Abstract
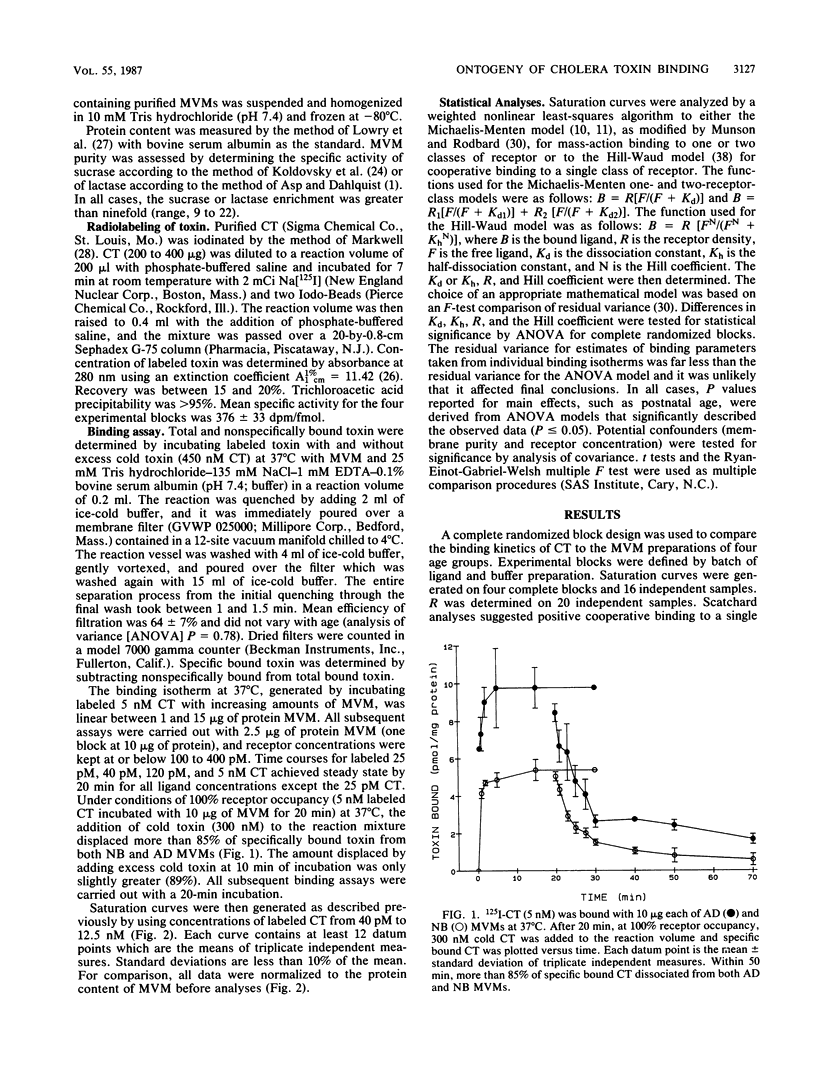
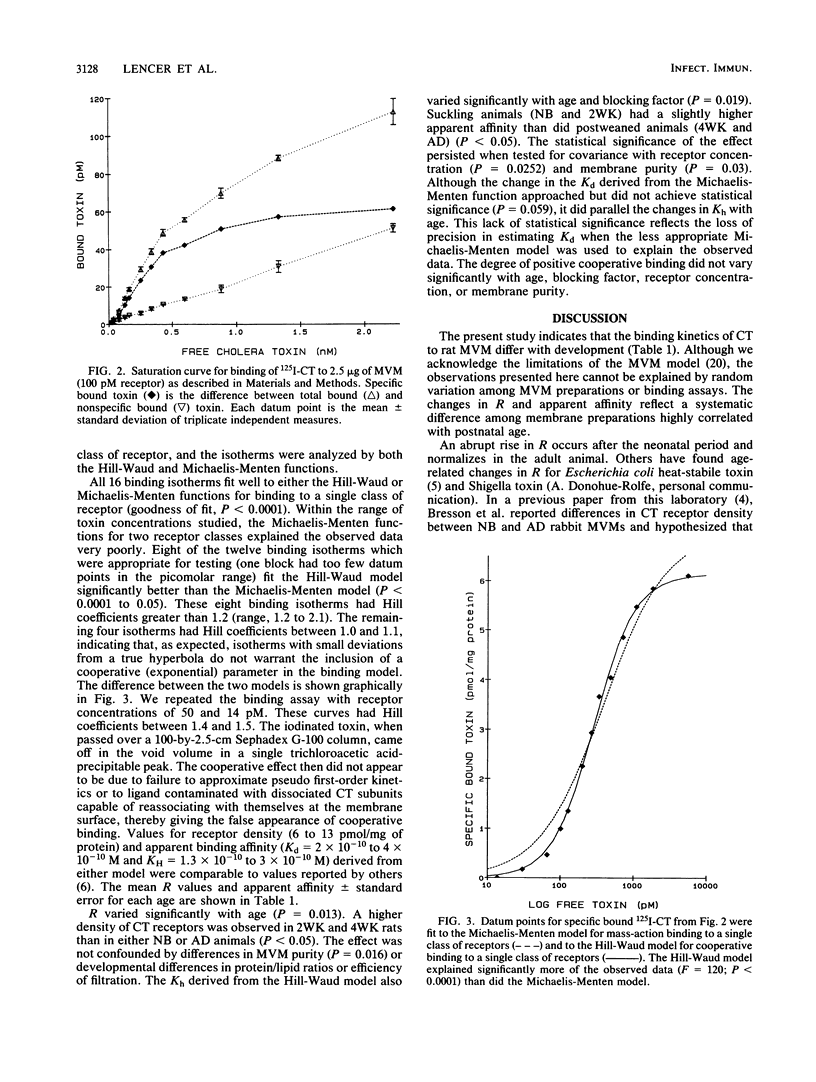
A complete randomized block design was used to compare the binding kinetics of cholera toxin to developing rat enterocyte microvillus membranes prepared from newborn, 2-week-old, 4-week-old, and adult animals. Saturation-binding isotherms were generated on 16 independent samples (four blocks) under steady-state and reversible conditions. Scatchard analyses suggested positive cooperative binding to a single class of receptors, and the isotherms were analyzed by both the Hill-Waud and Michaelis-Menten functions. Receptor density varied significantly with age (P = 0.013). An abrupt rise in receptor density occurred after the neonatal period and normalized in the adult animal. The half-dissociation constant also varied significantly with age (P = 0.019). Microvillus membranes from suckling animals had a slightly higher apparent affinity than those from weaned animals. Neither receptor concentration nor membrane purity confounded these observations. Whereas age-related changes in apparent affinity correlated with cellular responses, changes in receptor density did not. This study suggests that developmental changes in membrane structure which influence binding affinity but not receptor density may, in part, contribute to the increased sensitivity of suckling rats to cholera toxin exposure.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asp N. G., Dahlqvist A. Human small intestine -galactosidases: specific assay of three different enzymes. Anal Biochem. 1972 Jun;47(2):527–538. doi: 10.1016/0003-2697(72)90147-9. [DOI] [PubMed] [Google Scholar]
- Black R. E., Brown K. H., Becker S., Alim A. R., Huq I. Longitudinal studies of infectious diseases and physical growth of children in rural Bangladesh. II. Incidence of diarrhea and association with known pathogens. Am J Epidemiol. 1982 Mar;115(3):315–324. doi: 10.1093/oxfordjournals.aje.a113308. [DOI] [PubMed] [Google Scholar]
- Bouhours D., Bouhours J. F. Developmental changes of hematoside of rat small intestine. Postnatal hydroxylation of fatty acids and sialic acid. J Biol Chem. 1983 Jan 10;258(1):299–304. [PubMed] [Google Scholar]
- Bresson J. L., Pang K. Y., Walker W. A. Microvillus membrane differentiation: quantitative difference in cholera toxin binding to the intestinal surface of newborn and adult rabbits. Pediatr Res. 1984 Oct;18(10):984–987. doi: 10.1203/00006450-198410000-00015. [DOI] [PubMed] [Google Scholar]
