Abstract
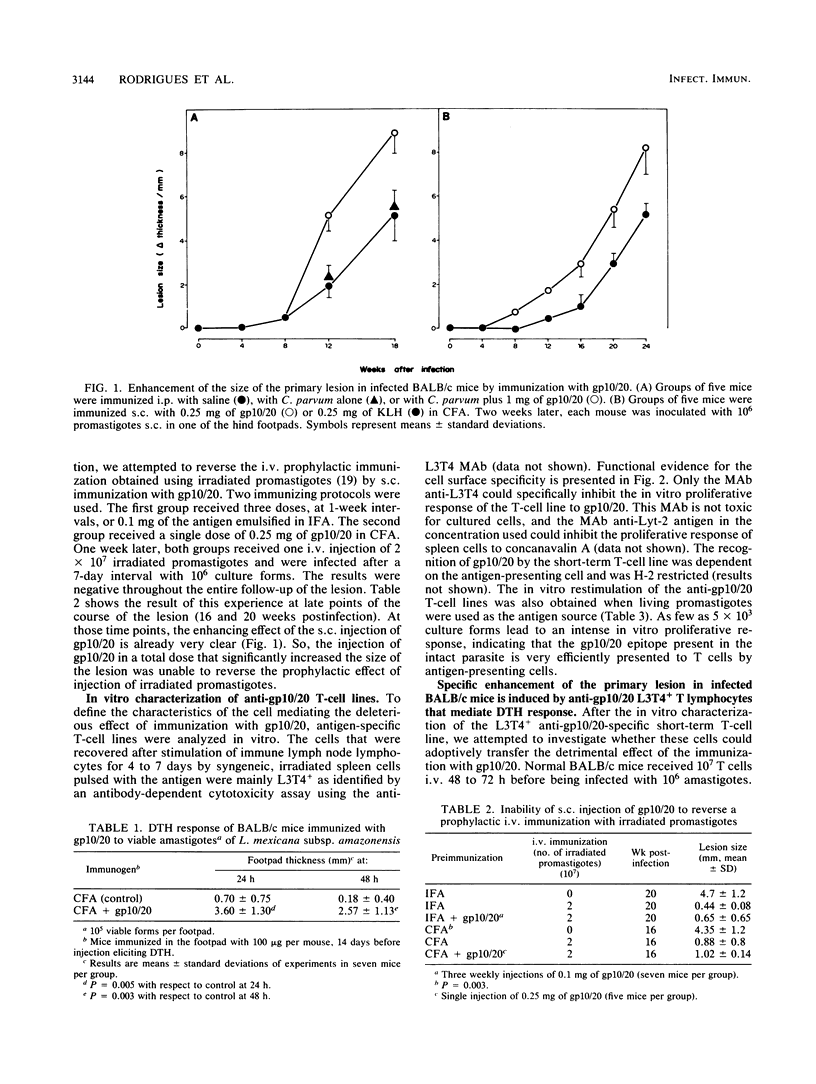
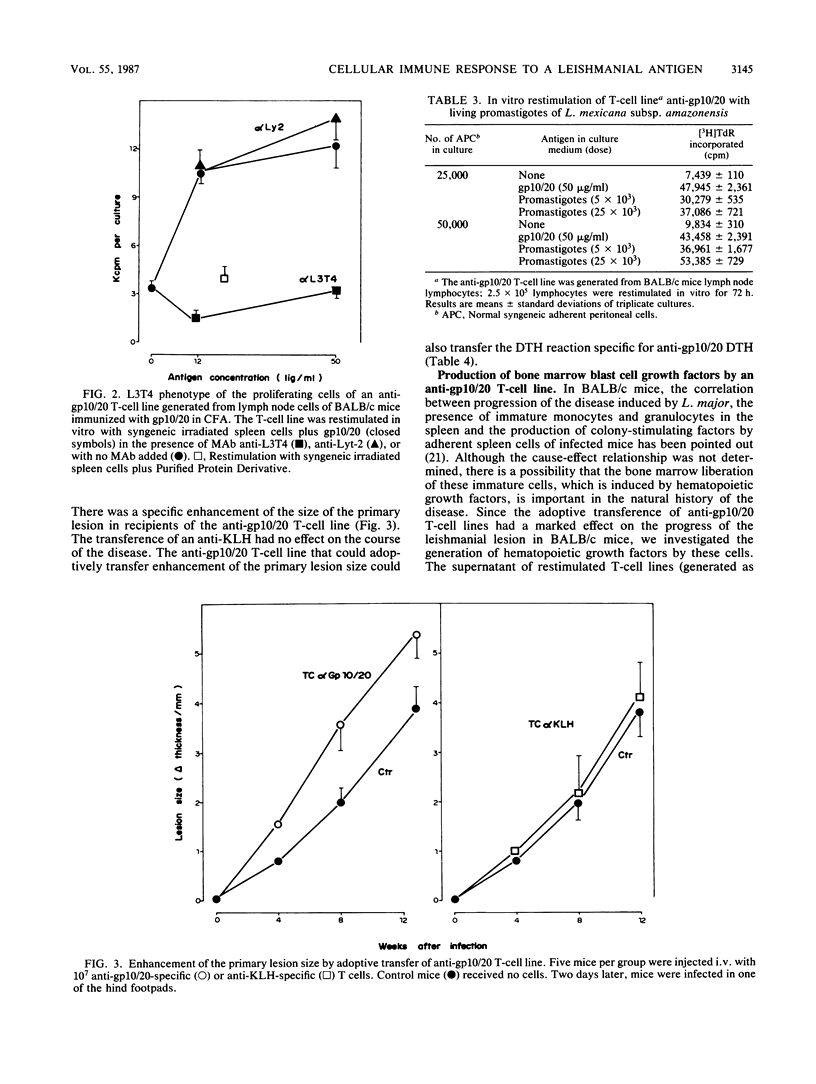
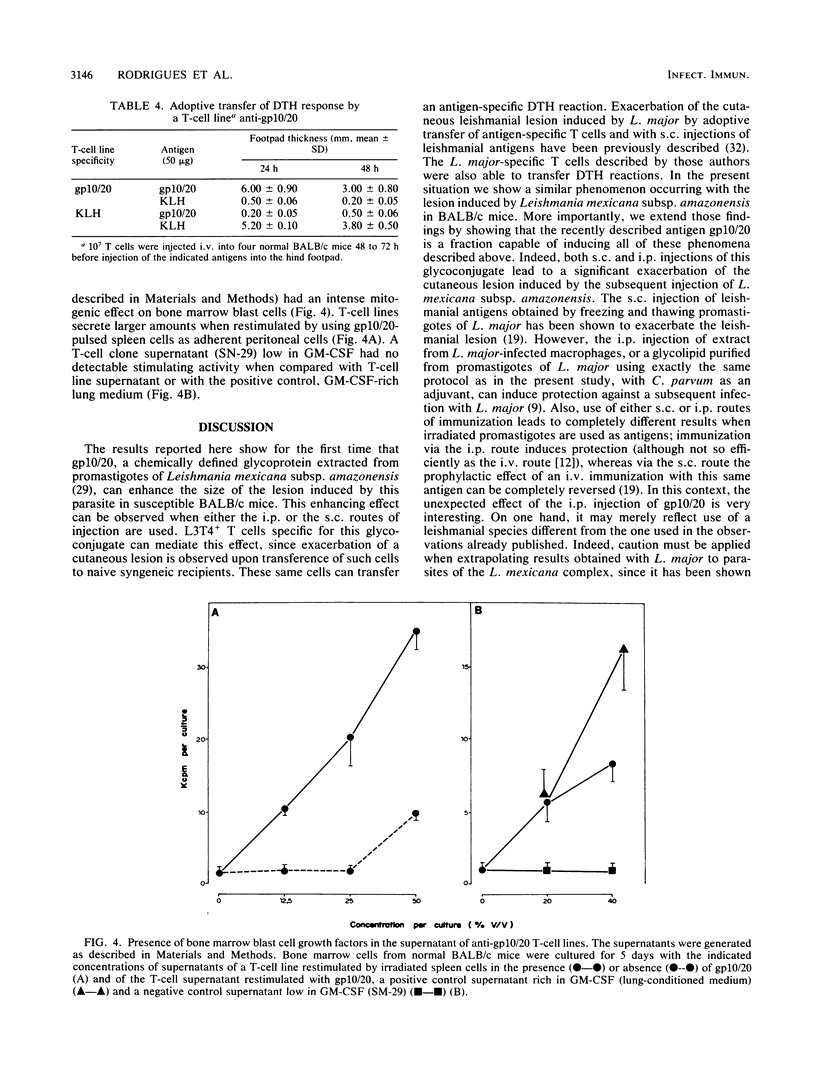
Immunization of BALB/c mice with gp10/20, a glycoconjugate purified from Leishmania mexicana subsp. amazonensis, induced a delayed-type hypersensitivity response to the antigen, and a significant increase was elicited in the size of the lesion induced by a subcutaneous infection with this parasite. The increase in the lesion size was observed when mice were immunized by the subcutaneous and the intraperitoneal routes. The subcutaneous immunization with gp10/20 was unable to reverse the prophylactic effect of an intravenous injection of irradiated promastigotes. An L3T4+ T-cell line specific for gp10/20 was able to transfer this lesion-enhancing effect and specific delayed-type hypersensitivity reactivity to normal syngeneic recipients. The same T-cell line was a good producer of a hematopoietic growth factor, granulocyte-macrophage colony-stimulating factor.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adorini L., Harvey M. A., Miller A., Sercarz E. E. Fine specificity of regulatory T cells. II. Suppressor and helper T cells are induced by different regions of hen egg-white lysozyme in a genetically nonresponder mouse strain. J Exp Med. 1979 Aug 1;150(2):293–306. doi: 10.1084/jem.150.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander J., Kaye P. M. Immunoregulatory pathways in murine leishmaniasis: different regulatory control during Leishmania mexicana mexicana and Leishmania major infections. Clin Exp Immunol. 1985 Sep;61(3):674–682. [PMC free article] [PubMed] [Google Scholar]
- Andrade Z. A., Reed S. G., Roters S. B., Sadigursky M. Immunopathology of experimental cutaneous leishmaniasis. Am J Pathol. 1984 Jan;114(1):137–148. [PMC free article] [PubMed] [Google Scholar]
- Arredondo B., Pérez H. Alterations of the immune response associated with chronic experimental leishmaniasis. Infect Immun. 1979 Jul;25(1):16–22. doi: 10.1128/iai.25.1.16-22.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borojevic R., Santos-da-Silva C., Carvalho E. A. Chronic schistosomiasis mansoni: splenic myelopoiesis and inhibition of neutrophil granulocytopoiesis mediated by the sera of patients. J Infect Dis. 1983 Sep;148(3):422–426. doi: 10.1093/infdis/148.3.422. [DOI] [PubMed] [Google Scholar]
