Abstract
During voluntary action, dorsal premotor cortex (PMd) may exert influences on motor regions in both hemispheres, but such inter-regional interactions are not well understood. We used transcranial magnetic stimulation (TMS) concurrently with event-related functional magnetic resonance imaging to study such interactions directly. We tested whether causal influences from left PMd upon contralateral (right) motor areas depend on the current state of the motor system, involving regions engaged in a current task. We applied short bursts (360 ms) of high or low intensity TMS to left PMd, during single isometric left-hand grips or during rest. TMS to left PMd affected activity in contralateral right PMd and primary motor cortex (M1), in a state-dependent manner. During active left-hand grip, high (vs low) intensity TMS led to activity increases in contralateral right PMd and M1, whereas activity decreases there due to TMS were observed during no-grip rest. Analyses of condition-dependent functional coupling confirmed topographically-specific stronger coupling between left PMd with right PMd (and right M1), when high intensity TMS was applied to left PMd during left-hand grip. We conclude that left PMd can exert state-dependent interhemispheric influences on contralateral cortical motor areas relevant for a current motor task.
Keywords: concurrent TMS-fMRI, effective connectivity, grip-force, motor preparation, transcallosal inhibition
Introduction
The dorsal premotor cortex (PMd) is a key structure for selection and initiation of voluntary actions (Scott and others 1997; Grafton and others 1998; Rushworth and others 2003; Hoshi and Tanji, 2004; Cisek and Kalaska 2005; Churchland and others 2006). It is reciprocally connected with ipsilateral (Lu and others 1994; Wise and others 1997; Dum and Strick, 2005) and contralateral (Marconi and others 2003) cortical motor areas, most notably primary motor cortex (M1), parietal cortex, and contralateral PMd. During simple unilateral hand movements, an increase in M1 activity is seen predominantly in the contralateral hemisphere (Evarts 1966), whereas the increase in PMd activity is typically bilateral in non-human primates (Tanji and others 1988; Cisek and others 2003) and humans (Ward and Frackowiak 2003). The potential importance of ipsilateral premotor cortex for voluntary action is further emphasized by findings that subcortical damage to corticospinal pathways can lead to a functionally relevant increase in PMd activity, during movement of the affected ipsilateral hand (Johansen-Berg and others 2002; Ward and others 2006, 2007). To date however, it remains unclear by what routes and in what functional manner ipsilateral PMd may exert its influences over contralateral cortical motor structures.
Several lines of evidence suggest the particular importance of left PMd and its dominance for selection of both ipsilateral and contralateral actions (Schluter and others 1998, 2001; Rushworth and others 2003). Recent transcranial magnetic stimulation (TMS) experiments have used an interhemispheric conditioning-test paradigm, with one coil over premotor cortex and the other over primary motor cortex in opposite hemispheres, finding that left PMd may change excitability of contralateral M1, possibly directly via transcallosal projections (Mochizuki and others 2004a, b; Koch and others 2006, 2007). This method therefore assesses the causal impact of one brain region upon an interconnected area, at high temporal precision, non-invasively in healthy human subjects. However, conditioning-test TMS approaches for studying influences of PMd on other areas must typically rely on the induced motor response to an M1-pulse as their dependent measure, and therefore must ultimately highlight influences converging on just M1 in particular. Moreover, they cannot disclose the spatial specificity across cortical regions of such influences. This is potentially important as left PMd also has anatomical connections with several cortical structures other than M1 in either hemisphere, notably with contralateral right PMd (Lu and others 1994; Wise and others 1997; Marconi and others 2003).
A very different approach has been to combine TMS with physiological measures of induced changes in brain activity, such as via PET (Paus and others 1997, 1998; Paus 1999), EEG (Paus and others 2001; Massimini and others 2005) or fMRI (Baudewig and others 2001; Bestmann and others 2004, 2005; Bohning and others 1998, 1999, 2000; Kemna and Gembris 2003; Ruff and others 2006). Using such approaches, it has been shown that stimulating left PMd with TMS at rest may affect activity in putatively interconnected motor structures, including contralateral (right) PMd, bilateral SMA, plus frontal and parietal association cortices (Chouinard and others 2003; Bestmann and others 2005).
However, to date such studies have not addressed any dependence of remote causal influences from left PMd on the current state of putatively interconnected motor regions. These studies did not vary state, due either to their use of prolonged TMS stimulation protocols prior to scanning (Chouinard and others 2003), and/or the absence of any varied task requirements during scanning (Bestmann and others 2005). Therefore, the nature and spatial distribution across brain regions of functional interplay between left PMd and interconnected cortical motor regions for the control of voluntary movement remains unclear.
Due to recent technical advances, such potentially state-dependent interplay between cortical regions can be studied non-invasively, by combining TMS on-line with event-related functional magnetic resonance imaging (fMRI) (Bohning and others 2000; Baudewig and others 2001; Kemna and Gembris 2003; Bestmann and others 2004; Sack and others 2007). This approach applies short TMS events or stimulation trains that can have immediate effects on brain activity lasting only a few seconds (Romeo and others 2000; Modugno and others 2001), during scanning. By concurrently assessing evoked activity changes throughout the brain with fMRI, this approach can thus provide a means of assessing inter-regional influences directly, by using the causal intervention or ‘perturbation’ of TMS and then measuring its remote effects as well as any local influences (Paus 2005).
Accordingly, we examined the causal impact of left PMd stimulation upon activity throughout the brain, during two different contexts (ipsilateral grip task or no-grip rest). We assessed any state-dependent remote effects of left PMd TMS, and whether they might be distributed across all putatively interconnected areas (potentially including, for example, ipsilateral left M1) or instead might be topographically restricted to task-relevant brain regions during the left-hand movement (including contralateral right M1 and right PMd). Such an outcome would indicate some state-specificity of causal influences from left PMd during control of voluntary ipsilateral movement. We specifically chose stimulation of left PMd, because of its suggested dominance for the selection and preparation of hand movements (Schluter and others 2001; Astafiev and others 2003; Rushworth and others 2003; Davare and others 2006).
We applied short bursts of left PMd stimulation either during an active force-generation grip task with the ipsilateral left hand, or during no-grip rest. We chose the grip-task as its neural substrates (in the absence of TMS) are already well characterized (e.g. Ehrsson and others 2000, 2001; Ward and Frackowiak 2003). Moreover, it allowed TMS application to overlap temporally with the active grip (see below), and because this task may also be of clinical interest (Ward and others 2007). Our data reveal that causal interactions of left PMd TMS on activity in interconnected regions (including contralateral right PMd and right M1) vary strongly with the current motor state, in a manner that is specific to relevant task-related motor cortical areas contralateral to the gripping hand, and thus contralateral to the applied left PMd TMS.
Materials and Methods
Participants
Twelve right-handed healthy volunteers participated (three females, mean age 45.33 yrs, range 28-73 yrs), with no history of neurological or psychiatric illness nor regular medication. Participants were naïve to the purpose of the experiment. Full written consent was obtained from all participants, in accord with local ethics approval. The wide age-range of participants (chosen here with a view to future possible clinical studies of patients) was taken into account in analysis of the results, which considered age as one possible factor.
Experimental paradigm
A continuous scanning session (25 min 44 s) was conducted to measure TMS influences in a 2 × 2 factorial event-related design with motor state (grip, nogrip) and TMS input to left PMd (TMShigh, TMSlow) as experimental factors. In addition to 20 experimental trials per condition, we also recorded 20 null events. These comprise trials without experimental treatment and thus provide a baseline measure of activity. All trials were pseudo-randomized so that each trial type occurred twice within eight consecutive trials, with an average inter-trial-interval (ITI) of 16.11 s to minimize any carry-over effects of TMS bursts between successive trials (Modugno and others 2001).
We varied the context in which TMS was applied using a simple left-hand dynamic isometric grip task (Ward and Frackowiak 2003; Ward and others 2007). The grip manipulandum consisted of two force transducers (Honeywell FSG15N1A; Honeywell, NJ, USA) moulded between two plastic bars (width 6 cm). Compression of the two bars during isometric hand grip generated a differential voltage, linearly proportional to the exerted force. The signal was fed into an amplifier (CED 1902), digitized (CED 1401; both Cambridge Electronic Design, Cambridge, UK), and stored on a personal computer. A video projector at the bore of the magnet projected visual stimuli onto a frosted screen, viewed via a mirror system mounted atop the MR head coil. A centrally presented visual columnar display gave visual feedback about any dynamic changes in recorded voltage (and hence grip-force) vertically. During scanning, a visual cue indicated that participants should either perform a single isometric left-hand grip, or maintain rest. Target force-levels for all grip-trials were set to 20% of individual maximum voluntary contraction (MVC) and displayed as a horizontal yellow bar on the red feedback column (Figure 1A). This grip task was chosen based on pilot work showing that participants could perform the task well even in the presence of TMS (thus avoiding contamination of any neural effects of TMS by overt behavioral changes, see Discussion). Furthermore, the extended duration of each hand grip in the present task allowed TMS to overlap with the active motor state, as confirmed below. Finally, the neural substrates of this particular grip task have been well characterised by prior fMRI studies (Ward and Frackowiak, 2003).
