Summary
Prior knowledge regarding the possible identity of an object facilitates its recognition from a degraded visual input, though the underlying mechanisms are unclear. Previous work implicated ventral visual cortex, but did not disambiguate whether activity-changes in these regions are causal to or merely reflect an effect of facilitated recognition. We used fMRI to study top-down influences on processing of gradually-revealed objects, by preceding each object with a name that was congruent or incongruent with the object. Congruently primed objects were recognised earlier than incongruently primed, and this was paralleled by shifts in activation profiles for ventral visual, parietal and prefrontal cortices. Prior to recognition, defined on a trial-by-trial basis, activity in ventral visual cortex rose gradually, but equivalently for congruently and incongruently primed objects. In contrast, pre-recognition activity was greater with congruent priming in lateral parietal, retrosplenial, and lateral prefrontal cortices, while functional coupling between parietal and ventral visual (and also left lateral prefrontal and parietal) cortices was enhanced in the same context. Thus, when controlling for recognition point and stimulus information, activity in ventral visual cortex mirrors recognition success, independent of condition. Facilitation by top-down cues involves lateral parietal cortex interacting with ventral visual areas, potentially explaining why parietal lesions can lead to deficits in recognising degraded objects even in the context of top-down knowledge.
Keywords: Object recognition, fMRI, fusiform, parietal, top-down, priming
Introduction
Success in visual object recognition depends not only on stimulus quality, but also on top-down influences that predict likely object identities. Priming by previously seen images, or expectancies due to visual or non-visual context, can facilitate recognition (Palmer, 1975; Biederman, 1972). This is particularly apparent with impoverished visual input, where appropriate top-down knowledge allows identification of an object from an otherwise uninterpretable image (Snodgrass and Feenan, 1990; Hirshman et al., 1990; Ramachandran, 1994; Sadr and Sinha, 2004).
Numerous studies have investigated neural correlates of priming for undegraded objects (e.g, Schacter and Buckner, 1998; Henson, 2003; van Turennout et al., 2000; Dehaene et al., 2001; Koutstaal et al., 2001; James et al., 2002; Vuilleumier et al., 2002, Simons et al., 2003), but fewer have addressed situations where priming induces a qualitative perceptual change for a degraded stimulus (Tovee et al., 1996; Dolan et al., 1997; George et al., 1996; Doniger et al., 2001). While the former case usually leads to repetition decreases in the fMRI signal in areas (such as ventral visual cortex) thought to be involved in neural representation of objects (Henson, 2003; Wiggs and Martin, 1998), in the latter case activity increases have been described, but their functional significance is poorly understood. In particular, it is unknown whether such increases in activity precede (and thus potentially cause) earlier identification, or are consequent to recognition success. Moreover, since in previous studies objects were always primed by an identical or similar visual stimulus, they could not separate the neural correlates of top-down, knowledge-based facilitation from those related to repetition of sensory information.
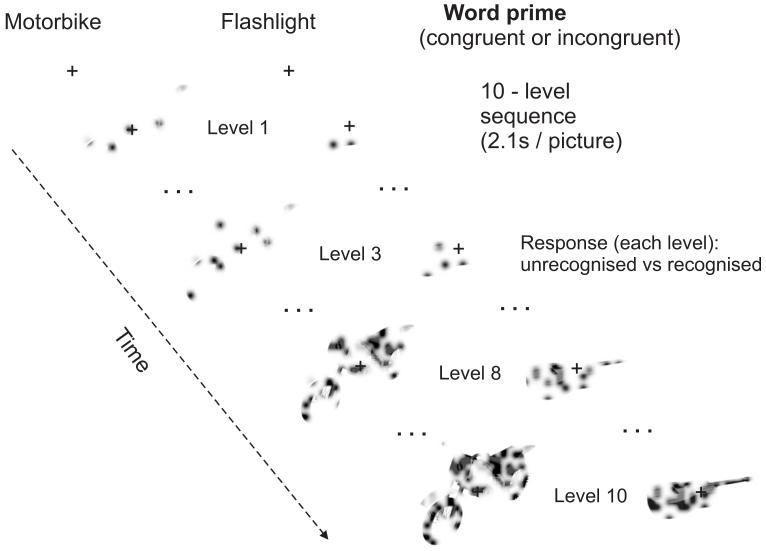
Here we addressed both issues by delaying recognition through gradually revealing objects over twenty seconds, from behind a mask of multiple Gaussian filters (Figure 1 and 2), and priming each of these sequences by a written word (i.e. not by a picture, as in previous work) that matched, or mismatched, the name of the subsequently revealed object with equal probability. Thus, no prior visual information about the specific instance of each object was given (e.g., on reading the word “guitar”, observers did not know whether to expect a Spanish Acoustic or Fender Stratocaster, nor from what viewpoint. Furthermore, a different object than a guitar was equally likely).
