae: detection of antibodies to surface proteins in sera of patients with gonorrhea. J Infect Dis. 1984 Feb;149(2):166–174. doi: 10.1093/infdis/149.2.166. [DOI] [PubMed] [Google Scholar]