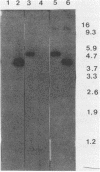
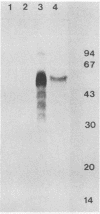
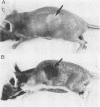
Abstract
The gene coding for protein A (spa) of Staphylococcus aureus 8325-4 has been inactivated by substituting part of the spa coding sequence for a DNA fragment specifying resistance to ethidium bromide. The in vitro-constructed spa::EtBrr substitution mutation was introduced into the S. aureus chromosome by recombinational allele replacement. Southern blot hybridization showed that the in vitro-constructed mutation was present in the chromosomal spa locus. We have previously reported the inactivation of the alpha-toxin gene (hly) by allele replacement with an in vitro-constructed hly::Emr (erythromycin resistance) mutation (M. O'Reilly, J.C.S. de Azavedo, S. Kennedy, and T.J. Foster, Microb. Pathogen. 1:125-138, 1986). A double Spa- Hly- mutant was constructed by transduction. The virulence of Spa- and Hly- mutants was tested by experimental infection of mice. When subcutaneous injections were given, Hly- mutants formed a flat, darkened lesion, whereas Hly+ strains caused a raised, cream lesion. Alpha-toxin was shown to be a major factor in forming subcutaneous lesions and in causing the death of mice injected intraperitoneally. Spa- mutants were slightly less virulent than their Spa+ counterparts, which suggests that protein A is also a virulence factor of S. aureus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adlam C., Ward P. D., McCartney A. C., Arbuthnott J. P., Thorley C. M. Effect immunization with highly purified alpha- and beta-toxins on staphylococcal mastitis in rabbits. Infect Immun. 1977 Aug;17(2):250–256. doi: 10.1128/iai.17.2.250-256.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleyard R K. Segregation of New Lysogenic Types during Growth of a Doubly Lysogenic Strain Derived from Escherichia Coli K12. Genetics. 1954 Jul;39(4):440–452. doi: 10.1093/genetics/39.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNHEIMER A. W., SCHWARTZ L. L. Isolation and composition of staphylococcal alpha toxin. J Gen Microbiol. 1963 Mar;30:455–468. doi: 10.1099/00221287-30-3-455. [DOI] [PubMed] [Google Scholar]
- Brenner D. G., Shaw W. V. The use of synthetic oligonucleotides with universal templates for rapid DNA sequencing: results with staphylococcal replicon pC221. EMBO J. 1985 Feb;4(2):561–568. doi: 10.1002/j.1460-2075.1985.tb03665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S., Cohen S. N. High frequency transformation of Bacillus subtilis protoplasts by plasmid DNA. Mol Gen Genet. 1979 Jan 5;168(1):111–115. doi: 10.1007/BF00267940. [DOI] [PubMed] [Google Scholar]
- Coleman D. C., Pomeroy H., Estridge J. K., Keane C. T., Cafferkey M. T., Hone R., Foster T. J. Susceptibility to antimicrobial agents and analysis of plasmids in gentamicin- and methicillin-resistant Staphylococcus aureus from Dublin hospitals. J Med Microbiol. 1985 Oct;20(2):157–167. doi: 10.1099/00222615-20-2-157. [DOI] [PubMed] [Google Scholar]
- Fairweather N., Kennedy S., Foster T. J., Kehoe M., Dougan G. Expression of a cloned Staphylococcus aureus alpha-hemolysin determinant in Bacillus subtilis and Staphylococcus aureus. Infect Immun. 1983 Sep;41(3):1112–1117. doi: 10.1128/iai.41.3.1112-1117.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guss B., Uhlén M., Nilsson B., Lindberg M., Sjöquist J., Sjödahl J. Region X, the cell-wall-attachment part of staphylococcal protein A. Eur J Biochem. 1984 Jan 16;138(2):413–420. doi: 10.1111/j.1432-1033.1984.tb07931.x. [DOI] [PubMed] [Google Scholar]
- Helfman D. M., Feramisco J. R., Fiddes J. C., Thomas G. P., Hughes S. H. Identification of clones that encode chicken tropomyosin by direct immunological screening of a cDNA expression library. Proc Natl Acad Sci U S A. 1983 Jan;80(1):31–35. doi: 10.1073/pnas.80.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson P., Lindberg M., Haraldsson I., Wadström T. Virulence of Staphylococcus aureus in a mouse mastitis model: studies of alpha hemolysin, coagulase, and protein A as possible virulence determinants with protoplast fusion and gene cloning. Infect Immun. 1985 Sep;49(3):765–769. doi: 10.1128/iai.49.3.765-769.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiswirth B. N., Löfdahl S., Betley M. J., O'Reilly M., Schlievert P. M., Bergdoll M. S., Novick R. P. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature. 1983 Oct 20;305(5936):709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langone J. J. Protein A of Staphylococcus aureus and related immunoglobulin receptors produced by streptococci and pneumonococci. Adv Immunol. 1982;32:157–252. [PubMed] [Google Scholar]
