Abstract
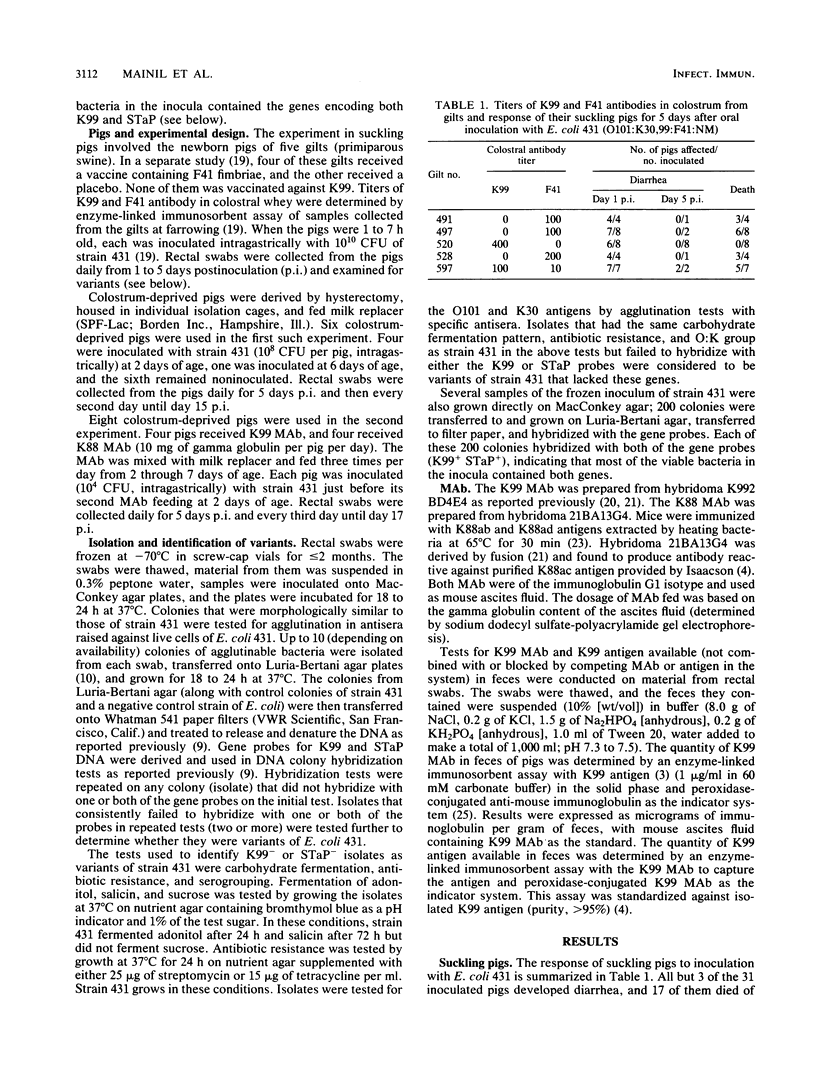
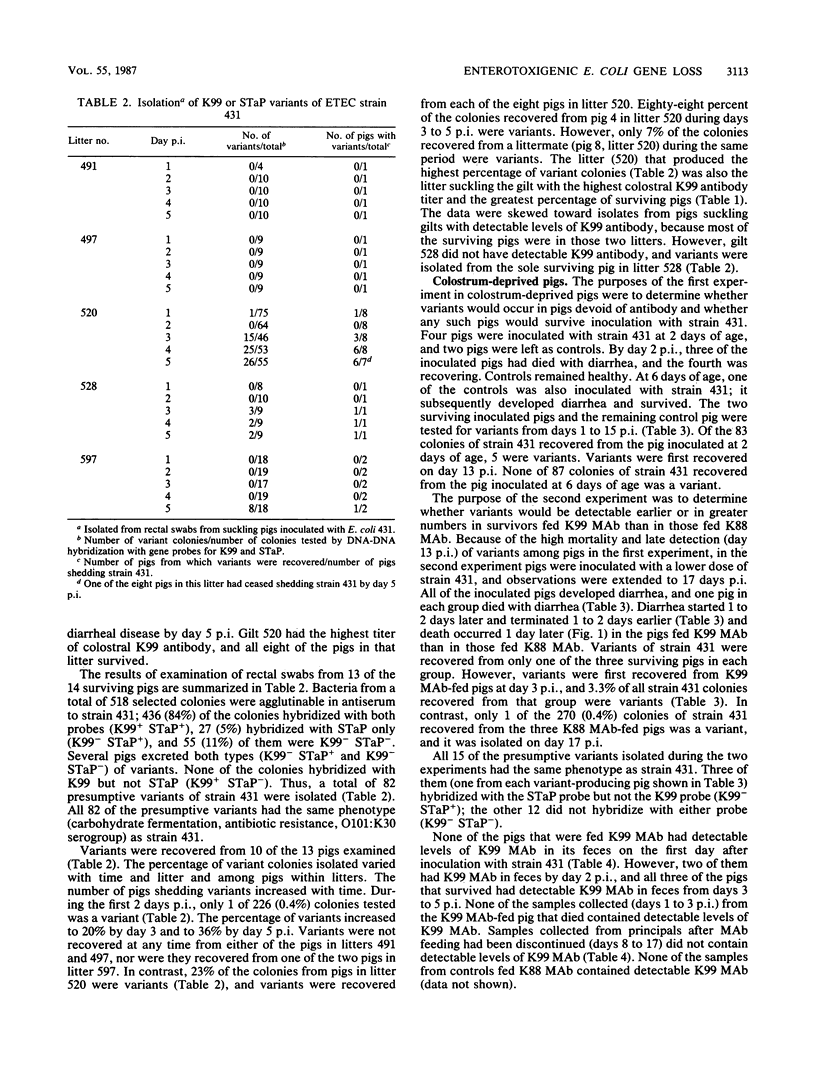
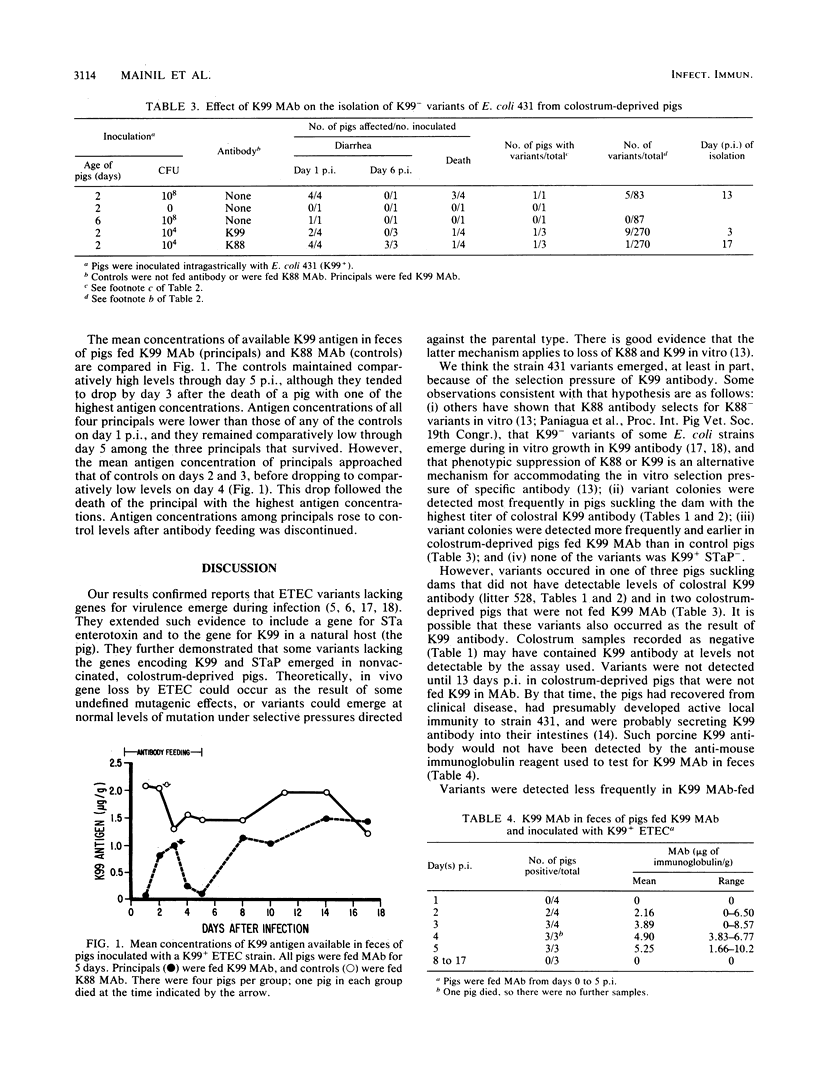
Neonatal pigs were inoculated with porcine enterotoxigenic Escherichia coli 431, which carries genes for K99 fimbriae and STaP enterotoxin. Colonies of strain 431 were recovered from feces of pigs for up to 17 days after inoculation and tested for hybridization with gene probes for K99 and STaP. Variants of strain 431 that did not hybridize with the probes were considered to have lost the genes. Variants were recovered from 10 of 13 suckling pigs that survived the infection. Only 0.4% of the isolates recovered during the first 2 days after inoculation were variants. Of the isolates recovered 3 to 5 days after inoculation, 20 to 36% were variants. Variant colonies were detected more frequently among pigs in some litters than in others. The litter with the highest number of variant-shedding pigs had the dam with the highest titer of K99 antibody in her colostrum. Variants also occurred in colostrum-deprived, artificially reared pigs. However, the number of variants detected was lower and they occurred later in the course of the infection in colostrum-deprived pigs than in suckling pigs. More variants were detected and they were detected earlier in colostrum-deprived pigs fed anti-K99 monoclonal antibody than in controls fed anti-K88 monoclonal antibody. Loss of STaP appeared to be secondary to loss of K99 in that some variants lacked only K99 (K99- STaP+) and some lacked both genes (K99- STaP-), but none was of the K99+ STaP- type. Our results confirmed reports of gene loss from enterotoxigenic E. coli during infection. They are consistent with the hypothesis that variants emerge under in vivo selection pressure of K99 antibody and with the speculation that gene loss may be an important component of protection in vaccinated populations. However, the emergence of variants did not appear to play a major role in the recovery of individual pigs from clinical disease.