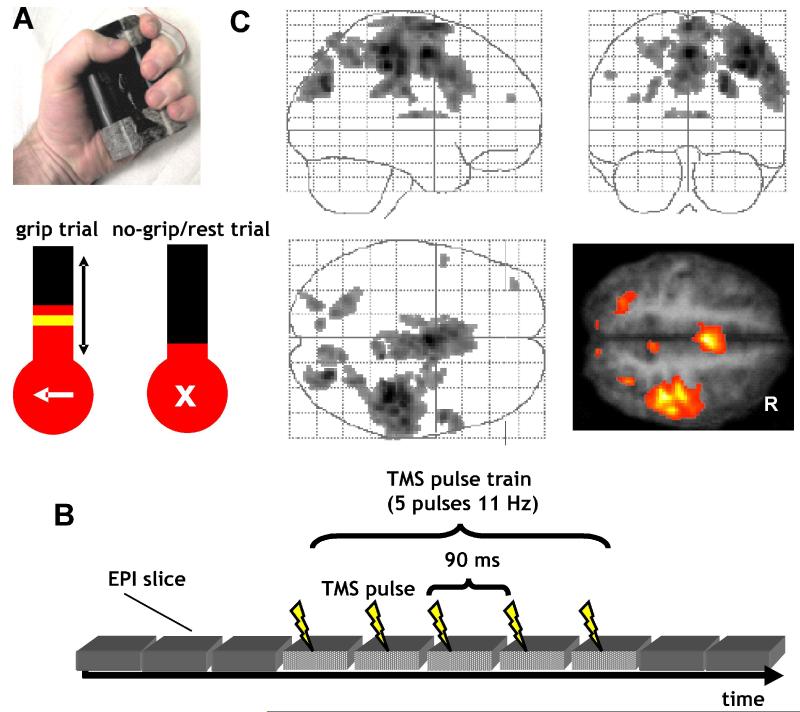
Figure 1.
Experimental setup and main effects of grip task.
A. Grip-force manipulandum and screen display for grip (arrow to left) and nogrip (central cross) trials. During grip trials, a yellow target bar indicated the required force level of 20% maximum voluntary contraction. The actual force exerted was indicated online by a red column. Participants were instructed to generate a non-ballistic force matching the displayed target bar using a gentle pace without major corrective movements. TMS was applied in a train, starting 900 ms after presentation of the target force bar, so as to overlap with performance of the grip.
B. Schematic of EPI-TMS pulse synchronization. On each trial, TMS was applied at one of two intensities (110% resting motor threshold or 70% active motor threshold). TMS pulses were applied every 90 ms during the waiting time between EPI navigator echoes and EPI data readout. Every slice was perturbed equally often throughout a scanning session. TMS pulses were temporally separated from any slice selection gradients or excitation pulses (fat saturation pulse, trigger pulse, RF pulse, slice selection gradient, refocusing gradient, navigator echo) and EPI data readout-gradients.
C. Simple fMRI main effects of grip > nogrip, irrespective of TMS. Results of the group random effects analysis are projected onto a transparent schematic of the MNI template brain and on a transverse section (z=54) of the averaged normalized structural scans of all participants. The height threshold was set at T > 4, uncorrected for multiple comparisons across whole brain, and the extent (or cluster) threshold set at P < 0.05, corrected for multiple comparisons across whole brain. Left hand grip evoked activity in right primary motor cortex, extending into adjacent dorsal and ventral premotor cortex, plus primary and secondary somatosensory cortex. Additional activity increases were found in superior parietal cortex and intraparietal sulcus, reflecting the visuomotor processing involved in hand grip-trials. Note that we focused imaging largely on the dorsal part of the brain. Therefore, any ventral visual activations due to visual aspects of the grip task were outside the field of view. R = right.
On an unpredictable 50% of trials (grip condition), a left-hand arrow as well as the grip-force target level was presented at trial onset (see Figure 1A). This cue started the trial, indicating that participants should perform a brief single left-hand grip, until the column representing force reached the horizontal target bar. The cue and target level were displayed for 4 s, superimposed on the force column that was present throughout the experiment. Participants were explicitly instructed that speed was not critical, and that they should generate a non-ballistic hand grip to approximately match the displayed target bar using a gentle pace without major corrective movements.
On the other unpredictable 50% of trials (nogrip condition), a cross (‘X’ character) was shown instead of an arrow at trial onset (Figure 1A). This indicated that participants should withhold from making a left-hand grip and instead keep the hands relaxed (no-grip rest condition). This forewarning for a nogrip trial also served to prevent unintentional grip-force being generated when TMS pulses occurred. Only left-hand grips were performed. However, participants held one manipulandum in the right hand to allow the assessment of any unintended mirror movements, or of undesired twitches during TMS. Neither was observed here.
Two TMS intensities were applied over left PMd: For the TMShigh condition, TMS was applied at 110% of individual resting motor (M1) threshold (RMT; mean intensity (± s.d) of 74% (± 7%) of maximal stimulator output, MSO), which is known to be effective in stimulating PMd (Mochizuki and others 2004a; Bestmann and others 2005; Praeg and others 2005; Baumer and others 2006; Koch and others 2006; O’Shea and others, 2007). RMT was determined for the right hand when stimulating over left M1. An unpredictable 50% of all trials during the fMRI experiment were in this TMShigh condition (orthogonally to the grip/nogrip manipulation). The other unpredictable half of trials during scanning provided the TMSlow condition, which had the TMS intensity set to 70% of individual active motor threshold (AMT), corresponding to a mean intensity (± s.d.) of 42% (± 4%) MSO. The seemingly rather high motor thresholds (in terms of MSO) in our study reflect increased impedance of the extended MR-compatible cabling running from the stimulator to the coil.
A TMS train (5 pulses at 11 Hz) was applied on all trials to left PMd (i.e. ipsilateral to the active hand for the grip-task), starting 900 ms (10 EPI slice acquisitions) after presentation of the instructional visual cue. We chose this fixed interval (rather than triggering TMS from the actual movement onset on each trial) because TMS-pulse application had to be synchronized and interleaved with high temporal precision in relation to each EPI slice acquisition (see below, and Figure 1B). We later verified that participants were indeed performing the grip task at the moment of TMS delivery on relevant trials (see Results), as in previous work with the same behavioral grip-task (Ward and Frackowiak 2003). In total, 400 TMS pulses were applied during each scanning session, half of these at the low intensity of 70% AMT. Our stimulation protocol thus conformed to published TMS guidelines (Wassermann 1998).
Prior to scanning, participants were pre-trained outside the scanner until comfortable with the grip task, without TMS being applied. Once inside the scanner the TMS coil was carefully located over left PMd (see below), and the task was briefly practiced again until participants performed brief and non-ballistic isometric hand grips that approximately reached the required force level on every trial. Some preliminary TMS trains were applied at 110% RMT while the participant was contracting their right hand at around 20% MVC (i.e., with the opposite hand to that used during the scanning paradigm). This ensured that participants were comfortable with the TMS stimulation protocol and also provided a check that no twitches were induced contralaterally to TMS. No overt muscle responses were observed in any participant, nor later reported by participants.
Magnetic resonance imaging
MRI was conducted with a 1.5T Magnetom SONATA system (Siemens Medical Solutions, Erlangen, Germany) using the body-transmit coil and the single-channel receive CP head array (30 cm diameter). Whole-head T1-weighted anatomical images were acquired after the experiment using a 3D MDEFT sequence with an isotropic resolution of 1 mm3 (Deichmann and others 2004). Each scanning session comprised functional T2*-weighted MRI transverse echo-planar images (EPI) with blood oxygenation level dependent (BOLD) contrast, covering the dorsal convexity of the brain down to the thalamus (848 volumes, 20 slices / volume, 64 × 96 matrix, 3 × 3 mm in-plane resolution with 50% oversampling in phase-encoding direction, 2.5 mm slice thickness plus 50% spatial gap between spatially adjacent slices, repetition time (TR) = 1800 ms; TE = 50 ms; α = 90°; echo-spacing 500 μs; 2298 Hz/pixel bandwidth; trapezoidal readout gradients with a ramp of 130 μs and a flat top of 240 μs; field of view: 192 × 192 mm; max slew rate 214.9 mT/m/ms). The oversampling in the phase-encoding direction shifted residual Nyquist ghosting (induced by the presence of the MR-compatible TMS coil inside the scanner) outside of the brain image, without compromising the spatial resolution of MR images. Physiological and technical artifacts were monitored online during scanning (Weiskopf et al., 2007). The first five volumes were discarded to allow for T1 equilibration effects. Following the main experiment, whole brain EPI volumes were acquired using the same orientation, to facilitate spatial normalization of the spatially restricted functional image series.