Figure 1.
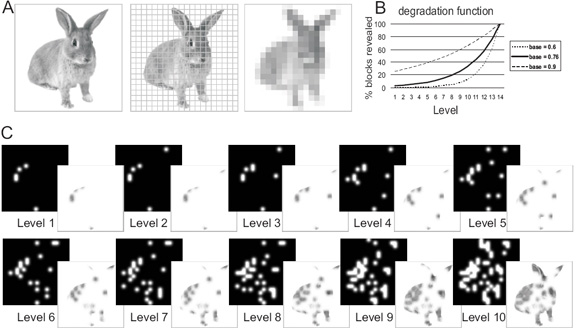
Illustration of stimulus generation: A) Pictures of everyday familiar objects were first divided into a 20 × 20 grid. Subsequently, grid elements containing object parts were identified (by calculating the mean of each element and finding those elements with mean > 1, corresponding to non-white blocks). The corresponding blocks were then randomly and cumulatively revealed by Gaussian apertures, with the number of apertures/level following an exponential function. B) shows the exponential function with the parameters used. The base value (0.76 used here) determines both the amount of image structure revealed at the lowest level, and the curvature of the function across levels. Lower base values would imply revealing less image structure/level at the beginning of the sequence and more image structure/level at the end of the sequence, the opposite being true for higher base values. The chosen value 0.76 implemented sufficiently slow revealing at the beginning of the sequence, while nevertheless ensuring that with all the objects used there was at least a difference of one aperture between levels. With the chosen function (see also Material and Methods) an aperture would be placed on each selected grid element within 14 levels. Only the first 10 levels of this were used in the experiment because within these objects usually became identifiable. C) shows the 10 levels of Gaussian aperture masks and resulting filtered images for the original picture in A).
Figure 2.
Illustration of experimental paradigm: Pictures were gradually revealed over 10 steps, with 2.1s presentation per image. Each degraded-object sequence was preceded either by a matching written name (congruently primed condition, as shown here for the motorbike) or an non-matching name (incongruently primed condition, as shown here for the guitar). Subjects were instructed to press one of two buttons for each degraded picture, to indicate whether they could or could not currently identify the object.
A similar paradigm of slowly revealing objects (but there using identical pictures rather than words as primes) has been employed in one previous fMRI study (James et al., 2000). That study assessed activity profiles averaged over primed or unprimed trials. As explained below in more detail, this leaves unresolved whether the earlier peak of activity found for primed objects in ventral visual cortex reflects changes in pre-recognition activity, or activity subsequent to recognition success on individual trials.
An initial goal here was to replicate differences in mean activation between primed and unprimed objects (James et al., 2000), but now we provided “top-down” word primes, rather than pictorial primes. Importantly, we also disambiguated effects due to congruent versus incongruent priming, and effects due to recognition success (and also separated both from any effects due to current level of stimulus degradation per se). This distinction was achieved by analysing our data relative to the point when observers indicated recognition on a trial-by-trial basis, while factoring out degradation level. If priming by a congruent versus incongruent word reflects pre-activation of regions such as ventral visual cortex (James et al., 2000), we expected increased activity there for the congruently primed condition prior to the recognition point. Alternatively, enhanced ventral visual activity might instead reflect recognition success (Grill-Spector et al., 2000; Bar et al., 2001), for both congruently and incongruently primed conditions alike. Finally, if facilitated recognition cannot be explained by changes in pre-recognition activity within ventral visual cortex, then its source may lie in the influence of other brain areas upon visual cortex, which we tested here with analyses of functional coupling.
