Abstract
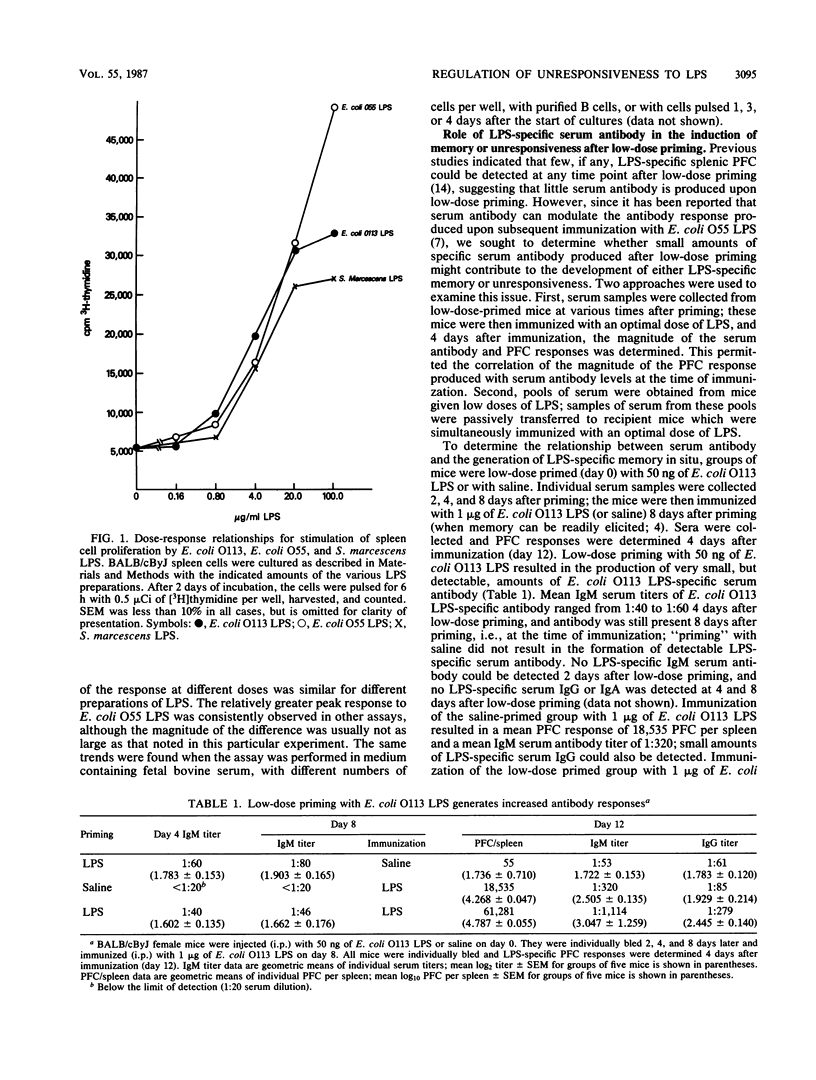
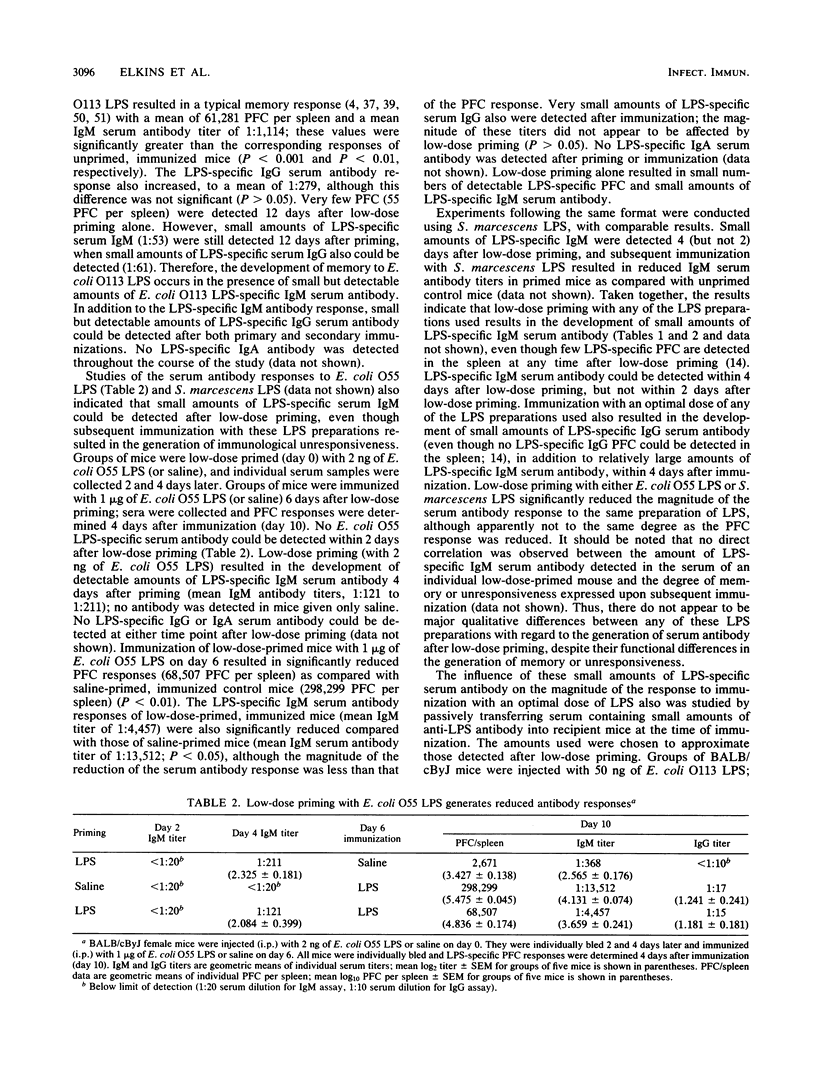
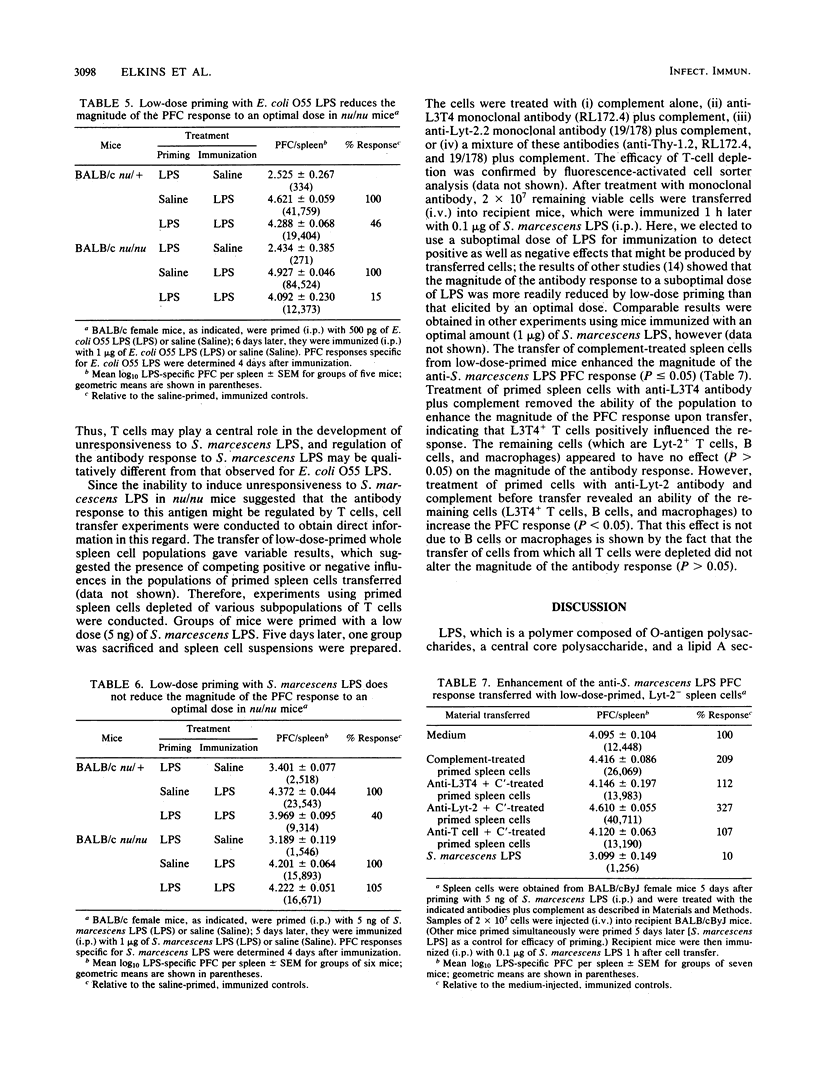
Low-dose priming of mice with Escherichia coli O113 lipopolysaccharide (LPS) results in the development of immunological memory, whereas low-dose priming with E. coli O55 LPS or Serratia marcescens LPS induces significant antigen-specific unresponsiveness. All three preparations of LPS induced proliferation of mouse splenocytes with similar time course and [3H]thymidine uptake. There was no correlation between the small amounts of serum antibody detected by enzyme-linked immunosorbent assay after low-dose priming and the subsequent generation of either memory or unresponsiveness. Further, the passive transfer of small amounts of LPS-specific antibody had no significant effect on the magnitude of the plaque-forming cell (PFC) response elicited after subsequent immunization. Reduction of the PFC response to E. coli O55 LPS occurred after low-dose priming of nu/nu (as well as nu/+) mice; however, unresponsiveness could not be generated in nu/nu mice by low-dose priming with S. marcescens LPS. Thus, although the development of low-dose unresponsiveness to S. marcescens LPS appears to involve T cells, the response of E. coli O55 LPS does not. Enhancement of the primary PFC response to S. marcescens LPS could be transferred with low-dose primed spleen cells depleted of Lyt-2+ T cells; this suggests that the magnitude of the PFC response to this preparation of LPS is negatively influenced by Lyt-2+ T cells and positively influenced by Lyt-2- spleen cells (i.e., L3T4+ T cells). These findings indicate that T cells appear to be involved in regulating the magnitude of the antibody response to some types of bacterial LPS.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amsbaugh D. F., Hansen C. T., Prescott B., Stashak P. W., Barthold D. R., Baker P. J. Genetic control of the antibody response to type 3 pneumococcal polysaccharide in mice. I. Evidence that an X-linked gene plays a decisive role in determining responsiveness. J Exp Med. 1972 Oct 1;136(4):931–949. doi: 10.1084/jem.136.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armerding D., Katz D. H. Activation of T and B lymphocytes in vitro. I. Regulatory influence of bacterial lipopolysaccharide (LPS) on specific T-cell helper function. J Exp Med. 1974 Jan 1;139(1):24–43. doi: 10.1084/jem.139.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. H., Stashak P. W. Quantitative and qualitative studies on the primary antibody response to pneumococcal polysaccharides at ehe cellular level. J Immunol. 1969 Dec;103(6):1342–1348. [PubMed] [Google Scholar]