- Chang C. S., Chang K. P. Monoclonal antibody affinity purification of a Leishmania membrane glycoprotein and its inhibition of leishmania-macrophage binding. Proc Natl Acad Sci U S A. 1986 Jan;83(1):100–104. doi: 10.1073/pnas.83.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cillari E., Liew F. Y., Lelchuk R. Suppression of interleukin-2 production by macrophages in genetically susceptible mice infected with Leishmania major. Infect Immun. 1986 Nov;54(2):386–394. doi: 10.1128/iai.54.2.386-394.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaliwal J. S., Liew F. Y., Cox F. E. Specific suppressor T cells for delayed-type hypersensitivity in susceptible mice immunized against cutaneous leishmaniasis. Infect Immun. 1985 Aug;49(2):417–423. doi: 10.1128/iai.49.2.417-423.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E., Mitchell G. F. Immunization with Leishmania receptor for macrophages protects mice against cutaneous leishmaniasis. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5910–5914. doi: 10.1073/pnas.82.17.5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak H., Turner A. R., Shaw A. R., Yau O. W. Stimulation of [3H]thymidine uptake in mouse marrow by granulocyte-macrophage colony stimulating factor from mouse lung conditioned medium. J Immunol Methods. 1983 Jan 28;56(2):253–260. doi: 10.1016/0022-1759(83)90417-9. [DOI] [PubMed] [Google Scholar]
- Howard J. G., Hale C., Chan-Liew W. L. Immunological regulation of experimental cutaneous leishmaniasis. 1. Immunogenetic aspects of susceptibility to Leishmania tropica in mice. Parasite Immunol. 1980 Winter;2(4):303–314. doi: 10.1111/j.1365-3024.1980.tb00061.x. [DOI] [PubMed] [Google Scholar]
- Jaffe C. L., Bennett E., Grimaldi G., Jr, McMahon-Pratt D. Production and characterization of species-specific monoclonal antibodies against Leishmania donovani for immunodiagnosis. J Immunol. 1984 Jul;133(1):440–447. [PubMed] [Google Scholar]