Interleaved TMS-fMRI
TMS was implemented using a MagStim Rapid system (The Magstim Company, Dyfed, UK) with a custom-built MR-compatible figure-of-eight stimulation coil (two windings of ten turns each; inner wing diameter 53 mm, distance between outer coil surface and windings of 2-3 mm (variation due to manufacturing tolerance); coil inductance, including cable, of 20 μH; maximal current at 100% stimulator output of ∼5kA). The stimulation unit was housed inside the scanner room in a shielded cabinet, from which the stimulation coil cable was fed through a custom filter box (The Magstim Company). Residual RF transmission along the coil cable was further suppressed using ferrite sleeves. The TMS coil was connected to the stimulator in parallel to a high voltage relay (Magstim ES9486, The Magstim Company). During EPI acquisition, the relay was in closed mode, thereby effectively preventing any residual leakage in current flow from the stimulator. The relay was opened 50 ms prior to TMS pulse discharge, and closed again 8 ms after termination of the last TMS pulse of a trial. The relay and TMS were controlled with a unit developed in-house based on a BASIC Stamp 2 micro-controller (Parallax Inc., Rocklin, California, USA). TMS pulses were applied during the dead time between the EPI navigator echoes and the EPI data readout, and separated from RF slice excitation pulses (Gaussian-like symmetric sync, 2560 ms duration, Bestmann and others 2003a). Throughout scanning, each slice coincided equally often with TMS pulses to avoid any systematic influences on slice-by-slice variance.
The stimulation site over the left dorsal PMd was determined as the scalp point 2 cm anterior and 1 cm medial to the so-called motor ‘hot-spot’ for evoking single muscle twitches in the contralateral first dorsal interosseous (Schluter and others 1999; Johansen-Berg and others 2002; O’Shea and others, 2007). Inside the scanner, the stimulation coil was placed over the marked location using an MR-compatible custom-built coil holder, allowing stable positioning of the TMS coil with several degrees of freedom. The coil was oriented tangential to the scalp, at approximately 45 degrees from the midline, inducing a biphasic current with an initial antero-posterior induced direction. Foam-padded cushions were used to restrict head-movements.
All visual stimulation, grip-force data acquisition, TMS triggering and intensity regulation, and relay settings were controlled using the toolbox Cogent 2000 (Wellcome Department of Imaging Neuroscience, London, UK; http://www.fil.ion.ucl.ac.uk/cogent) running under Matlab (The Mathworks, Natick, Massachusetts, USA). Participants wore earplugs (SNR = 36dB) to reduce acoustic noise from the scanner and the TMS discharge sound.
Behavioral and fMRI data analysis
For each grip trial, we measured the movement onset, grip duration, and peak force. Movement ‘onset’ was defined as the latency between the onset of the visual cue indicating a grip trial, and the point when grip force exceeded 20% of baseline value. This, however, means that the cortical activity related to grip force production probably started several tens or hundreds of milliseconds before this level was reached. Moreover, the extended nature of the TMS train (360 ms duration) ensured that it would overlap with grip-related brain states during the grip task. Grip duration was defined between the two successive time points at which grip force exceeded the 20% of baseline boundary, and the maximum force was determined during this period. Paired two-tailed t-tests were used for comparison of these parameters across participants and stimulation conditions.
Following trajectory-based online reconstruction of functional images (Josephs and others 2000), EPI slices coinciding with TMS pulses were replaced by interpolation between the previous and subsequent acquisition of the same slice (Ruff and others 2006). Additionally, any remaining slices whose mean signal deviated from the mean signal of the slice time series by more than 1.5 SD were replaced in the same way (only 0.19% in total).
Imaging data were analyzed using Statistical Parametric Mapping (SPM5, http://www.fil.ion.ucl.ac.uk/spm) implemented in Matlab 6. The first five volumes were discarded, and all subsequent volumes were realigned to the first volume in order to correct for interscan movement. Additional unwanted variance related to interactions of head motion and geometric distortions was removed using the ‘unwarp’ toolbox as implemented in SPM5 (Andersson and others 2001).
The whole-brain EPI images were normalized to a standard EPI template based on the Montreal Neurological Institute (MNI) reference brain in Talairach space, using 4th-degree b-spline interpolation, and re-sampled to 2 × 2 × 2 mm3 voxels. The resulting transformation parameters were applied to the image volumes of the main experiment. Preprocessing included detrending of time-series in each voxel with a linear model of the global signal (Macey and others 2004); grand mean scaling of 100 over voxels and scans, AR(1)-model to account for serial autocorrelations of the data; and spatial smoothing of normalized images with an isotropic 8 mm FWHM Gaussian kernel to allow for valid statistical inference according to Gaussian random field theory.
Statistical analysis followed a two-stage procedure. First, a single subject fixed-effects model was computed for each participant by multiple regression of the voxel-wise time-series onto a composite model containing the covariates of interest. Each of the four event-related trial types (TMShigh-grip, TMSlow-grip, TMShigh-nogrip, TMSlow-nogrip) were modeled as delta functions, with onsets defined as the first TMS pulse, and were included as separate covariates. To account for any additional variance induced by any slight trial-by-trial and or intersubject variations in grip-force and grip duration, additional covariates comprising a delta function scaled by the actual peak force and duration exerted for each hand grip were included in the model. All covariates were convolved with a canonical synthetic hemodynamic response function in a general linear model (Friston and others 1995; Friston and others 1998), together with a single covariate representing the mean (constant) term over scans. Thus for each participant, voxel-wise parameter estimates for each covariate were calculated, resulting from the weighted least squares (WLS) fit of the model to the data. The statistical parametric maps (SPMs) of the t statistic resulting from linear contrasts of each covariate were then generated and stored as separate images for each participant.
At a second level of analysis, contrast images for each participant and covariate were entered into a one sample t-test for each covariate of interest, in a random-effects analysis across participants. Any age-related changes in cortical connectivity (Della-Maggiore and others 2000; Rowe and others 2006) or PMd function (Ward and Frackowiak 2003) were considered by including the age of each participant as a covariate in this second level model. This revealed no age-related influences for the critical findings, namely for the main effect of TMS; the main effect of grip; or for their interaction, in any grip-task-related areas for the present dataset. The height threshold for SPM{t} maps was set at T > 4, uncorrected for multiple comparisons across the whole brain, and the extent (cluster) threshold set at P < 0.05, corrected for multiple comparisons across the whole brain.
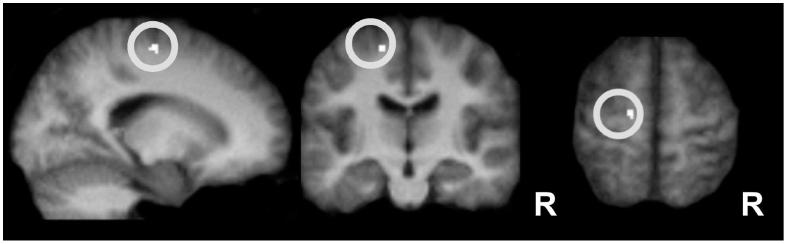
The site of stimulation (left PMd) around the left precentral sulcus (x,y,z=-26,-14,62; see (Amiez and others 2006) was a priori defined as a region of interest (ROI) for our fMRI analysis. This region corresponds well to previously reported activity changes at the putative site of stimulation following premotor rTMS (Rowe and others 2006). Small volume correction for this ROI was applied by using a spherical mask around this location with a diameter of 15 mm. Activity within this ROI is reported at p < 0.05, cluster level corrected for multiple comparisons within the spherical ROI.
Analysis of condition-dependent functional coupling between stimulated left PMd and other brain regions
In addition to the standard event-related analysis of fMRI activity by condition described above, we also ran a supplementary analysis testing for any changes in ‘functional coupling’ or effective connectivity between areas, as a function of condition (Friston and others 1997; Gitelman and others 2003). Specifically, we tested for any regions showing higher functional coupling with the targeted left PMd site when stimulated with high versus low TMS, under the active grip versus no-grip conditions. To do so, we used the well-established ‘psychophysiological interaction’ or PPI approach (e.g. Friston and others 1997; Gitelman and others 2003; Lee and others 2003; Egner and Hirsch 2005; Taniwaki and others 2006; Wolbers and others 2006), a data-driven analysis that makes relatively minimal assumptions. We tested for areas showing stronger functional coupling with left PMd (i.e. higher covariation in residual activity patterns with the time course of left PMd, once the mean condition-specific activations identified by the main SPM analysis, as above, had been factored out from all regions).
Specifically, we extracted the first eigenvariate of the fMRI signal from an 8 mm ROI sphere, centered around the group peak voxel for the main effect of high vs low intensity TMS in the basic SPM analysis (x,y,z = -18, -16, 62). We then tested for a PPI interaction, namely a stronger covariation of left PMd with other areas related to the motor task that was specific to high versus low TMS, during the active grip-task versus the no-grip condition. To focus on the task-related areas of interest, we restricted this PPI analysis to those regions which had shown an overall activation for grip versus no-grip (inclusive masking at p<0.05, uncorrected, but with the PPI itself thresholded at our conventional threshold of T > 4 and extent (or cluster) threshold set at P < 0.05, corrected for multiple comparisons across the whole brain). At the second level model, we included the age of each participant as a covariate. Again, no age-related influences for the critical contrast were observed.
For all fMRI analyses (except for the ROI consideration of left PMd), allocation of BOLD signal changes to anatomical regions was made according to anatomical landmarks identified from the mean T1-weighted structural image of all participants with the aid of the atlas of Duvernoy (Duvernoy 1991) as well as by using a three-dimensional probabilistic computerized cytoarchitectonic atlas (Eickhoff and others 2005).