- Cohen M. B., Moyer M. S., Luttrell M., Giannella R. A. The immature rat small intestine exhibits an increased sensitivity and response to Escherichia coli heat-stable enterotoxin. Pediatr Res. 1986 Jun;20(6):555–560. doi: 10.1203/00006450-198606000-00017. [DOI] [PubMed] [Google Scholar]
- Critchley D. R., Magnani J. L., Fishman P. H. Interaction of cholera toxin with rat intestinal brush border membranes. Relative roles of gangliosides and galactoproteins as toxin receptors. J Biol Chem. 1981 Aug 25;256(16):8724–8731. [PubMed] [Google Scholar]
- Cuatrecasas P. Gangliosides and membrane receptors for cholera toxin. Biochemistry. 1973 Aug 28;12(18):3558–3566. doi: 10.1021/bi00742a032. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Interaction of Vibrio cholerae enterotoxin with cell membranes. Biochemistry. 1973 Aug 28;12(18):3547–3558. doi: 10.1021/bi00742a031. [DOI] [PubMed] [Google Scholar]
- Feldman H., Rodbard D., Levine D. Mathematical theory of cross-reactive radioimmunoassay and ligand-binding systems of equilibrium. Anal Biochem. 1972 Feb;45(2):530–556. doi: 10.1016/0003-2697(72)90216-3. [DOI] [PubMed] [Google Scholar]
- Fishman P. H., Atikkan E. E. Mechanism of action of cholera toxin: effect of receptor density and multivalent binding on activation of adenylate cyclase. J Membr Biol. 1980;54(1):51–60. doi: 10.1007/BF01875376. [DOI] [PubMed] [Google Scholar]
- Fishman P. H. Mechanism of action of cholera toxin: studies on the lag period. J Membr Biol. 1980;54(1):61–72. doi: 10.1007/BF01875377. [DOI] [PubMed] [Google Scholar]
- Fishman P. H., Pacuszka T., Hom B., Moss J. Modification of ganglioside GM1. Effect of lipid moiety on choleragen action. J Biol Chem. 1980 Aug 25;255(16):7657–7664. [PubMed] [Google Scholar]
- Fishman P. H. Role of membrane gangliosides in the binding and action of bacterial toxins. J Membr Biol. 1982;69(2):85–97. doi: 10.1007/BF01872268. [DOI] [PubMed] [Google Scholar]
- Gill D. M. Multiple roles of erythrocyte supernatant in the activation of adenylate cyclase by Vibrio cholerae toxin in vitro. J Infect Dis. 1976 Mar;133 (Suppl):55–63. doi: 10.1093/infdis/133.supplement_1.s55. [DOI] [PubMed] [Google Scholar]