- Baker P. J., Amsbaugh D. F., Stashak P. W., Caldes G., Prescott B. Regulation of the antibody response to pneumococcal polysaccharide by thymus-derived cells. Rev Infect Dis. 1981 Mar-Apr;3(2):332–341. doi: 10.1093/clinids/3.2.332. [DOI] [PubMed] [Google Scholar]
- Baker P. J., Hiernaux J. R., Stashak P. W., Rudbach J. A. Cyclic development of immunological memory to bacterial lipopolysaccharide. Infect Immun. 1985 Apr;48(1):1–6. doi: 10.1128/iai.48.1.1-6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Prescott B. Use of erythrocytes sensitized with purified pneumococcal polysaccharides for the assay of antibody and antibody-producing cells. Appl Microbiol. 1969 Mar;17(3):422–426. doi: 10.1128/am.17.3.422-426.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton S., Möller G. Regulation of antibody synthesis against Escherichia coli endotoxin. I. Suppressive effect of endogenously produced and passively transferred antibodies. J Immunol. 1968 Jun;100(6):1326–1334. [PubMed] [Google Scholar]
- Britton S., Wepsic T., Möller G. Persistence of immunogenicity of two complex antigens retained in vivo. Immunology. 1968 Apr;14(4):491–501. [PMC free article] [PubMed] [Google Scholar]
- Ceredig R., Lowenthal J. W., Nabholz M., MacDonald H. R. Expression of interleukin-2 receptors as a differentiation marker on intrathymic stem cells. Nature. 1985 Mar 7;314(6006):98–100. doi: 10.1038/314098a0. [DOI] [PubMed] [Google Scholar]
- Colwell D. E., Michalek S. M., Briles D. E., Jirillo E., McGhee J. R. Monoclonal antibodies to Salmonella lipopolysaccharide: anti-O-polysaccharide antibodies protect C3H mice against challenge with virulent Salmonella typhimurium. J Immunol. 1984 Aug;133(2):950–957. [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Gillis S., Union N. A., Baker P. E., Smith K. A. The in vitro generation and sustained culture of nude mouse cytolytic T-lymphocytes. J Exp Med. 1979 Jun 1;149(6):1460–1476. doi: 10.1084/jem.149.6.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. C., Leive L. Heterogeneity of antigenic-side-chain length in lipopolysaccharide from Escherichia coli 0111 and Salmonella typhimurium LT2. Eur J Biochem. 1980;107(1):145–153. doi: 10.1111/j.1432-1033.1980.tb04635.x. [DOI] [PubMed] [Google Scholar]
- Goodman S. A., Vukajlovich S. W., Munkenbeck P., Morrison D. C. Selective interaction between lymphocytes and lipid A subunits in lipopolysaccharide macromolecular aggregates. Rev Infect Dis. 1984 Jul-Aug;6(4):511–518. doi: 10.1093/clinids/6.4.511. [DOI] [PubMed] [Google Scholar]