Results
Behavioral results
None of the participants reported any adverse side-effects. Participants reached the desired target force-level accurately on grip-trials (mean ± s.d.) 20.3 ± 1.5% of MVC), with left-hand grip movements exceeding our criterion for movement onset (> 20% of baseline) on average 1.13 ± 0.21 s after the instructional cue, and lasting for 1.1 ± 0.5 s. Note that here we used a conservative measure for movement onset (>20% of baseline). Accordingly, neural changes related to grip probably started several tens or hundreds of milliseconds prior to this, and given the extended train of TMS (360 ms duration), some of this train must overlap with neural states associated with the active grip. Force level, movement onset, and movement duration within participants were highly correlated across the two TMS stimulation conditions within single subjects (all r > 0.7, p < 0.05), indicating that performance was comparable across conditions. There were no behavioral differences between the high and low TMS condition in peak force (TMSlow mean ± s.e.m.: 20.3 ± 1.5% MVC, TMShigh: 20.3 ± 1.6% MVC, t11 = -0.24, p = 0.81), grip duration (TMSlow: 1.12 ± 0.45 s, TMShigh: 1.09 ± 0.43 s, t11 = 1.97, p = 0.08), or grip onset time (TMSlow: 1.13 ± 0.16 s, TMShigh: 1.15 ± 0.15 s, t11 = -1.02, p = 0.33). This was important for our design, as our aim was that the motor task here should serve to manipulate the ‘motor state’ of the cortical network when high versus low TMS was applied, rather than high TMS serving to disrupt performance of the motor task (which might then have complicated interpretation of any fMRI changes). Instead, the approach here was to apply TMS as a physiological ‘perturbation’ during one motor state (active grip) versus another (rest), and then measure the impact of this on remote but potentially interconnected brain regions with fMRI. No mirror movements of the right hand occurred during TMS, as confirmed by the right hand grip-force recordings, in addition to participants’ self-report.
Functional imaging results: activations by condition
Figure 1C shows the main effect of the grip-force motor task as compared with the no-grip condition, when pooling across the two TMS intensities: (TMShigh-grip + TMSlow-grip) - (TMShigh-nogrip + TMSlow-nogrip). In accord with prior fMRI work with this grip-task (Ward and Frackowiak 2003), left-hand grip induced largely right lateralized activity in primary sensorimotor cortex, right PMd, supplementary and cingulate motor area, ventral premotor cortex, secondary somatosensory cortex, and intraparietal sulcus (see Figure 1C; see also Table 1, grip>nogrip). Additional activity increases were observed in left PMd (peak: x,y,z=-30, -8, 60), albeit at a lower threshold of p<0.001, uncorrected for multiple comparisons across the brain.
TABLE.
Imaging results
| Anatomical / functional region | MNI coordinates | Peak voxel | Peak voxel | Cluster | ||
|---|---|---|---|---|---|---|
| X | Y | Z | Z-score | T-score | P-value | |
| Simple effect grip > nogrip (irrespective of TMS) | ||||||
| R. primary motor cortex/dorsal premotor cortex | 38 | -22 | 54 | 4.85 | 10.29 | <0.001 |
| R postcentral sulcus | 40 | -34 | 54 | 5.56 | 15.37 | <0.001 |
| supplementary motor area / cingulate motor area | 2 | -4 | 54 | 5.28 | 13.05 | <0.001 |
| BL supplementary motor area | 0 | -4 | 70 | 5.07 | 11.62 | <0.001 |
| BL cingulate gyrus | 0 | -22 | 48 | 4.42 | 8.17 | <0.001 |
| R posterior superior parietal cortex | 8 | -78 | 52 | 3.79 | 5.90 | 0.002 |
| R posterior intraparietal sulcus | 24 | -74 | 30 | 5.02 | 11.32 | <0.001 |
| R ventral premotor cortex | 54 | 6 | 38 | 4.35 | 7.87 | <0.001 |
| L parieto-occiptal junction | -24 | -86 | 26 | 3.79 | 5.91 | <0.02 |
| L superior parietal cortex | -26 | -60 | 54 | 4.33 | 7.79 | <0.001 |
| R thalamus | 8 | -12 | 10 | 3.92 | 6.31 | <0.02 |
| Simple effect nogrip > grip (irrespective of TMS) | ||||||
| L medial frontal gyrus | -32 | 28 | 52 | 4.93 | 10.75 | <0.001 |
| R medial frontal gyrus | 32 | 36 | 50 | 4.32 | 7.74 | <0.01 |
| Simple effect TMShigh > TMSlow (irrespective of grip) | ||||||
| R inferior temporal gyrus | 46 | -20 | 8 | 3.80 | 5.93 | <0.05 |
| L precentral sulcus | -18 | -16 | 62 | 3.21 | 4.40 | <0.05 π |
| Simple effect TMSlow > TMShigh (irrespective of grip) | ||||||
| R anterior inferior parietal cortex | 54 | -58 | 42 | 4.48 | 8.44 | <0.005 |
| L superior parietal cortex | -26 | -46 | 66 | 4.35 | 7.87 | <0.05 |
| Interaction (TMShighnogrip - TMSlownogrip) - (TMShighgrip - TMSlowgrip) | ||||||
| R precentral gyrus | 34 | -16 | 58 | 4.58 | 8.88 | <0.05 |
| R central sulcus | 36 | -26 | 62 | 4.11 | 6.93 | <0.05 |
Height threshold of T > 4, uncorrected for multiple comparisons across whole brain, and extent (or cluster) threshold set at P < 0.05, corrected for multiple comparisons across whole brain
p < 0.05 cluster level corrected for multiple comparisons across whole across small volume of interest in a priori defined area of stimulation
By contrast, when participants had to withhold movements (i.e. following appearance of an ‘X’ symbol, rather than a leftward arrow, at the start of nogrip trials, see Figure 1A) activity was significantly larger in left and right dorsolateral prefrontal cortex, as compared to grip trials (see Table 1, nogrip>grip), possibly reflecting response inhibition in these no-grip trials.
Turning to the impact of high vs low intensity TMS [i.e. (TMShigh-grip + TMShigh-nogrip) - (TMSlow-grip + TMSlow-nogrip)], given that the TMS probe was located over left PMd, we considered an a priori ROI for inspection of local TMS effects (see Methods above). As shown in Figure 2, the ROI analysis of left PMd revealed a significant activity increase for TMShigh (versus TMSlow), which was attributed to premotor Area 6, with 60% probability, by a computerized cytoarchitectonic atlas designed for use with SPM (Eickhoff and others 2005). This assignment would thus correspond to the dorsal premotor cortex (Picard and Strick 2001; Chouinard and Paus 2006; Mayka and others 2006) (see Table 1). Therefore, TMS at high (versus low) intensity elicited a small yet significant relative activity increase at the putative site of stimulation.
Figure 2.
Local effect of TMS stimulation on BOLD signal from left PMd Region of interest (ROI) analysis for TMShigh > TMSlow, irrespective of grip, in an a priori defined ROI (sphere of 15 mm diameter, illustrated by the white circle) centered around the site of stimulation in the left PMd (x,y,z: -24, -14, 62). TMShigh vs TMSlow induced more activity in this area (P < 0.05, corrected for multiple comparisons at the cluster level within ROI). Thus, TMS at 110% of resting motor threshold evoked a local activity increase, relative to stimulation at the low intensity (70% active motor threshold). Activations are projected onto the average normalized structural scans of all participants. R = right.
Comparing both types of TMS event (high and low together, 50% of which contained grip trials at each TMS intensity) jointly against null events confirmed that presence versus absence of any TMS stimulation activated bilateral auditory cortex, as would be expected, due to the click sound inevitably associated with TMS application (see supplemental Figure 1, supplemental Table 1). This is consistent with all prior combined TMS-neuroimaging studies (e.g. Siebner and others 1999). However, such considerations are tangential to the hypothesis-driven focus on motor-related regions here, and cannot explain our other fMRI findings.
The critical question in the present study was how the high>low TMS manipulation (which activated the target site in left PMd, see Fig. 2) would interact with the current motor state, i.e. (TMShigh-grip - TMSlow-grip) - (TMShigh-nogrip - TMSlow-nogrip). Critically, we found (Fig. 3A) a significant crossover interaction between motor state and TMS intensity to left PMd, arising in right M1 hand region (BA4a) and the caudal aspect of right PMd (BA6). Figure 3B plots the parameter estimates (proportional to % signal change) for the peak voxels within each of these areas, for each of the four main conditions. This reveals that during the grip-task that activated the motor network, TMShigh (versus TMSlow) to left PMd led to relative increases in contralateral right PMd and M1. By contrast, when TMShigh was applied to left PMd during no-grip rest, this led to relative decreases instead in contralateral motor and premotor activity, as compared with TMSlow (see Figure 3B). Note that due to its ‘crossover’ nature, this interaction cannot simply be explained away by scaling effects, changes in baseline activity per se; nor can it be explained by age-dependent changes in PMd function, as we had assessed any such influences in our analysis, and the critical interaction did not depend on the age of our subjects.
