Abstract
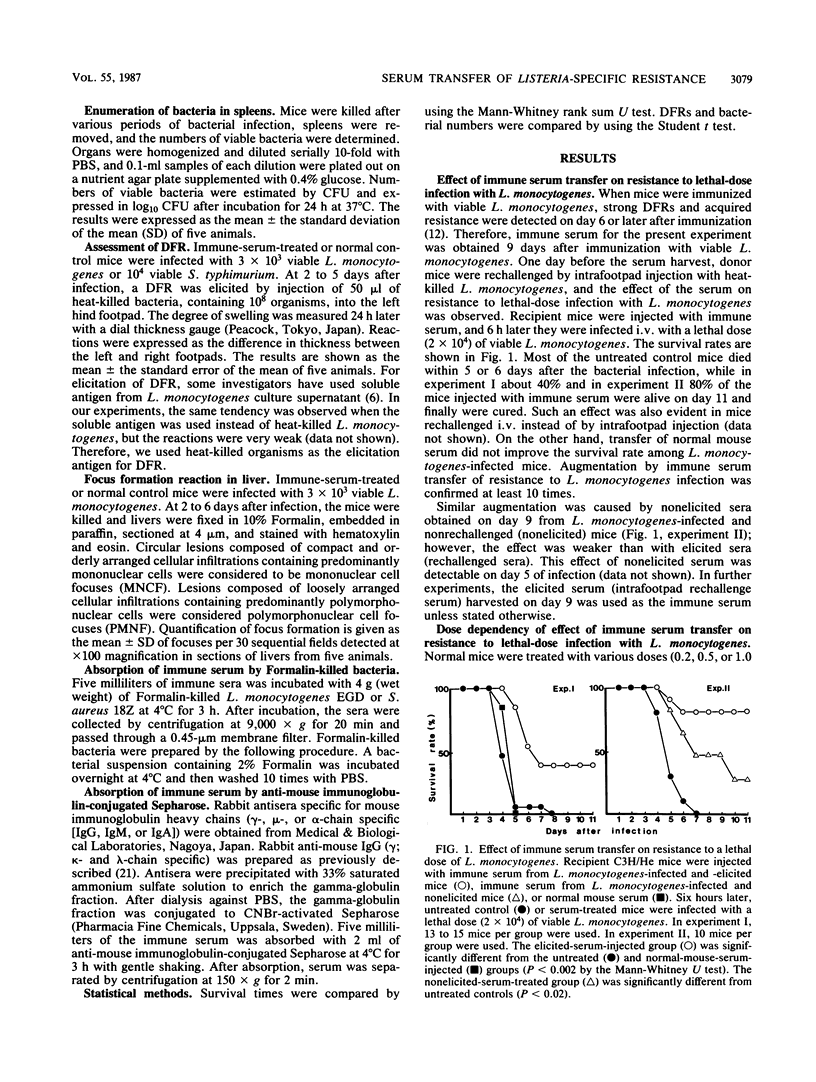
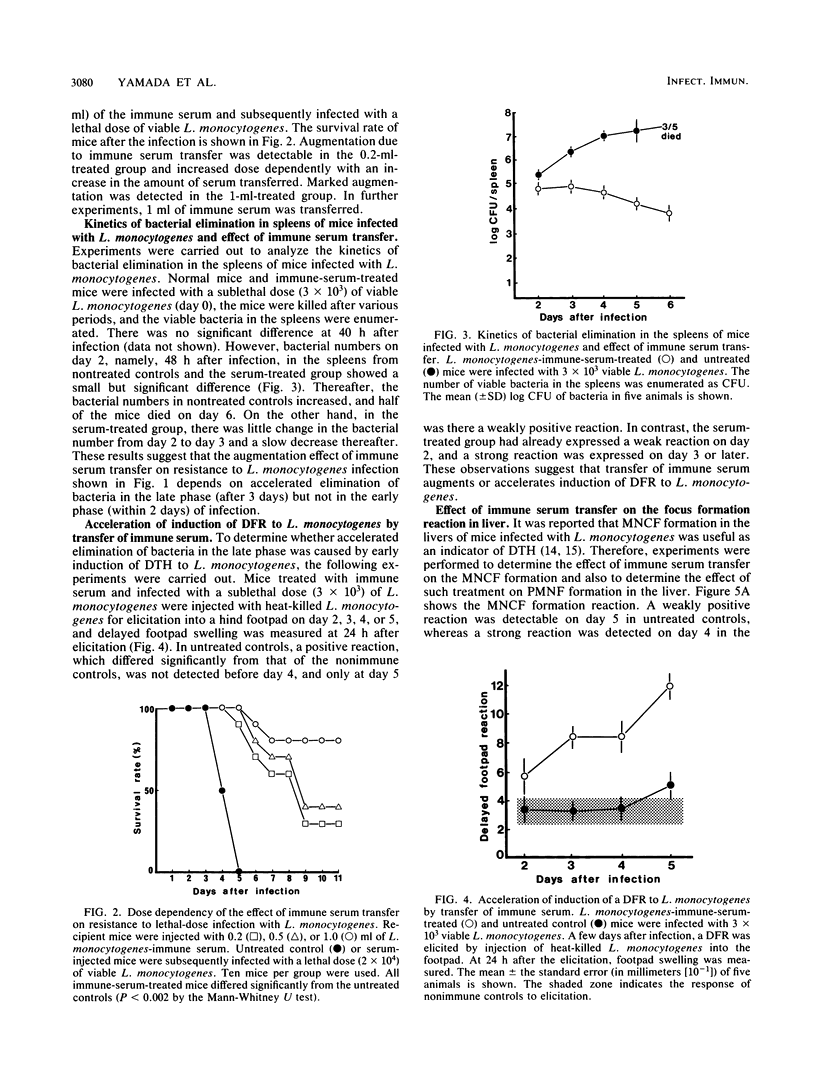
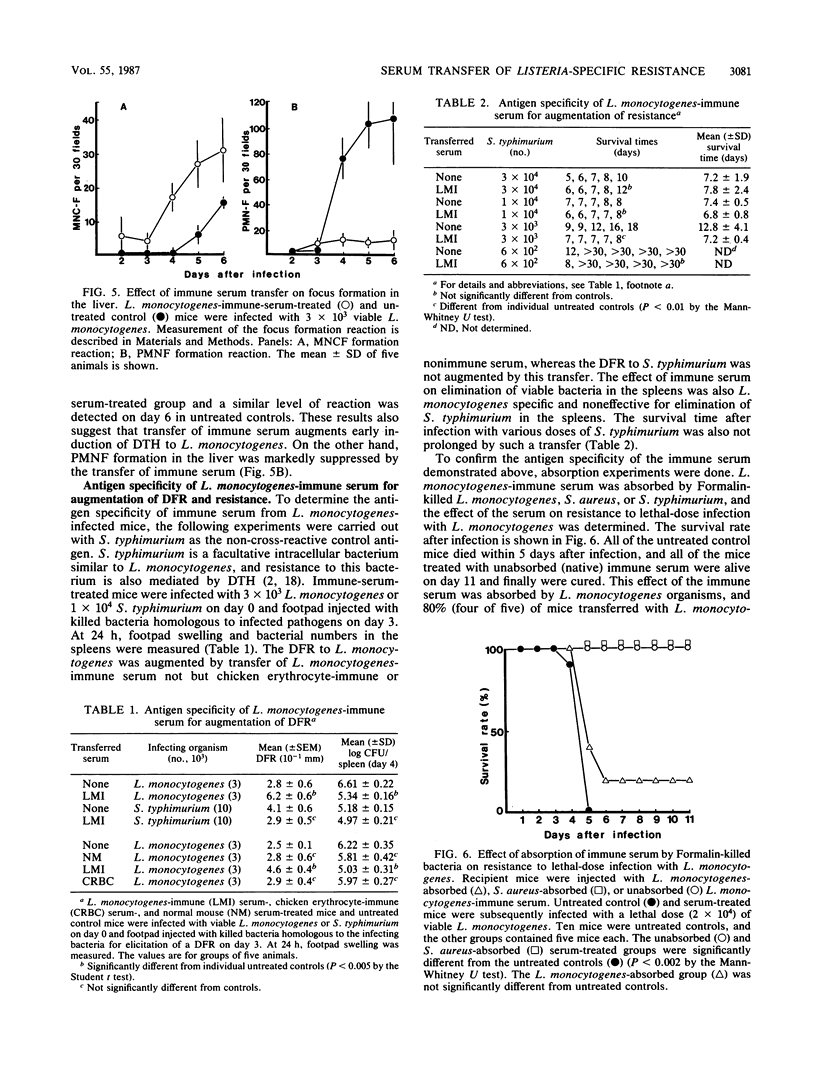
We found a new phenomenon which differs from previous reports on experimental listeriosis, that is, failure of passive transfer of serum from Listeria monocytogenes-infected mice to convey resistance to the bacterium. Transfer of immune serum from L. monocytogenes-infected mice markedly augmented resistance to the bacterium, and mechanisms of the transfer of L. monocytogenes-immune serum were investigated. Transfer of immune serum prevented L. monocytogenes lethality. This effect of the immune serum was transferred dose dependently. Augmentation of resistance to L. monocytogenes also appeared in elimination of bacteria from the spleen. The growth of bacteria within 2 days in the spleen was not inhibited. Transfer of the immune serum augmented and accelerated induction of a delayed footpad reaction. Delayed hypersensitivity-dependent accumulation of mononuclear cells, detected by focus formation reaction in the liver, was also augmented. In contrast, polymorphonuclear cell accumulation in the liver was suppressed. Development of delayed hypersensitivity reactions was correlated with the elimination of bacteria in the spleens. These effects of the immune serum were expressed antigen specifically; however, the effector molecule(s) in the immune serum differs from immunoglobulin molecules.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheers C., McKenzie I. F., Pavlov H., Waid C., York J. Resistance and susceptibility of mice to bacterial infection: course of listeriosis in resistant or susceptible mice. Infect Immun. 1978 Mar;19(3):763–770. doi: 10.1128/iai.19.3.763-770.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Mackaness G. B. Delayed hypersensitivity and arthus reactivity in relation to host resistance in salmonella-infected mice. J Immunol. 1968 Nov;101(5):830–845. [PubMed] [Google Scholar]