Materials and Methods
Participants and Data acquisition
13 healthy right-handed volunteers (5 men and 8 women, mean age 23.2 +/- 5.3 years) had normal or corrected vision. fMRI data from 4 additional scanned volunteers were excluded due to substantantial movement evident in online monitoring during scanning (1 case), or a high number of unrecognised items (more than 30% of all trials in either condition - 3 cases). The study was approved by the Joint Ethics Commitee of the National Hospital and Institute of Neurology, London. Functional images were acquired on a 3 Tesla MR system with standard head coil (Siemens Allegra, Erlangen, Germany) as T2* weighted echo-planar image (EPI) volumes every 2.1 s (TE 30 ms, flip angle 90 deg, FOV 192 mm, 32 transversal slices with 10 deg anterior-posterior angulation (up at front), voxel size 3 × 3 × 2 mm, skip 1 mm).
Stimuli and Design
90 greyscale photographs or realistic renderings of objects from different sources (Object Databank - http://www.cog.brown.edu/~tarr/; MasterClips image collection; Hemera Photo Clipart) were used to create stimuli for this study.
For the degradation procedure, stimuli were revealed from behind a mask consisting of multiple embedded Gaussian filters randomly positioned on those parts of the image that contained object structure (Figure 1). A given image of 200 × 200 pixels was first subdivided into a 20 × 20 array and those grid elements containing object parts were cumulatively chosen as targets for Gaussian apertures with a standard deviation big enough to allow smooth blending together of the apertures at neighbouring locations in the array. The number of grid elements to be revealed at each level of degradation depended exponentially on the overall area covered by the object (total number of grid elements):
in accordance with previous behavioural studies with degraded line drawings (Snodgrass et al., 1990), see Figure legend 1 for further details. The degradation method based on Gaussian filters allows realistic grey-scale objects to be revealed smoothly, without artificial edge effects. This method was previously used in combination with a reverse correlation approach (Gosselin and Schyns, 2001), but here the sole aim was to gradually reveal objects (as previously done with line drawings, Snodgrass et al., 1990), in a manner that was constant across the priming manipulation. The stimuli were divided into 6 sets balanced for object categories (such as animals, tools, vehicles, furniture, other household items, musical instruments, food) and stimulus extent (area). The 6 sets were rotated around participants for use as written primes, or as pictures in the congruently and incongruently primed conditions. A given object thus only appeared in one condition for a given observer. This procedure resulted in stimuli being close to counterbalanced with the 13 subjects included (fully counterbalanced within 12 subjects). Furthermore, since the 6 stimulus sets were equated as mentioned above, the effects that a non-fully-counterbalanced assignment of stimuli to conditions would have were most likely kept minimal.
Experimental protocol and task
Stimuli were back-projected onto a translucent screen located ∼60 cm above the subjects’ head and viewed via a mirror on the head coil. The pictures subtended approximately 5 ° of visual angle. The experimental paradigm (see Figure 2) consisted of presentation of a written word for 2.1 s, followed by 2.1 s fixation baseline and subsequently a 10-level sequence of degraded images, for 2.1 s each, from least to most complete for a given object, followed by 8 s of fixation baseline before the start of the next trial. The observers’ task was to press one of two buttons (for every 2.1 s image in the sequence) depending on whether they could identify (covertly name) the object at the basic level or not (they were instructed that when seeing e.g. an animal, they were supposed to press the button for “yes” only when they could identify the type of animal, for example cat or dog). Participants were further requested to try to maintain a constant level of confidence in the recognition judgement, and to indicate recognition irrespective of whether the image seemed to “match” the preceding word or not, possibilities that were equally likely.
Behavioural pilot experiments (6 subjects) using an overt naming task in addition to button-presses, and otherwise identical instructions, produced comparable results regarding the average degradation level at recognition for both prime conditions (congruent condition: level 6 +/- 0.7, incongruent condition level 7.8 +/- 0.8). In these pilots, nearly all objects were responded to within the 10-level sequence (congruent condition 97.9 +/- 1.5 %, incongruent condition 94.2 +/- 4.0 %). Among those trials with a response, naming accuracy was high (counting exact name matches and synonyms as correct: congruent condition: 99.4 +/- 1.1 %, incongruent condition: 97.5 +/- 1.6 %).