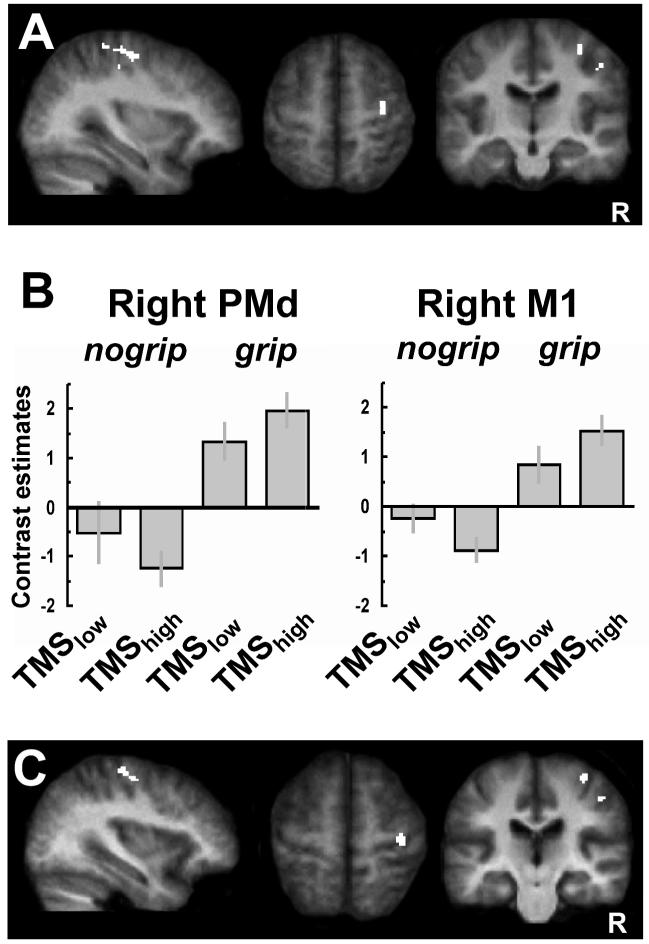
Figure 3.
Interaction between TMS intensity and current motor state for BOLD signals. A. SPMs showing a significant interaction [(TMShighnogrip - TMSlownogrip) - (TMShigh-grip - TMSlow-grip)] in right PMd contralateral to TMS stimulation (x,y,z: 34, -16, 58; assigned to Area 6 with a 60% probability, [Eickhoff and others 2006]) and right M1 (36, -26, 62; assigned to Area 4a with a 60% probability). These same regions were also modulated by the grip task (i.e. were active during left-hand grip), see Table 1, Figure 1C.
B. SPM parameter estimates showing that TMShigh to left PMd at rest led to a relative activity decrease in contralateral PMd and M1 (BA4a), as compared to TMSlow. By contrast, when applied during active left-hand grip, TMShigh to left PMd now led instead to a relative increase in activity in these regions. This illustrates the state-dependent influence of left PMD TMS on contralateral motor areas which are implicated in hand movement control.
C. Regions implicated in the grip task that also show changes in functional coupling (as revealed by an independent psychophysiological interaction analysis) with the site of TMS stimulation (left PMd), as a function of event-related condition. A significant context-dependent covariation with left (stimulated) PMd was revealed in two similar clusters as for the SPM interaction analysis, located in contralateral right PMd (x, y, z = 36, -14, 56) and right M1 (x, y, z = 36, -24, 64). Thus, the functional coupling between left PMd and right PMd and right M1 was stronger for TMShigh versus TMSlow during grip than during no-grip. Results are projected onto the average normalized structural scans of all participants (random effects analysis, corrected for multiple comparisons across the whole brain at T>4 and extent (cluster) threshold of P < 0.05). R = right.
Functional imaging results: condition-specific functional coupling with left PMd
In addition to assessing the overall activations per condition, as analyzed above, we also implemented a PPI analysis for any condition-specific functional coupling between TMS-stimulated left PMd (which provided the ‘seed’ area) and other brain regions, with a particular interest in those related to the grip-task. The resulting SPM for the PPI seeded with left PMd (i.e. testing for covariations in any other brain regions, with the residual time-course of left PMd) confirmed that TMS-stimulated left PMd showed significantly higher effective connectivity with contralateral PMd (x, y, z = 36, -14, 56) and contralateral M1 (x, y, z = 36, -24, 64) during grip, than for the no-grip condition (see Fig. 3C). Thus, these right-hemispheric motor regions showed stronger functional coupling with the stimulated left PMd during voluntary control of left-hand grip, than in the no-grip condition
Discussion
Using short bursts of TMS to left PMd during fMRI, we showed state-dependent causal influences of left PMd on activity levels in remote anatomically interconnected regions (right PMd and M1). While previous work had shown that more extended TMS (for 10 sec or more) to PMd can produce some remote activity increases in cortical motor structures distant to the stimulation site (Chouinard and others 2003; Bestmann and others 2005), the new finding here was that such remote influences can vary depending on the current motor state. Moreover, they can do so in an event-related fashion, when the motor state changes unpredictably from trial to trial, and TMS is applied in short bursts.
This state-dependence arose predominantly for motor structures engaged in the current motor behavior (i.e. within the network activated by the left-hand grip task), rather than being broadly distributed across the entire range of potentially interconnected brain regions (including ipsilateral M1). While TMS is known to produce some unspecific secondary activity changes (see Supplementary Figure 1), as for example in auditory cortex (Siebner and others 1999), the present findings cannot be readily explained away or attributed merely to such undesired stimulation effects. In particular, the crossover interaction (see Fig. 3B) from our random-effects analysis shows that the effect of TMShigh versus TMSlow to left PMd on right PMd and right M1 depends on the current motor-state. This is also unlikely to be accounted for by any scaling effects, changes in baseline activity per se, or any conceivable artifacts generated by the TMS pulse.
We argue below that the present findings most likely reflect event-related such changes in the effective connectivity of left PMd with contralateral (right) motor areas. Note also that our TMS-fMRI study demonstrated state-dependent interactions not only between left PMd and right M1 (potentially consistent with Koch and others 2006, 2007 work with conditioning-test double-coil approaches), but also for left PMd influences on right PMd. This goes beyond what could be achieved with conditioning-test double-coil TMS techniques alone, as those must always rely on an induced EMG response and thus are ultimately confined to considering possible effects on M1.
The contralateral activity decrease resulting from PMd stimulation reported here, under no-grip rest, seems broadly compatible with previously described transcallosal inhibition between primary motor cortex in both hemispheres during rest (Ferbert and others 1992). For example, recent conditioning-test TMS studies show that PMd stimulation at rest can decrease contralateral corticospinal excitability, as measured by the EMG response to a subsequent TMS pulse over M1 of the other hemisphere (Mochizuki and others 2004a, 2004b; Baumer and others 2006; Koch and others 2006, 2007). The specific latencies at which PMd stimulation influences contralateral M1 excitability favor a direct transcallosal mechanism (Mochizuki and others 2004a, 2004b; Baumer and others 2006; Koch and others 2006, 2007). While callosal fibers are predominantly excitatory (Innocenti 1986; Bloom and Hynd 2005), transcallosal inhibition is thought to be mediated by these excitatory fibers projecting onto GABA-ergic inhibitory neurons (Ferbert and others 1992).
In our particular case, this would suggest that the contralateral reduction in BOLD fMRI signal observed for TMShigh versus TMSlow during the no-grip condition may reflect a transcallosally mediated net activity decrease contralateral to the site of stimulation. This finding is similar to the relative contralateral BOLD signal reductions previously observed at rest when using more prolonged TMS to M1 (Bestmann and others 2003b, 2004). However, here we demonstrate that this can arise in both right PMd and right M1 due to left PMd TMS, even for short stimulation periods of just a few hundred milliseconds applied in an event-related manner.
By contrast, when participants were performing a single isometric left-hand grip we now found instead a relative activity increase (rather than decrease, i.e. the opposite BOLD effect) in PMd and M1 contralateral to the site of stimulation, for TMShigh versus TMSlow to left PMd. Thus, during a left-hand grip task, left PMd TMShigh now led to a boost in activity in contralateral motor regions. Our data thus lend credence to the concept of transcallosal signaling as a dynamic process, such that at a functional level either (or both) interhemispheric transcallosal inhibition and excitation may arise, with the relative dominance of these depending on the current state (Bloom and Hynd 2005). In our case, interhemispheric facilitatory effects of PMd stimulation become more influential during voluntary left-hand action. Facilitatory interneurons in M1/PMd may then have a lowered threshold for being activated by transcallosal pathways, as targeted by our PMd stimulation. Recent findings from conditioning-test double-coil studies corroborate this idea: inhibitory and excitatory interactions from left PMd to contralateral right M1 interact differentially with motor state, during movement of the left hand (Koch and others 2006), as opposed to rest. Notably, here we found analogous BOLD consequences for right PMd as well as right M1. We suggest that here either left PMd stimulation may have direct effects on both right M1 and PMd, or that the stimulation effect may first be transmitted to transcallosally to right PMd, and then propagated to right M1. These different possibilities might be differentiated by applying the present new paradigm to patients with right PMd lesions that spare right M1. We should note, however, that relating BOLD-signal increases or decreases to actual inhibitory or excitatory neural activity is a complex issue, that cannot be resolved directly by fMRI alone, but inevitably requires combination of more invasive recordings of neural activity with fMRI (Logothetis and Wandell, 2004; Logothetis and others 2001). While the present study found that remote BOLD effects of left PMd TMS could reverse in direction with current motor-state, in apparent analogy to recent twin-coil findings (Koch and others 2006), the relation between BOLD changes and neural firing must always be considered with care. We note also that TMS to left PMD did not alter grip-behavior, although we did observe a clear effect on BOLD signal in right PMd and right M1 that reversed during rest. Our design has in fact aimed to avoid any behavioral changes due to TMS, as otherwise those might have further complicated interpretation of the BOLD effects. We therefore conclude that the observed BOLD changes were caused by the physiological TMS intervention here, rather than reflecting differences in behavior.