- Loenen W. A., Brammar W. J. A bacteriophage lambda vector for cloning large DNA fragments made with several restriction enzymes. Gene. 1980 Aug;10(3):249–259. doi: 10.1016/0378-1119(80)90054-2. [DOI] [PubMed] [Google Scholar]
- Löfdahl S., Guss B., Uhlén M., Philipson L., Lindberg M. Gene for staphylococcal protein A. Proc Natl Acad Sci U S A. 1983 Feb;80(3):697–701. doi: 10.1073/pnas.80.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movitz J. A study on the biosynthesis of protein A in Staphylococcus aureus. Eur J Biochem. 1974 Oct 1;48(1):131–136. doi: 10.1111/j.1432-1033.1974.tb03750.x. [DOI] [PubMed] [Google Scholar]
- Nordström K., Lindberg M. Effects of streptomycin and novobiocin on Staphylococcus aureus gene expression. J Bacteriol. 1978 Feb;133(2):614–620. doi: 10.1128/jb.133.2.614-620.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967 Sep;33(1):155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- O'Reilly M., de Azavedo J. C., Kennedy S., Foster T. J. Inactivation of the alpha-haemolysin gene of Staphylococcus aureus 8325-4 by site-directed mutagenesis and studies on the expression of its haemolysins. Microb Pathog. 1986 Apr;1(2):125–138. doi: 10.1016/0882-4010(86)90015-x. [DOI] [PubMed] [Google Scholar]
- O'Toole P. W., Foster T. J. Molecular cloning and expression of the epidermolytic toxin A gene of Staphylococcus aureus. Microb Pathog. 1986 Dec;1(6):583–594. doi: 10.1016/0882-4010(86)90043-4. [DOI] [PubMed] [Google Scholar]
- Pattee P. A. Chromosomal map location of the alpha-hemolysin structural gene in Staphylococcus aureus NCTC 8325. Infect Immun. 1986 Nov;54(2):593–596. doi: 10.1128/iai.54.2.593-596.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recsei P., Kreiswirth B., O'Reilly M., Schlievert P., Gruss A., Novick R. P. Regulation of exoprotein gene expression in Staphylococcus aureus by agar. Mol Gen Genet. 1986 Jan;202(1):58–61. doi: 10.1007/BF00330517. [DOI] [PubMed] [Google Scholar]
- Russell R. J., Wilkinson P. C., McInroy R. J., McKay S., McCartney A. C., Arbuthnott J. P. Effects of staphylococcal products on locomotion and chemotaxis of human blood neutrophils and monocytes. J Med Microbiol. 1976 Nov;9(4):433–439. doi: 10.1099/00222615-9-4-433. [DOI] [PubMed] [Google Scholar]
- Russell R. R., Coleman D., Dougan G. Expression of a gene for glucan-binding protein from Streptococcus mutans in Escherichia coli. J Gen Microbiol. 1985 Feb;131(2):295–299. doi: 10.1099/00221287-131-2-295. [DOI] [PubMed] [Google Scholar]
- Shortle D. A genetic system for analysis of staphylococcal nuclease. Gene. 1983 May-Jun;22(2-3):181–189. doi: 10.1016/0378-1119(83)90102-6. [DOI] [PubMed] [Google Scholar]
- Sjöquist J., Movitz J., Johansson I. B., Hjelm H. Localization of protein A in the bacteria. Eur J Biochem. 1972 Oct 17;30(1):190–194. doi: 10.1111/j.1432-1033.1972.tb02086.x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Uhlén M., Guss B., Nilsson B., Gatenbeck S., Philipson L., Lindberg M. Complete sequence of the staphylococcal gene encoding protein A. A gene evolved through multiple duplications. J Biol Chem. 1984 Feb 10;259(3):1695–1702. [PubMed] [Google Scholar]
- Uhlén M., Guss B., Nilsson B., Götz F., Lindberg M. Expression of the gene encoding protein A in Staphylococcus aureus and coagulase-negative staphylococci. J Bacteriol. 1984 Aug;159(2):713–719. doi: 10.1128/jb.159.2.713-719.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. D., Adlam C., McCartney A. C., Arbuthnott J. P., Thorley C. M. A histopathological study of the effects of highly purified staphlococcal alpha and beta toxins on the lactating mammary gland and skin of the rabbit. J Comp Pathol. 1979 Apr;89(2):169–177. doi: 10.1016/0021-9975(79)90056-2. [DOI] [PubMed] [Google Scholar]
- Winkler K. C., de Waart J., Grootsen C. Lysogenic conversion of staphylococci to loss of beta-toxin. J Gen Microbiol. 1965 Jun;39(3):321–333. doi: 10.1099/00221287-39-3-321. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]