In an additional scanning session of ∼ 6 mins length, object-responsive areas were determined for all subjects with a standard LOC localiser (Grill-Spector, 2003), comparing the objects pictures to scrambled versions of the same pictures (created by dividing the image into a 20 × 20 grid and randomly permuting grid elements), in blocked presentations with 500 ms per picture every 1 s, and block length of 12 s (6 s baseline) during which subjects performed a one-back repetition detection task. This functional localiser for object-responsive visual regions served as a mask and/or small-volume correction for some of the fMRI comparisons performed, as described below.
Image processing and data analysis
Analysis of the imaging data used SPM2 (http://www.fil.ion.ucl.ac.uk/spm/software/spm2). Image preprocessing included realignment and unwarping; slice-time correction with middle slice as reference; spatial normalisation (EPI-template); and spatial smoothing (10 mm FWHM Gaussian kernel). A Finite Impulse Response (FIR) model was used to assess the effects of experimental manipulations (see e.g. Henson, 2004). This model was chosen because it can accommodate unusual shapes of fMRI response that are not well described by a single assumed hemodynamic response function (HRF), as is the case in the present slow stimulus revealing process. An FIR model is effectively performing selective averaging of fMRI time courses, but within the context of the general linear model in SPM, using as basis functions a set of timebins of prespecified width.
For the overall difference between congruently and incongruently primed conditions, 10 FIR bins of 2.1s (=1TR) bin width, corresponding to the 10 undegradation steps, were used for each condition separately. All FIR regressors were delayed by 2 TRs (4.2s) to account for the time-lag of the hemodynamic response, and all word primes were modelled by an additional 2.1s bin. For the analysis assessing activity relative to indicated recognition on a trial-by-trial basis, 10 FIR bins of 1 TR width were modelled for congruently and incongruently primed conditions together, corresponding to the overall effect of degradation level. Note that this model does not assume a given (e.g. linear) response to degradation level, but can account for any shape. Further bins were modelled separately for congruently and incongruently primed trials, corresponding to the time bin relative to recognition for each individual trial. These bins varied from -7 to +2 relative to recognition (chosen as the maximum range possible across subjects). A temporal high-pass filter (cut-off 256 s) was applied, and temporal autocorrelation was modelled as an AR(1) process. F-tests were used to test for significance across subjects of the parameter estimates of one or more selected time bins (i.e. random-effects analysis).
For analysis of interregional functional coupling, or “effective connectivity” (Friston et al., 1997; Stephan et al., 2003), the same model including effects of degradation level and recognition-related levels was used, but now extended with further regressors: one for the BOLD signal time course of a given seed region (see Results for details), and a further interaction term that corresponded to a product (1 for congruently and -1 for incongruently primed, thus testing for stronger covariation in the congruently than incongruently primed condition) between 5 bins (4 prerecognition plus one at recognition) and that time course data. To derive the time course data for the PPI seed, a volume of interest of 10 mm radius was defined around each subject’s individual maximum nearest to a given focus derived from a group analysis (see Results for details) and the first eigenvariate of the voxel time courses was extracted (adjusted for the session mean and drift terms as modelled by the high-pass filter). Significant coupling was assessed by a t-contrast on the interaction term averaging over the 5 included time bins, corresponding to a test for stronger regression of the seeded activity timecourse on any other region in the congruently compared to the incongruently primed condition. Note that this regression is tested after discounting effects that non-specifically covary with the overall signal time course of the seed region, or directly with experimental manipulations (congruent vs incongruent priming, degradation level), since all of these are included in this extended model. Since our aim was to test the specific hypothesis of functional coupling affecting ventral object-selective cortex for the congruently primed condition, we restricted interrogation of the coupling data by the mask for object-responsive regions as determined by the LOC-localiser group contrast, within which we report effects at p<.001, uncorrected.
Results
Behavioural data
Each object was gradually revealed over a sequence of 10 pictures presented successively every 2.1s. This sequence was preceded unpredictably by either a congruent or incongruent word (Figure 2). Subjects responded to each picture in the sequence, using a button press to indicate whether or not they could identify (covertly name) the object (see Materials and Methods for details). On average across subjects, congruently primed objects were recognised at an earlier level; at level 6.6 (+/-0.85), compared to level 8.2 (+/-0.56) for incongruently primed, where level 1 represents the most incomplete and level 10 the most complete image. This difference in recognition level was highly reliable (t(12) = 8.99, p<.001). The percentage of items that remained unrecognised even at level 10 also differed significantly (only 1.0 +/- 1.3 for congruently, but 5.2 +/-2.9 for incongruently primed, t(12) = 5.81, p<.001).