Our further PPI analysis revealed significantly enhanced functional coupling (covariation in BOLD signal) between left PMd and right PMd/M1, specifically for TMShigh versus TMSlow in the grip versus no-grip conditions. That is, functional coupling between these regions was strongest when high intensity TMS was applied to left PMd during the active grip condition, consistent with variations in activity for this stimulated region then propagating in a corresponding manner to right PMd and right M1, in the context of the left-hand grip task. This provides further evidence that a voluntary motor task with the left hand leads to facilitatory interactions between left PMd and right PMd/M1, rather than just transcallosal inhibition as is typically found at rest. Moreover, our data suggest that the topography of these inter-regional influences is specific to brain regions engaged in the current task. This implies that while the effects of TMS are not restricted to the site of stimulation, they are also not broadcast across the entire range of putatively interconnected brain regions, as, for example, ipsilateral M1. In addition, TMS here predominantly influenced currently task-relevant brain regions that are intimately connected to the stimulation site and concerned with the generation of movement (Ehrsson and others 2000, 2001; Ward and others 2007), whereas other regions activated during the task such as contralateral parietal and somatosensory cortex were not influenced by TMS in a context-dependent way.
The present study demonstrates how concurrent TMS-fMRI can be used to characterize context- or state-dependent interactions between remote but interconnected regions of the motor system. TMS-fMRI may have some advantages over classical TMS approaches relying on EMG measures after stimulation of M1, and of other sites as in conditioning-test approaches, in addition to allowing measurement of activity levels in numerous brain regions concurrently. Quantification of peripheral muscle responses during contraction can become ambiguous, since different I-waves (Amassian and Stewart 2003) are recruited during motor behavior than at rest. During voluntary action, it can thus remain unclear whether any changes in peripheral muscle responses (e.g. due to M1 TMS, in the context of a conditioning-pulse elsewhere or not) reflect different I-wave recruitment or the stimulation of different intracortical pathways. While the BOLD signal itself has to be interpreted with some caution (Logothetis and Wandell, 2004), it can nevertheless provide a very different, potentially complementary measure of the cortical areas affected during stimulation of primary and secondary motor areas, and of their functional interplay, without having to rely on peripheral muscle responses or direct M1 stimulation.
We had chosen to target left PMd with TMS here, while varying the requirements for an ipsilateral-hand grip-task, precisely because we did not seek to disrupt performance via TMS, but rather to ‘inject’ a well-controlled input into PMd and then measure its remote causal influences with fMRI, while varying context in an event-related manner. The present approach thus extends previous repetitive TMS (Plewnia and others 2003; Schambra and others 2003) or ‘off-line’ TMS studies that were combined with neuroimaging measures (Paus 1999; Siebner and others 2003, 2003; Chouinard and others 2003; Tegenthoff and others 2005), which had disclosed some network activity changes following extended stimulation of sensorimotor areas. The more ‘on-line’ combination of fMRI with interleaved short bursts of TMS in the scanner, as used here, can evidently disclose rapid event-related effects and their spatial topography within the sensorimotor network. While we had specifically chosen to stimulate left PMd here because of its suggested dominance for the selection and preparation of hand movements (Schluter and others 2001; Astafiev and others 2003; Rushworth and others 2003; Davare and others 2006), we would not make any strong claims for hemispheric specialisation based on the current data. It may be useful to extend the same approach as introduced here to stimulation of right PMd instead in future work, and indeed to stimulation of numerous different motor sites.
Other approaches in fMRI research without TMS (e.g. Worsley and others 1998; Friston and others 2003; Roebroeck and others 2005; Patel and others 2006) have also emphasized that ‘functional coupling’ or ‘effective connectivity’ between interconnected brain regions may vary with the current state. This may dynamically reconfigure how much influence a given brain region has upon certain others. The present combined TMS- fMRI approach may provide a particularly direct empirical approach to such theoretical issues concerning inter-regional influences. We thus think our study establishes and corroborates a methodological approach that may be of more general use, in studying how motor networks may be reconfigured as a function of the current state (Massimini and others 2005; Paus 2005).
In conclusion, by obtaining fMRI measures of brain activity during event-related TMS, rather than relying on peripheral muscle activity (as required in the standard conditioning-test TMS method), we were able not only to confirm previous hypotheses of inter-hemispheric causal influences between (left) PMd and contralateral (right) M1, but also to reveal these for contralateral right PMd. Moreover, we showed that controlling hand movements engages inter-hemispheric interplay specifically within a network of movement-related cortical motor structures. Finally, we found that the nature of this functional interplay between interconnected motor areas depended critically on the current motor state. For future studies, the current approach might be extended to clinical applications, to assess how the observed interplay between interconnected brain regions might be affected in disease states; how this reacts to damage of other parts of the brain, after stroke; and how the observed remote effects of PMd TMS might change in patients with recovery, either spontaneously over time since brain injury, or after specific interventions.
Supplementary Material
Acknowledgements
Supported by the Wellcome Trust (SB, CCR, FB, JD, NSW, NW, OJ), the Medical Research Council (JCR, JD), and the Patrick Berthoud Charitable Trust (OS). We thank Victor Baller for construction of the TMS coil holder, Peter Aston, and Eric Featherstone for technical support.
References
- Amassian VE, Stewart M. Motor cortical and other cortical interneuronal networks that generate very high frequency waves. Suppl Clin Neurophysiol. 2003;56:119–142. doi: 10.1016/s1567-424x(09)70214-4. [DOI] [PubMed] [Google Scholar]
- Amiez C, Kostopoulos P, Champod AS, Petrides M. Local morphology predicts functional organization of the dorsal premotor region in the human brain. J Neurosci. 2006;26:2724–2731. doi: 10.1523/JNEUROSCI.4739-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JL, Hutton C, Ashburner J, Turner R, Friston K. Modeling geometric deformations in EPI time series. Neuroimage. 2001;13:903–919. doi: 10.1006/nimg.2001.0746. [DOI] [PubMed] [Google Scholar]
- Astafiev SV, Shulman GL, Stanley CM, Snyder AZ, Van Essen DC, Corbetta M. Functional organization of human intraparietal and frontal cortex for attending, looking, and pointing. J Neurosci. 2003;23:4689–4699. doi: 10.1523/JNEUROSCI.23-11-04689.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudewig J, Siebner HR, Bestmann S, Tergau F, Tings T, Paulus W, Frahm J. Functional MRI of cortical activations induced by transcranial magnetic stimulation (TMS) Neuroreport. 2001;12:3543–3548. doi: 10.1097/00001756-200111160-00034. [DOI] [PubMed] [Google Scholar]
- Baumer T, Bock F, Koch G, Lange R, Rothwell JC, Siebner HR, Munchau A. Magnetic stimulation of human premotor or motor cortex produces interhemispheric facilitation through distinct pathways. J Physiol. 2006;572:857–868. doi: 10.1113/jphysiol.2006.104901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestmann S, Baudewig J, Frahm J. On the synchronization of transcranial magnetic stimulation and functional echo-planar imaging. J Magn Reson Imaging. 2003a;17:309–16. doi: 10.1002/jmri.10260. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J. Subthreshold high-frequency TMS of human primary motor cortex modulates interconnected frontal motor areas as detected by interleaved fMRI-TMS. Neuroimage. 2003b;20:1685–1696. doi: 10.1016/j.neuroimage.2003.07.028. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J. Functional MRI of the immediate impact of transcranial magnetic stimulation on cortical and subcortical motor circuits. Eur J Neurosci. 2004;19:1950–1962. doi: 10.1111/j.1460-9568.2004.03277.x. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J. BOLD MRI responses to repetitive TMS over human dorsal premotor cortex. Neuroimage. 2005;28:22–29. doi: 10.1016/j.neuroimage.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Bloom JS, Hynd GW. The role of the corpus callosum in interhemispheric transfer of information: excitation or inhibition? Neuropsychol Rev. 2005;15:59–71. doi: 10.1007/s11065-005-6252-y. [DOI] [PubMed] [Google Scholar]