- Gonnella P. A., Neutra M. R. Membrane-bound and fluid-phase macromolecules enter separate prelysosomal compartments in absorptive cells of suckling rat ileum. J Cell Biol. 1984 Sep;99(3):909–917. doi: 10.1083/jcb.99.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann J., Fishman P. H. Detergent extraction of cholera toxin and gangliosides from cultured cells and isolated membranes. Biochim Biophys Acta. 1982 Apr 29;720(2):181–187. doi: 10.1016/0167-4889(82)90010-6. [DOI] [PubMed] [Google Scholar]
- Hagmann J., Fishman P. H. Inhibitors of protein synthesis block action of cholera toxin. Biochem Biophys Res Commun. 1981 Feb 12;98(3):677–684. doi: 10.1016/0006-291x(81)91167-0. [DOI] [PubMed] [Google Scholar]
- Hopfer U. Characterization of microvillus membrane vesicles. J Pediatr Gastroenterol Nutr. 1985 Dec;4(6):865–867. [PubMed] [Google Scholar]
- Hyun C. S., Kimmich G. A. Interaction of cholera toxin and Escherichia coli enterotoxin with isolated intestinal epithelial cells. Am J Physiol. 1984 Dec;247(6 Pt 1):G623–G631. doi: 10.1152/ajpgi.1984.247.6.G623. [DOI] [PubMed] [Google Scholar]
- Kellie S., Patel B., Pierce E. J., Critchley D. R. Capping of cholera toxin-ganglioside GM1 complexes on mouse lymphocytes is accompanied by co-capping of alpha-actinin. J Cell Biol. 1983 Aug;97(2):447–454. doi: 10.1083/jcb.97.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler M., Acuto O., Storelli C., Murer H., Müller M., Semenza G. A modified procedure for the rapid preparation of efficiently transporting vesicles from small intestinal brush border membranes. Their use in investigating some properties of D-glucose and choline transport systems. Biochim Biophys Acta. 1978 Jan 4;506(1):136–154. doi: 10.1016/0005-2736(78)90440-6. [DOI] [PubMed] [Google Scholar]
- Koldovský O., Asp N. G., Dahlqvist A. A method for the separate assay of "neutral" and "acid" beta-galactosidase in homogenates of rat small-intestinal mucosa. Anal Biochem. 1969 Mar;27(3):409–418. doi: 10.1016/0003-2697(69)90054-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lospalluto J. J., Finkelstein R. A. Chemical and physical properties of cholera exo-enterotoxin (choleragen) and its spontaneously formed toxoid (choleragenoid). Biochim Biophys Acta. 1972 Jan 26;257(1):158–166. doi: 10.1016/0005-2795(72)90265-6. [DOI] [PubMed] [Google Scholar]