- Gottlieb C. F. Application of transformations to normalize the distribution of plaque-forming cells. J Immunol. 1974 Jul;113(1):51–57. [PubMed] [Google Scholar]
- Hiernaux J. R., Baker P. J., Delisi C., Rudbach J. A. Modulation of the immune response to lipopolysaccharide. J Immunol. 1982 Mar;128(3):1054–1058. [PubMed] [Google Scholar]
- Hiernaux J. R., Jones J. M., Rudbach J. A., Rollwagen F., Baker P. J. Antibody response of immunodeficient (xid) CBA/N mice to Escherichia coli 0113 lipopolysaccharide, a thymus-independent antigen. J Exp Med. 1983 Apr 1;157(4):1197–1207. doi: 10.1084/jem.157.4.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämmerling G. J., Hämmerling U., Flaherty L. Qat-4 and Qat-5, new murine T-cell antigens governed by the Tla region and identified by monoclonal antibodies. J Exp Med. 1979 Jul 1;150(1):108–116. doi: 10.1084/jem.150.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns M., Skehill A., McCabe W. R. Immunization with rough mutants of Salmonella minnesota. IV. Protection by antisera to O and rough antigens against endotoxin. J Infect Dis. 1983 Jan;147(1):57–67. doi: 10.1093/infdis/147.1.57. [DOI] [PubMed] [Google Scholar]
- Kotani S., Takada H., Tsujimoto M., Ogawa T., Takahashi I., Ikeda T., Otsuka K., Shimauchi H., Kasai N., Mashimo J. Synthetic lipid A with endotoxic and related biological activities comparable to those of a natural lipid A from an Escherichia coli re-mutant. Infect Immun. 1985 Jul;49(1):225–237. doi: 10.1128/iai.49.1.225-237.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüderitz O., Tanamoto K., Galanos C., McKenzie G. R., Brade H., Zähringer U., Rietschel E. T., Kusumoto S., Shiba T. Lipopolysaccharides: structural principles and biologic activities. Rev Infect Dis. 1984 Jul-Aug;6(4):428–431. doi: 10.1093/clinids/6.4.428. [DOI] [PubMed] [Google Scholar]
- McGhee J. R., Kiyono H., Michalek S. M., Babb J. L., Rosenstreich D. L., Mergenhagen S. E. Lipopolysaccharide (LPS) regulation of the immune response: T lymphocytes from normal mice suppress mitogenic and immunogenic responses to LPS. J Immunol. 1980 Apr;124(4):1603–1611. [PubMed] [Google Scholar]
- Milner E. C., Rudbach J. A., Voneschen K. B. Cellular responses to bacterial lipopolysaccharide: T cells recognize LPS determinants. Scand J Immunol. 1983 Jul;18(1):21–28. doi: 10.1111/j.1365-3083.1983.tb00831.x. [DOI] [PubMed] [Google Scholar]
- Mita A., Ohta H., Mita T. Induction of splenic T cell proliferation by lipid A in mice immunized with sheep red blood cells. J Immunol. 1982 Apr;128(4):1709–1711. [PubMed] [Google Scholar]