Despite the relatively small variability in mean recognition times across subjects, there was considerable variability in the recognition times across different objects within each subject. Thus, the average min/max range of recognition points across subjects was 3.4-9.4 for congruently, and 5.6-9.9 for incongruently primed objects. It was this variability in recognition times across trials that allowed us to separate the effects of recognition from those of degradation level per se in the fMRI analyses below.
Functional imaging data
Overall effects of prime condition
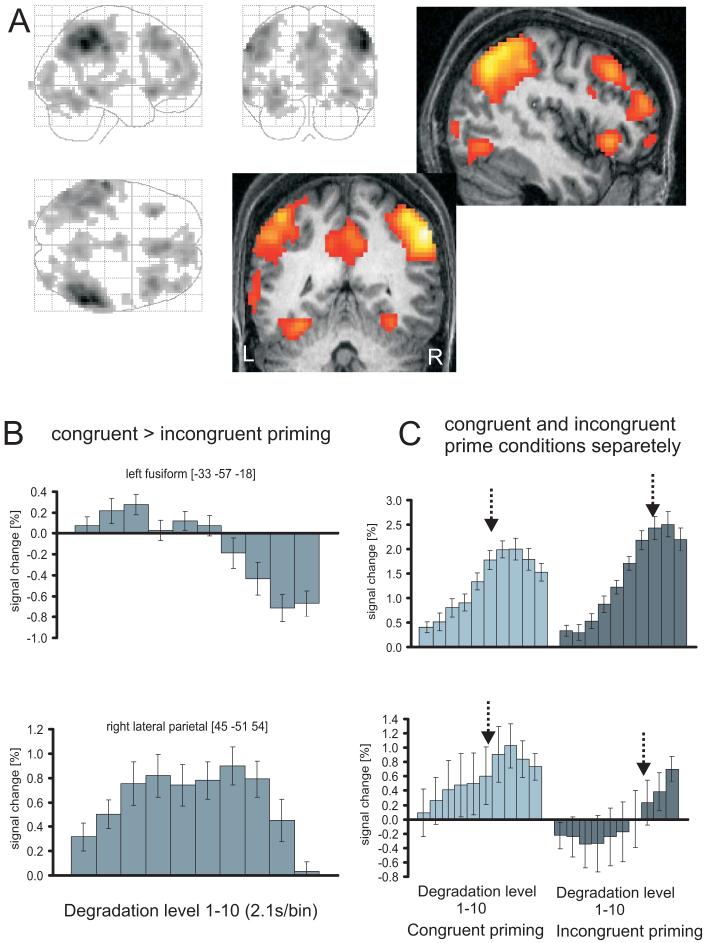
The first step in our fMRI analyses compared mean activity for congruently and incongruently primed objects across all 10 steps of the object-revealing sequence. An F-test assessing any difference in BOLD activity across all degradation levels for congruently versus incongruently primed objects (see Materials and Methods) revealed differences in regions that included lateral parietal, medial parietal (posterior cingulate/retrosplenial) and fusiform ventral visual cortices (Figure 3, Table 1), all bilaterally. As the plots for the fusiform and lateral parietal regions in Figure 3B&C illustrate, activity tended to peak earlier for congruently than incongruently primed sequences. This initial result is similar to those reported previously (James et al., 2000), but now extends those results to conceptual priming by written words rather than identical pictures.
Figure 3.
Results of fMRI group analysis (N=13) testing for any difference in the BOLD response for congruently vs incongruently primed picture sequences (F-test for primed-unprimed across the 10 degradation levels). A - Group activation maps at p<.001, uncorrected, superimposed on the normalised structural scan of one participant, and revealing significant activation maxima in lateral and medial parietal, fusiform, and frontal regions. B - Subtracted (congruent minus incongruent) response profiles (estimates of % signal change, relative to mean over all voxels and scans, delayed by 2 TR to account for the delay of the peak hemodynamic response) for right lateral parietal and left fusiform activation maxima, showing that congruently primed responses tended to be larger than incongruently primed during the first bins (and also somewhat smaller towards the end of the sequence in the fusiform). The parietal region shown expressed more pronounced responses for congruently than incongruently primed over most of the sequence. The statistical results for the overall effect of prime condition are related to any effect in these differential responses across the 10 bins. For completeness, unsubtracted profiles (C) are also shown revealing an earlier peaking response for congruently as compared to incongruently primed objects. The arrows indicate mean point at which recognition was indicated when averaged over subjects.