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gaastra W., de Graaf F. K. Host-specific fimbrial adhesins of noninvasive enterotoxigenic Escherichia coli strains. Microbiol Rev. 1982 Jun;46(2):129–161. doi: 10.1128/mr.46.2.129-161.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnett N. M., Gyles C. L. Enterotoxin plasmids in bovine and porcine enterotoxigenic Escherichia coli of O groups 9, 20, 64 and 101. Can J Comp Med. 1985 Jan;49(1):79–87. [PMC free article] [PubMed] [Google Scholar]
- Isaacson R. E. K99 surface antigen of Escherichia coli: purification and partial characterization. Infect Immun. 1977 Jan;15(1):272–279. doi: 10.1128/iai.15.1.272-279.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanata C. F., Kaper J. B., Baldini M. M., Black R. E., Levine M. M. Sensitivity and specificity of DNA probes with the stool blot technique for detection of Escherichia coli enterotoxins. J Infect Dis. 1985 Nov;152(5):1087–1090. doi: 10.1093/infdis/152.5.1087. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Nataro J. P., Karch H., Baldini M. M., Kaper J. B., Black R. E., Clements M. L., O'Brien A. D. The diarrheal response of humans to some classic serotypes of enteropathogenic Escherichia coli is dependent on a plasmid encoding an enteroadhesiveness factor. J Infect Dis. 1985 Sep;152(3):550–559. doi: 10.1093/infdis/152.3.550. [DOI] [PubMed] [Google Scholar]
- Linggood M. A., Ellis M. L., Porter P. An examination of the O and K specificity involved in the antibody-induced loss of the K88 plasmid from porcine enteropathogenic strains of Escherichia coli. Immunology. 1979 Sep;38(1):123–127. [PMC free article] [PubMed] [Google Scholar]