- Jaffe C. L., McMahon-Pratt D. Monoclonal antibodies specific for Leishmania tropica. I. Characterization of antigens associated with stage- and species-specific determinants. J Immunol. 1983 Oct;131(4):1987–1993. [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Krammer P. H., Echtenacher B., Gemsa D., Hamann U., Hültner L., Kaltmann B., Kees U., Kubelka C., Marcucci F. Immune-interferon (IFN-gamma), macrophage-activating factors (MAFs), and colony-stimulating factors (CSFs) secreted by T cell clones in limiting dilution microcultures, long-term cultures, and by T cell hybridomas. Immunol Rev. 1983;76:5–28. doi: 10.1111/j.1600-065x.1983.tb01095.x. [DOI] [PubMed] [Google Scholar]
- Li Z. Q., Giegé R., Jacrot B., Oberthür R., Thierry J. C., Zaccaï G. Structure of phenylalanine-accepting transfer ribonucleic acid and of its environment in aqueous solvents with different salts. Biochemistry. 1983 Sep 13;22(19):4380–4388. doi: 10.1021/bi00288a006. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Hale C., Howard J. G. Immunologic regulation of experimental cutaneous leishmaniasis. V. Characterization of effector and specific suppressor T cells. J Immunol. 1982 Apr;128(4):1917–1922. [PubMed] [Google Scholar]
- Liew F. Y., Singleton A., Cillari E., Howard J. G. Prophylactic immunization against experimental leishmaniasis. V. Mechanism of the anti-protective blocking effect induced by subcutaneous immunization against Leishmania major infection. J Immunol. 1985 Sep;135(3):2102–2107. [PubMed] [Google Scholar]
- Liew F. Y. Specific suppression of responses to Leishmania tropica by a cloned T-cell line. Nature. 1983 Oct 13;305(5935):630–632. doi: 10.1038/305630a0. [DOI] [PubMed] [Google Scholar]
- Milon G., Titus R. G., Cerottini J. C., Marchal G., Louis J. A. Higher frequency of Leishmania major-specific L3T4+ T cells in susceptible BALB/c as compared with resistant CBA mice. J Immunol. 1986 Feb 15;136(4):1467–1471. [PubMed] [Google Scholar]
- Mirkovich A. M., Galelli A., Allison A. C., Modabber F. Z. Increased myelopoiesis during Leishmania major infection in mice: generation of 'safe targets', a possible way to evade the effector immune mechanism. Clin Exp Immunol. 1986 Apr;64(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- Mitchell G. F., Curtis J. M., Scollay R. G., Handman E. Resistance and abrogation of resistance to cutaneous leishmaniasis in reconstituted BALB/c nude mice. Aust J Exp Biol Med Sci. 1981 Oct;59(Pt 5):539–554. doi: 10.1038/icb.1981.47. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F., Handman E. T-lymphocytes recognise Leishmania glycoconjugates. Parasitol Today. 1985 Aug;1(2):61–63. doi: 10.1016/0169-4758(85)90117-6. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F., Handman E. The glycoconjugate derived from a Leishmania major receptor for macrophages is a suppressogenic, disease-promoting antigen in murine cutaneous leishmaniasis. Parasite Immunol. 1986 May;8(3):255–263. doi: 10.1111/j.1365-3024.1986.tb01037.x. [DOI] [PubMed] [Google Scholar]
- Preston P. M., Carter R. L., Leuchars E., Davies A. J., Dumonde D. C. Experimental cutaneous leishmaniasis. 3. Effects of thymectomy on the course of infection of CBA mice with Leishmania tropica. Clin Exp Immunol. 1972 Feb;10(2):337–357. [PMC free article] [PubMed] [Google Scholar]
- Pérez H., Labrador F., Torrealba J. W. Variations in the response of five strains of mice to Leishmania mexicana. Int J Parasitol. 1979 Feb;9(1):27–32. doi: 10.1016/0020-7519(79)90062-6. [DOI] [PubMed] [Google Scholar]
- Rodrigues M. M., Xavier M. T., Previato L. M., Barcinski M. A. Characterization of cellular immune response to chemically defined glycoconjugates from Leishmania mexicana subsp. amazonensis. Infect Immun. 1986 Jan;51(1):80–86. doi: 10.1128/iai.51.1.80-86.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. G., Wilhelm H. The involvement of the major surface glycoprotein (gp63) of Leishmania promastigotes in attachment to macrophages. J Immunol. 1986 Apr 1;136(7):2613–2620. [PubMed] [Google Scholar]
- Titus R. G., Ceredig R., Cerottini J. C., Louis J. A. Therapeutic effect of anti-L3T4 monoclonal antibody GK1.5 on cutaneous leishmaniasis in genetically-susceptible BALB/c mice. J Immunol. 1985 Sep;135(3):2108–2114. [PubMed] [Google Scholar]
- Titus R. G., Lima G. C., Engers H. D., Louis J. A. Exacerbation of murine cutaneous leishmaniasis by adoptive transfer of parasite-specific helper T cell populations capable of mediating Leishmania major-specific delayed-type hypersensitivity. J Immunol. 1984 Sep;133(3):1594–1600. [PubMed] [Google Scholar]