- Markwell M. A. A new solid-state reagent to iodinate proteins. I. Conditions for the efficient labeling of antiserum. Anal Biochem. 1982 Sep 15;125(2):427–432. doi: 10.1016/0003-2697(82)90025-2. [DOI] [PubMed] [Google Scholar]
- Martin A. R., Mosley W. H., Sau B. B., Ahmed S., Huq I. Epidemiologic analysis of endemic cholera in urban East Pakistan, 1964-1966. Am J Epidemiol. 1969 May;89(5):572–582. doi: 10.1093/oxfordjournals.aje.a120970. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Pang K. Y., Bresson J. L., Walker W. A. Development of gastrointestinal surface. VIII. Lectin identification of carbohydrate differences. Am J Physiol. 1987 May;252(5 Pt 1):G685–G691. doi: 10.1152/ajpgi.1987.252.5.G685. [DOI] [PubMed] [Google Scholar]
- Pang K. Y., Bresson J. L., Walker W. A. Development of the gastrointestinal mucosal barrier. Evidence for structural differences in microvillus membranes from newborn and adult rabbits. Biochim Biophys Acta. 1983 Jan 5;727(1):201–208. doi: 10.1016/0005-2736(83)90385-1. [DOI] [PubMed] [Google Scholar]
- Révész T., Greaves M. Ligand-induced redistribution of lymphocyte membrane ganglioside GM1. Nature. 1975 Sep 11;257(5522):103–106. doi: 10.1038/257103a0. [DOI] [PubMed] [Google Scholar]
- Schwarz S. M., Hostetler B., Ling S., Mone M., Watkins J. B. Intestinal membrane lipid composition and fluidity during development in the rat. Am J Physiol. 1985 Feb;248(2 Pt 1):G200–G207. doi: 10.1152/ajpgi.1985.248.2.G200. [DOI] [PubMed] [Google Scholar]
- Siminoski K., Gonnella P., Bernanke J., Owen L., Neutra M., Murphy R. A. Uptake and transepithelial transport of nerve growth factor in suckling rat ileum. J Cell Biol. 1986 Nov;103(5):1979–1990. doi: 10.1083/jcb.103.5.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder J. D., Merson M. H. The magnitude of the global problem of acute diarrhoeal disease: a review of active surveillance data. Bull World Health Organ. 1982;60(4):605–613. [PMC free article] [PubMed] [Google Scholar]
- Streuli C. H., Patel B., Critchley D. R. The cholera toxin receptor ganglioside GM remains associated with triton X-100 cytoskeletons of BALB/c-3T3 cells. Exp Cell Res. 1981 Dec;136(2):247–254. doi: 10.1016/0014-4827(81)90002-1. [DOI] [PubMed] [Google Scholar]
- Wissig S. L., Graney D. O. Membrane modifications in the apical endocytic complex of ileal epithelial cells. J Cell Biol. 1968 Dec;39(3):564–579. doi: 10.1083/jcb.39.3.564. [DOI] [PMC free article] [PubMed] [Google Scholar]