- Mainil J. G., Moseley S. L., Schneider R. A., Sutch K., Casey T. A., Moon H. W. Hybridization of bovine Escherichia coli isolates with gene probes for four enterotoxins (STaP, STaH, STb, LT) and one adhesion factor (K99). Am J Vet Res. 1986 May;47(5):1145–1148. [PubMed] [Google Scholar]
- Moon H. W., Schneider R. A., Moseley S. L. Comparative prevalence of four enterotoxin genes among Escherichia coli isolated from swine. Am J Vet Res. 1986 Feb;47(2):210–212. [PubMed] [Google Scholar]
- Morgan R. L., Isaacson R. E., Moon H. W., Brinton C. C., To C. C. Immunization of suckling pigs against enterotoxigenic Escherichia coli-induced diarrheal disease by vaccinating dams with purified 987 or K99 pili: protection correlates with pilus homology of vaccine and challenge. Infect Immun. 1978 Dec;22(3):771–777. doi: 10.1128/iai.22.3.771-777.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy L. K., Mackenzie T., Pickard D. J., Dougan G. Effects of immune colostrum on the expression of a K88 plasmid encoded determinant: role of plasmid stability and influence of phenotypic expression of K88 fimbriae. J Gen Microbiol. 1986 Sep;132(9):2497–2503. doi: 10.1099/00221287-132-9-2497. [DOI] [PubMed] [Google Scholar]
- Olsson E., Smyth C. J., Söderlind O., Svennerholm A. M., Möllby R. Development of intestinal antibodies against Escherichia coli antigens in piglets with experimental neonatal E. coli diarrhoea. Vet Microbiol. 1986 Jul;12(2):119–133. doi: 10.1016/0378-1135(86)90074-x. [DOI] [PubMed] [Google Scholar]
- Orskov I., Orskov F. Serology of Escherichia coli fimbriae. Prog Allergy. 1983;33:80–105. [PubMed] [Google Scholar]
- Peñaranda M. E., Evans D. G., Murray B. E., Evans D. J., Jr ST:LT:CFA/II plasmids in enterotoxigenic Escherichia coli belonging to serogroups O6, O8, O80, O85, and O139. J Bacteriol. 1983 May;154(2):980–983. doi: 10.1128/jb.154.2.980-983.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter P., Linggood M. A. Development of oral vaccines for preventing diarrhoea caused by enteropathogenic Escherichia coli. J Infect. 1983 Mar;6(2):111–121. doi: 10.1016/s0163-4453(83)92632-4. [DOI] [PubMed] [Google Scholar]