- Bohning DE, Shastri A, Nahas Z, Lorberbaum JP, Andersen SW, Dannels WR, Haxthausen EU, Vincent DJ, George MS. Echoplanar BOLD fMRI of brain activation induced by concurrent transcranial magnetic stimulation. Invest Radiol. 1998;33:336–340. doi: 10.1097/00004424-199806000-00004. [DOI] [PubMed] [Google Scholar]
- Bohning DE, Shastri A, McConnell KA, Nahas Z, Lorberbaum JP, Roberts DR, Teneback C, Vincent DJ, George MS. A combined TMS/fMRI study of intensity-dependent TMS over motor cortex. Biol Psychiatry. 1999;45:385–394. doi: 10.1016/s0006-3223(98)00368-0. [DOI] [PubMed] [Google Scholar]
- Bohning DE, Shastri A, Wassermann EM, Ziemann U, Lorberbaum JP, Nahas Z, Lomarev MP, George MS. BOLD-f MRI response to single-pulse transcranial magnetic stimulation (TMS) J Magn Reson Imaging. 2000;11:569–574. doi: 10.1002/1522-2586(200006)11:6<569::aid-jmri1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Chouinard PA, Van Der Werf YD, Leonard G, Paus T. Modulating neural networks with transcranial magnetic stimulation applied over the dorsal premotor and primary motor cortices. J Neurophysiol. 2003;90:1071–1083. doi: 10.1152/jn.01105.2002. [DOI] [PubMed] [Google Scholar]
- Chouinard PA, Paus T. The primary motor and premotor areas of the human cerebral cortex. Neuroscientist. 2006;12:143–152. doi: 10.1177/1073858405284255. [DOI] [PubMed] [Google Scholar]
- Churchland MM, Yu BM, Ryu SI, Santhanam G, Shenoy KV. Neural variability in premotor cortex provides a signature of motor preparation. J Neurosci. 2006;26:3697–3712. doi: 10.1523/JNEUROSCI.3762-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P, Crammond DJ, Kalaska JF. Neural activity in primary motor and dorsal premotor cortex in reaching tasks with the contralateral versus ipsilateral arm. J Neurophysiol. 2003;89:922–942. doi: 10.1152/jn.00607.2002. [DOI] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural correlates of reaching decisions in dorsal premotor cortex: specification of multiple direction choices and final selection of action. Neuron. 2005;45:801–814. doi: 10.1016/j.neuron.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Deichmann R, Schwarzbauer C, Turner R. Optimisation of the 3D MDEFT sequence for anatomical brain imaging:technical implications at 1.5 and 3 T. Neuroimage. 2004;21:757–767. doi: 10.1016/j.neuroimage.2003.09.062. [DOI] [PubMed] [Google Scholar]
- Davare M, Andres, Cosnard G, Thonnard JL, Olivier E. Dissociating the role of the ventral and dorsal premotor cortex in precision grasping. J Neurosci. 2006;26:2260–2268. doi: 10.1523/JNEUROSCI.3386-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della-Maggiore V, Sekuler AB, Grady CL, Bennett PJ, Sekuler R, McIntosh AR. Corticolimbic interactions associated with performance on a short-term memory task are modified by age. J Neurosci. 2000;20:8410–8416. doi: 10.1523/JNEUROSCI.20-22-08410.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL. Frontal lobe inputs to the digit representations of the motor areas on the lateral surface of the hemisphere. J Neurosci. 2005;25:1375–1386. doi: 10.1523/JNEUROSCI.3902-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy HM. The human brain: surface, blood supply, and three-dimensional anatomy. New York: Springer-Verlag; 1991. [Google Scholar]
- Egner T, Hirsch J. The neural correlates and functional integration of cognitive control in a Stroop task. Neuroimage. 2005;24:539–547. doi: 10.1016/j.neuroimage.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Fagergren A, Jonsson T, Westling G, Johansson RS, Forssberg H. Cortical activity in precision- versus power-grip tasks: an fMRI study. J Neurophysiol. 2000;83:528–536. doi: 10.1152/jn.2000.83.1.528. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Fagergren E, Forssberg H. Differential fronto-parietal activation depending on force used in a precision grip task: an fMRI study. J Neurophysiol. 2001;85:2613–2623. doi: 10.1152/jn.2001.85.6.2613. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Evarts EV. Pyramidal tract activity associated with a conditioned hand movement in the monkey. J Neurophysiol. 1966;29:1011–1027. doi: 10.1152/jn.1966.29.6.1011. [DOI] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, Turner R. Analysis of fMRI time-series revisited. Neuroimage. 1995;2:45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI:characterizing differential responses. Neuroimage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19:1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Penny WD, Ashburner J, Friston KJ. Modeling regional and psychophysiologic interactions in fMRI:the importance of hemodynamic deconvolution. Neuroimage. 2003;19:200–207. doi: 10.1016/s1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Fagg AH, Arbib MA. Dorsal premotor cortex and conditional movement selection: A PET functional mapping study. J Neurophysiol. 1998;79:1092–1097. doi: 10.1152/jn.1998.79.2.1092. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tanji J. Functional specialization in dorsal and ventral premotor areas. Prog Brain Res. 2004;143:507–11. doi: 10.1016/S0079-6123(03)43047-1. [DOI] [PubMed] [Google Scholar]
- Innocenti GM. The general organization of callosal connections in the cerebral cortex. In: Jones EG, Peters AA, editors. Cerebral Cortex. New York: Plenum Press; 1986. pp. 291–354. [Google Scholar]
- Johansen-Berg H, Rushworth MF, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci U S A. 2002;99:14518–14523. doi: 10.1073/pnas.222536799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs O, Deichmann R, Turner R. Trajectory measurement and generalised reconstruction in rectilinear EPI. Proceedings of ISMRM. 2000;8:1517. [Google Scholar]
- Kemna LJ, Gembris D. Repetitive transcranial magnetic stimulation induces different responses in different cortical areas: a functional magnetic resonance study in humans. Neurosci Lett. 2003;336:85–88. doi: 10.1016/s0304-3940(02)01195-3. [DOI] [PubMed] [Google Scholar]
- Koch G, Franca M, Del Olmo MF, Cheeran B, Milton R, Alvarez SM, Rothwell JC. Time course of functional connectivity between dorsal premotor and contralateral motor cortex during movement selection. J Neurosci. 2006;26:7452–7459. doi: 10.1523/JNEUROSCI.1158-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Franca M, Mochizuki H, Marconi B, Caltagirone C, Rothwell JC. Interactions between pairs of transcranial magnetic stimuli over the human left dorsal premotor cortex differ from those seen in primary motor cortex. J Physiol. 2007;578:551–562. doi: 10.1113/jphysiol.2006.123562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L, Siebner HR, Rowe JB, Rizzo V, Rothwell JC, Frackowiak RS, Friston KJ. Acute remapping within the motor system induced by low-frequency repetitive transcranial magnetic stimulation. J Neurosci. 2003;23:5308–5318. doi: 10.1523/JNEUROSCI.23-12-05308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Wandell BA. Interpreting the BOLD signal. Annu Rev Physiol. 2004;66:735–769. doi: 10.1146/annurev.physiol.66.082602.092845. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Lu MT, Preston JB, Strick PL. Interconnections between the prefrontal cortex and the premotor areas in the frontal lobe. J Comp Neurol. 1994;341:375–392. doi: 10.1002/cne.903410308. [DOI] [PubMed] [Google Scholar]
- Macey PM, Macey KE, Kumar R, Harper RM. A method for removal of global effects from fMRI time series. Neuroimage. 2004;22:360–366. doi: 10.1016/j.neuroimage.2003.12.042. [DOI] [PubMed] [Google Scholar]
- Marconi B, Genovesio A, Giannetti S, Molinari M, Caminiti R. Callosal connections of dorso-lateral premotor cortex. Eur J Neurosci. 2003;18:775–788. doi: 10.1046/j.1460-9568.2003.02807.x. [DOI] [PubMed] [Google Scholar]
- Massimini M, Ferrarelli F, Huber R, Esser SK, Singh H, Tononi G. Breakdown of cortical effective connectivity during sleep. Science. 2005;309:2228–2232. doi: 10.1126/science.1117256. [DOI] [PubMed] [Google Scholar]
- Mayka MA, Corcos DM, Leurgans SE, Vaillancourt DE. Three-dimensional locations and boundaries of motor and premotor cortices as defined by functional brain imaging: A meta-analysis. Neuroimage. 2006;31:1453–1474. doi: 10.1016/j.neuroimage.2006.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki H, Huang YZ, Rothwell JC. Interhemispheric interaction between human dorsal premotor and contralateral primary motor cortex. J Physiol. 2004a;561:331–338. doi: 10.1113/jphysiol.2004.072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki H, Terao Y, Okabe S, Furubayashi T, Arai N, Iwata NK, Hanajima R, Kamakura K, Motoyoshi K, Ugawa Y. Effects of motor cortical stimulation on the excitability of contralateral motor and sensory cortices. Exp Brain Res. 2004b;158:519–526. doi: 10.1007/s00221-004-1923-0. [DOI] [PubMed] [Google Scholar]