- Morrison D. C., Ryan J. L. Bacterial endotoxins and host immune responses. Adv Immunol. 1979;28:293–450. doi: 10.1016/s0065-2776(08)60802-0. [DOI] [PubMed] [Google Scholar]
- Mosier D. E., Mond J. J., Goldings E. A. The ontogeny of thymic independent antibody responses in vitro in normal mice and mice with an X-linked B cell defect. J Immunol. 1977 Dec;119(6):1874–1878. [PubMed] [Google Scholar]
- O'Brien A. D., Scher I., Metcalf E. S. Genetically conferred defect in anti-Salmonella antibody formation renders CBA/N mice innately susceptible to Salmonella typhimurium infection. J Immunol. 1981 Apr;126(4):1368–1372. [PubMed] [Google Scholar]
- Ono S., Yaffe L. J., Ryan J. L., Singer A. Functional heterogeneity of the Lyb-5- B cell subpopulation: mutant xid B cells and normal Lyb-5- B cells differ in their responsiveness to phenol-extracted lipopolysaccharide. J Immunol. 1983 May;130(5):2014–2021. [PubMed] [Google Scholar]
- Palva E. T., Mäkelä P. H. Lipopolysaccharide heterogeneity in Salmonella typhimurium analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Eur J Biochem. 1980;107(1):137–143. doi: 10.1111/j.1432-1033.1980.tb04634.x. [DOI] [PubMed] [Google Scholar]
- Pluschke G., Achtman M. Antibodies to O-antigen of lipopolysaccharide are protective against neonatal infection with Escherichia coli K1. Infect Immun. 1985 Aug;49(2):365–370. doi: 10.1128/iai.49.2.365-370.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudbach J. A., Akiya F. I., Elin R. J., Hochstein H. D., Luoma M. K., Milner E. C., Milner K. C., Thomas K. R. Preparation and properties of a national reference endotoxin. J Clin Microbiol. 1976 Jan;3(1):21–25. doi: 10.1128/jcm.3.1.21-25.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudbach J. A. Molecular immunogenicity of bacterial lipopolysaccharide antigens: establishing a quantitative system. J Immunol. 1971 Apr;106(4):993–1001. [PubMed] [Google Scholar]
- Rudbach J. A., Reed N. D. Immunological responses of mice to lipopolysaccharide: lack of secondary responsiveness by C3H/HeJ mice. Infect Immun. 1977 May;16(2):513–517. doi: 10.1128/iai.16.2.513-517.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada S., Suzuki M., Kawamura T., Fujinaga S., Masuho Y., Tomibe K. Protection against infection with Pseudomonas aeruginosa by passive transfer of monoclonal antibodies to lipopolysaccharides and outer membrane proteins. J Infect Dis. 1984 Oct;150(4):570–576. doi: 10.1093/infdis/150.4.570. [DOI] [PubMed] [Google Scholar]