- Porter P., Linggood M. A. Novel mucosal anti-microbial functions interfering with the plasmid-mediated virulence determinants of adherence and drug resistance. Ann N Y Acad Sci. 1983 Jun 30;409:564–579. doi: 10.1111/j.1749-6632.1983.tb26899.x. [DOI] [PubMed] [Google Scholar]
- Runnels P. L., Moseley S. L., Moon H. W. F41 pili as protective antigens of enterotoxigenic Escherichia coli that produce F41, K99, or both pilus antigens. Infect Immun. 1987 Mar;55(3):555–558. doi: 10.1128/iai.55.3.555-558.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski P. L., Acres S. D., Sherman D. M. Monoclonal antibody for the protection of neonatal pigs and calves for toxic diarrhea. Basic Life Sci. 1983;25:93–99. doi: 10.1007/978-1-4684-4460-5_7. [DOI] [PubMed] [Google Scholar]
- Sherman D. M., Acres S. D., Sadowski P. L., Springer J. A., Bray B., Raybould T. J., Muscoplat C. C. Protection of calves against fatal enteric colibacillosis by orally administered Escherichia coli K99-specific monoclonal antibody. Infect Immun. 1983 Nov;42(2):653–658. doi: 10.1128/iai.42.2.653-658.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So M., Heffron F., McCarthy B. J. The E. coli gene encoding heat stable toxin is a bacterial transposon flanked by inverted repeats of IS1. Nature. 1979 Feb 8;277(5696):453–456. doi: 10.1038/277453a0. [DOI] [PubMed] [Google Scholar]
- Stirm S., Orskov F., Orskov I., Mansa B. Episome-carried surface antigen K88 of Escherichia coli. II. Isolation and chemical analysis. J Bacteriol. 1967 Feb;93(2):731–739. doi: 10.1128/jb.93.2.731-739.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weström B. R., Svendsen J., Karlsson B. W. Protease inhibitor levels in porcine mammary secretions. Biol Neonate. 1982;42(3-4):185–194. doi: 10.1159/000241597. [DOI] [PubMed] [Google Scholar]