- Modugno N, Nakamura Y, MacKinnon CD, Filipovic SR, Bestmann S, Berardelli A, Rothwell JC. Motor cortex excitability following short trains of repetitive magnetic stimuli. Exp Brain Res. 2001;140:453–459. doi: 10.1007/s002210100843. [DOI] [PubMed] [Google Scholar]
- Lu MT, Preston JB, Strick PL. Interconnections between the prefrontal cortex and the premotor areas in the frontal lobe. J Comp Neurol. 1994;341:375–392. doi: 10.1002/cne.903410308. [DOI] [PubMed] [Google Scholar]
- O’Shea J, Johansen-Berg H, Trief D, Göbel S, Rushworth M. Functionally specific reorganization in human premotor cortex. Neuron. 2007;54:479–490. doi: 10.1016/j.neuron.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Patel RS, Bowman FD, Rilling JK. A Bayesian approach to determining connectivity of the human brain. Hum Brain Mapp. 2006;27:267–276. doi: 10.1002/hbm.20182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Jech R, Thompson CJ, Comeau R, Peters T, Evans AC. Transcranial magnetic stimulation during positron emission tomography:a new method for studying connectivity of the human cerebral cortex. J Neurosci. 1997;17:3178–3184. doi: 10.1523/JNEUROSCI.17-09-03178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Jech R, Thompson CJ, Comeau R, Peters T, Evans AC. Dose-dependent reduction of cerebral blood flow during rapid-rate transcranial magnetic stimulation of the human sensorimotor cortex. J Neurophysiol. 1998;79:1102–1107. doi: 10.1152/jn.1998.79.2.1102. [DOI] [PubMed] [Google Scholar]
- Paus T. Imaging the brain before, during, and after transcranial magnetic stimulation. Neuropsychologia. 1999;37:219–224. doi: 10.1016/s0028-3932(98)00096-7. [DOI] [PubMed] [Google Scholar]
- Paus T, Sipila PK, Strafella AP. Synchronization of neuronal activity in the human primary motor cortex by transcranial magnetic stimulation:an EEG study. J Neurophysiol. 2001;86:1983–1990. doi: 10.1152/jn.2001.86.4.1983. [DOI] [PubMed] [Google Scholar]
- Paus T. Inferring causality in brain images:a perturbation approach. Philos Trans R Soc Lond B Biol Sci. 2005;360:1109–1114. doi: 10.1098/rstb.2005.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard N, Strick PL. Imaging the premotor areas. Curr Opin Neurobiol. 2001;11:663–672. doi: 10.1016/s0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Plewnia C, Lotze M, Gerloff C. Disinhibition of the contralateral motor cortex by low-frequency rTMS. Neuroreport. 2003;14:609–612. doi: 10.1097/00001756-200303240-00017. [DOI] [PubMed] [Google Scholar]
- Praeg E, Herwig U, Lutz K, Jancke L. The role of the right dorsal premotor cortex in visuomotor learning:a transcranial magnetic stimulation study. Neuroreport. 2005;16:1715–1718. doi: 10.1097/01.wnr.0000183333.36488.1c. [DOI] [PubMed] [Google Scholar]
- Roebroeck A, Formisano E, Goebel R. Mapping directed influence over the brain using Granger causality and fMRI. Neuroimage. 2005;25:230–242. doi: 10.1016/j.neuroimage.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Romeo S, Gilio F, Pedace F, Ozkaynak S, Inghilleri M, Manfredi M, Berardelli A. Changes in the cortical silent period after repetitive magnetic stimulation of cortical motor areas. Exp Brain Res. 2000;135:504–510. doi: 10.1007/s002210000541. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Siebner H, Filipovic SR, Cordivari C, Gerschlager W, Rothwell J, Frackowiak R. Aging is associated with contrasting changes in local and distant cortical connectivity in the human motor system. Neuroimage. 2006;32:747–760. doi: 10.1016/j.neuroimage.2006.03.061. [DOI] [PubMed] [Google Scholar]
- Ruff CC, Blankenburg F, Bjoertomt O, Bestmann S, Freeman E, Haynes JD, Rees G, Josephs O, Deichmann R, Driver J. Concurrent TMS-fMRI and Psychophysics Reveal Frontal Influences on Human Retinotopic Visual Cortex. Curr Biol. 2006;16:1479–1488. doi: 10.1016/j.cub.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Johansen-Berg H, Gobel SM, Devlin JT. The left parietal and premotor cortices:motor attention and selection. Neuroimage. 2003;20(Suppl 1):S89–100. doi: 10.1016/j.neuroimage.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Sack AT, Kohler A, Bestmann S, Linden DE, Dechent P, Goebel R, Baudewig J. Imaging the Brain Activity Changes Underlying Impaired Visuospatial Judgments: Simultaneous fMRI, TMS, and Behavioral Studies. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm013. in press. [DOI] [PubMed] [Google Scholar]
- Schambra HM, Sawaki L, Cohen LG. Modulation of excitability of human motor cortex (M1) by 1 Hz transcranial magnetic stimulation of the contralateral M1. Clin Neurophysiol. 2003;114:130–133. doi: 10.1016/s1388-2457(02)00342-5. [DOI] [PubMed] [Google Scholar]
- Schluter ND, Rushworth MF, Passingham RE, Mills KR. Temporary interference in human lateral premotor cortex suggests dominance for the selection of movements. A study using transcranial magnetic stimulation. Brain. 1998;121:785–799. doi: 10.1093/brain/121.5.785. [DOI] [PubMed] [Google Scholar]
- Schluter ND, Krams M, Rushworth MF, Passingham RE. Cerebral dominance for action in the human brain: the selection of actions. Neuropsychologia. 2001;39:105–113. doi: 10.1016/s0028-3932(00)00105-6. [DOI] [PubMed] [Google Scholar]
- Scott SH, Sergio LE, Kalaska JF. Reaching movements with similar hand paths but different arm orientations. II. Activity of individual cells in dorsal premotor cortex and parietal area 5. J Neurophysiol. 1997;78:2413–2426. doi: 10.1152/jn.1997.78.5.2413. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Peller M, Willoch F, Auer C, Bartenstein P, Drzezga A, Schwaiger M, Conrad B. Imaging functional activation of the auditory cortex during focal repetitive transcranial magnetic stimulation of the primary motor cortex in normal subjects. Neurosci Lett. 1999;270:37–40. doi: 10.1016/s0304-3940(99)00454-1. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Filipovic SR, Rowe JB, Cordivari C, Gerschlager W, Rothwell JC, Frackowiak RS, Bhatia KP. Patients with focal arm dystonia have increased sensitivity to slow-frequency repetitive TMS of the dorsal premotor cortex. Brain. 2003;126:2710–2725. doi: 10.1093/brain/awg282. [DOI] [PubMed] [Google Scholar]
- Taniwaki T, Okayama A, Yoshiura T, Nakamura Y, Goto Y, Kira J, Tobimatsu S. Reappraisal of the motor role of basal ganglia:a functional magnetic resonance image study. J Neurosci. 2003;23:3432–3438. doi: 10.1523/JNEUROSCI.23-08-03432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji J, Okano K, Sato KC. Neuronal activity in cortical motor areas related to ipsilateral, contralateral, and bilateral digit movements of the monkey. J Neurophysiol. 1988;60:325–343. doi: 10.1152/jn.1988.60.1.325. [DOI] [PubMed] [Google Scholar]
- Tegenthoff M, Ragert P, Pleger B, Schwenkreis P, Forster AF, Nicolas V, Dinse HR. Improvement of tactile discrimination performance and enlargement of cortical somatosensory maps after 5 Hz rTMS. PLoS Biol. 2005;3:e362. doi: 10.1371/journal.pbio.0030362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Frackowiak RS. Age-related changes in the neural correlates of motor performance. Brain. 2003;126:873–888. doi: 10.1093/brain/awg071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Newton JM, Swayne OB, Lee L, Thompson AJ, Greenwood RJ, Rothwell JC, Frackowiak RS. Motor system activation after subcortical stroke depends on corticospinal system integrity. Brain. 2006;129:809–819. doi: 10.1093/brain/awl002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Newton JM, Swayne OB, Lee L, Frackowiak RS, Thompson AJ, Greenwood RJ, Rothwell JC. The relationship between brain activity and peak grip force is modulated by corticospinal system integrity after subcortical stroke. Eur J Neurosci. 2007;25:1865–1873. doi: 10.1111/j.1460-9568.2007.05434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation:report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5-7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- Weiskopf N, Sitaram R, Josephs O, Veit R, Scharnowski F, Goebel R, Birbaumer N, Deichmann R, Mathiak K. Real-time functional magnetic resonance imaging: methods and applications. Magn Reson Imaging. 2007 doi: 10.1016/j.mri.2007.02.007. in press. [DOI] [PubMed] [Google Scholar]
- Wise SP, Boussaoud D, Johnson PB, Caminiti R. Premotor and parietal cortex:corticocortical connectivity and combinatorial computations. Annu Rev Neurosci. 1997;20:25–42. doi: 10.1146/annurev.neuro.20.1.25. [DOI] [PubMed] [Google Scholar]
- Wolbers T, Schoell ED, Verleger R, Kraft S, McNamara A, Jaskowski P, Buchel C. Changes in connectivity profiles as a mechanism for strategic control over interfering subliminal information. Cereb Cortex. 2006;16:857–864. doi: 10.1093/cercor/bhj029. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Cao J, Paus T, Petrides M, Evans AC. Applications of random field theory to functional connectivity. Hum Brain Mapp. 1998;6:364–367. doi: 10.1002/(SICI)1097-0193(1998)6:5/6<364::AID-HBM6>3.0.CO;2-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.