- Scheid M. P., Hoffmann M. K., Komuro K., Hämmerling U., Abbott J., Boyse E. A., Cohen G. H., Hooper J. A., Schulof R. S., Goldstein A. L. Differentiation of T cells induced by preparations from thymus and by nonthymic agents. J Exp Med. 1973 Oct 1;138(4):1027–1032. doi: 10.1084/jem.138.4.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher I. CBA/N immune defective mice; evidence for the failure of a B cell subpopulation to be expressed. Immunol Rev. 1982;64:117–136. doi: 10.1111/j.1600-065x.1982.tb00421.x. [DOI] [PubMed] [Google Scholar]
- Singer A., Morrissey P. J., Hathcock K. S., Ahmed A., Scher I., Hodes R. J. Role of the major histocompatibility complex in T cell activation of B cell subpopulations Lyb-5+ and Lyb-5- B cell subpopulations differ in their requirement for major histocompatibility complex-restricted T cell recognition. J Exp Med. 1981 Aug 1;154(2):501–516. doi: 10.1084/jem.154.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada H., Kotani S., Tsujimoto M., Ogawa T., Takahashi I., Harada K., Katsukawa C., Tanaka S., Shiba T., Kusumoto S. Immunopharmacological activities of a synthetic counterpart of a biosynthetic lipid A precursor molecule and of its analogs. Infect Immun. 1985 Apr;48(1):219–227. doi: 10.1128/iai.48.1.219-227.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Uhr J. W., Möller G. Regulatory effect of antibody on the immune response. Adv Immunol. 1968;8:81–127. doi: 10.1016/s0065-2776(08)60465-4. [DOI] [PubMed] [Google Scholar]
- Vogel S. N., Hilfiker M. L., Caulfield M. J. Endotoxin-induced T lymphocyte proliferation. J Immunol. 1983 Apr;130(4):1774–1779. [PubMed] [Google Scholar]
- Vogel S. N., Madonna G. S., Wahl L. M., Rick P. D. In vitro stimulation of C3H/HeJ spleen cells and macrophages by a lipid A precursor molecule derived from Salmonella typhimurium. J Immunol. 1984 Jan;132(1):347–353. [PubMed] [Google Scholar]
- Von Eschen K. B., Rudbach J. A. Dissociation of the anti-hapten and anti-carrier responses of mice injected with dinitrophenylated lipopolysaccharide. Infect Immun. 1981 Jan;31(1):327–333. doi: 10.1128/iai.31.1.327-333.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Eschen K. B., Rudbach J. A. Immunological responses of mice to native protoplasmic polysaccharide and lipopolysaccharide: functional separation of the two signals required to stimulate a secondary antibody response. J Exp Med. 1974 Dec 1;140(6):1604–1614. doi: 10.1084/jem.140.6.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukajlovich S. W., Morrison D. C. Activation of murine spleen cells by lipid A: negative modulation of lipid A mitogenic activity by O-antigen polysaccharide. J Immunol. 1985 Oct;135(4):2546–2550. [PubMed] [Google Scholar]