- Himeno K., Yamada A., Miyata H., Nanishi F., Nomoto K. Antigen-specific augmentation of delayed-type hypersensitivity by immune serum factor in mice. Cell Immunol. 1985 Oct 1;95(1):35–45. doi: 10.1016/0008-8749(85)90292-8. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Eichmann K., Müller I., Wrazel L. J. Vaccination against the intracellular bacterium Listeria monocytogenes with a clonotypic antiserum. J Immunol. 1985 Jun;134(6):4123–4127. [PubMed] [Google Scholar]
- Kaufmann S. H., Hug E., Väth U., Müller I. Effective protection against Listeria monocytogenes and delayed-type hypersensitivity to listerial antigens depend on cooperation between specific L3T4+ and Lyt 2+ T cells. Infect Immun. 1985 Apr;48(1):263–266. doi: 10.1128/iai.48.1.263-266.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Simon M. M., Hahn H. Specific Lyt 123 cells are involved in protection against Listeria monocytogenes and in delayed-type hypersensitivity to listerial antigens. J Exp Med. 1979 Oct 1;150(4):1033–1038. doi: 10.1084/jem.150.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostiala A. A., McGregor D. D. The mediator of cellular immunity. IX. The relationship between cellular hypersensitivity and acquired cellular resistance in rats infected with Listeria monocytogenes. J Exp Med. 1975 Jun 1;141(6):1249–1260. doi: 10.1084/jem.141.6.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane F. C., Unanue E. R. Requirement of thymus (T) lymphocytes for resistance to listeriosis. J Exp Med. 1972 May 1;135(5):1104–1112. doi: 10.1084/jem.135.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIKI K., MACKANESS G. B. THE PASSIVE TRANSFER OF ACQUIRED RESISTANCE TO LISTERIA MONOCYTOGENES. J Exp Med. 1964 Jul 1;120:93–103. doi: 10.1084/jem.120.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuyama M., Takeya K., Nomoto K., Shimotori S. Three phases of phagocyte contribution to resistance against Listeria monocytogenes. J Gen Microbiol. 1978 May;106(1):165–171. doi: 10.1099/00221287-106-1-165. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Himeno K., Yamada A., Mitani M., Nomoto K. Antigen-specific augmentation of delayed-type hypersensitivity by immune serum factor in mice: augmentation of anti-tumor cytostatic activity. Cell Immunol. 1986 Dec;103(2):311–325. doi: 10.1016/0008-8749(86)90092-4. [DOI] [PubMed] [Google Scholar]
- Näher H., Sperling U., Hahn H. H-2K-restricted granuloma formation by Ly-2+ T cells in antibacterial protection to facultative intracellular bacteria. J Immunol. 1985 Jan;134(1):569–572. [PubMed] [Google Scholar]
- Näher H., Sperling U., Skupin-Schüssler H., Hahn H. An adoptive transfer system for the investigation of granuloma formation in murine listeriosis. Med Microbiol Immunol. 1985;173(6):311–318. doi: 10.1007/BF02125035. [DOI] [PubMed] [Google Scholar]
- OSEBOLD J. W., SAWYER M. T. Immunization studies on listeriosis in mice. J Immunol. 1957 Apr;78(4):262–268. [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Natural resistance to Salmonella infection, delayed hypersensitivity and Ir genes in different strains of mice. Nature. 1974 Mar 22;248(446):345–347. doi: 10.1038/248345a0. [DOI] [PubMed] [Google Scholar]
- Yamada A., Himeno K., Kumazawa Y., Nomoto K. Antigen-specific augmentation of delayed-type hypersensitivity by a humoral factor in the culture supernatant of immune spleen cells. Cell Immunol. 1984 Mar;84(1):206–209. doi: 10.1016/0008-8749(84)90092-3. [DOI] [PubMed] [Google Scholar]
- Yamada A., Himeno K., Kumazawa Y., Nomoto K. Antigen-specific augmentation of murine immediate hypersensitivity-like footpad reaction by a T-cell factor in the culture supernatant of immune spleen cells. Cell Immunol. 1985 May;92(2):350–358. doi: 10.1016/0008-8749(85)90016-4. [DOI] [PubMed] [Google Scholar]
- Yamada A., Himeno K., Miyata H., Kumazawa Y., Nomoto K. Antigen-specific augmentation factor involved in murine delayed-type footpad reaction. I. Nature of augmentation factor. Cell Immunol. 1984 Oct 1;88(1):184–192. doi: 10.1016/0008-8749(84)90063-7. [DOI] [PubMed] [Google Scholar]