Table 1.
Initial overall comparison of congruently and incongruently primed conditions: Results of an F-test across the 10 degradation levels, corresponding to any difference in the activation profile between prime conditions. Effects are reported if either significant at p<.05, corrected for the whole brain (**) or at p.<05, small-volume-corrected (*) for object-responsive areas as determined by the separate LOC-localiser thresholded at p<.001. Up to 3 maxima per cluster are listed
| Region | MNI coords | Z | ||
|---|---|---|---|---|
| Right IPL | 57 | -48 | 42 | 7.57 ** |
| 57 | -39 | 51 | 7.25 ** | |
| 45 | -51 | 54 | 6.26 ** | |
| Left IPL | -63 | -27 | 39 | 6.44 ** |
| -45 | -39 | 54 | 5.77 ** | |
| -51 | -66 | 33 | 5.43 ** | |
| Right inferior LPF | 39 | 21 | -6 | 5.61 ** |
| Left inferior LPF | -33 | 24 | -6 | 5.43 ** |
| Right superior LPF | 42 | 24 | 42 | 5.40 ** |
| 45 | 45 | 21 | 4.77 ** | |
| Ant. (para)-cingulate | 0 | 36 | 42 | 5.46 ** |
| 3 | 24 | 45 | 5.28 ** | |
| 6 | 24 | 57 | 5.26 ** | |
| Medial prefrontal | 6 | 54 | 3 | 4.73 ** |
| Right fusiform | 48 | -63 | -9 | 4.89 ** |
| 33 | -54 | -15 | 4.48 * | |
| Left fusiform | -33 | -57 | -18 | 5.05 ** |
| -39 | -78 | -12 | 4.62 * | |
| Retro-splenial | 6 | -54 | 42 | 4.80 ** |
| -9 | -54 | 36 | 4.21 * | |
| Medial cingulate | -3 | -12 | 42 | 4.80 ** |
| Left middle temporal | -66 | -42 | -6 | 5.59 ** |
LPF - lateral prefrontal cortex, IPL - inferior parietal lobule
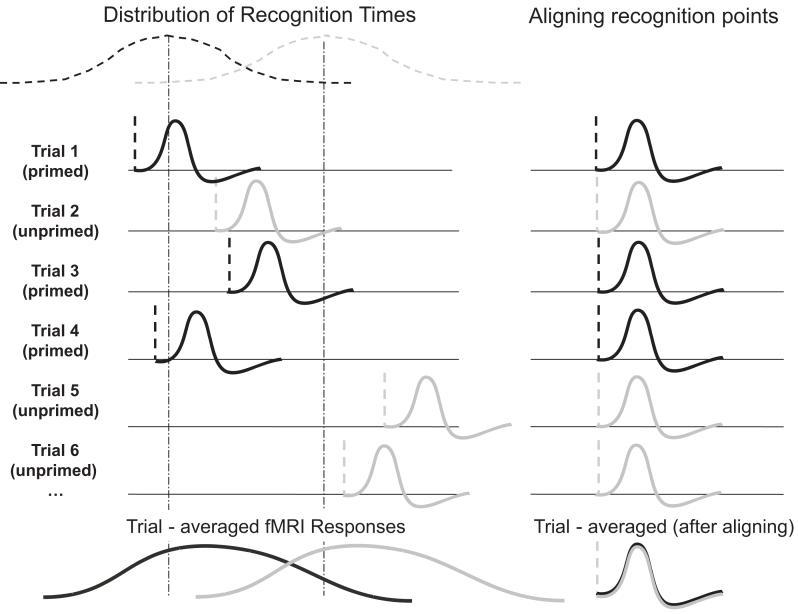
However, this initial analysis, as the one in James et al. (2000), does not take into account the recognition points for each individual trial. Hence the tendency for activity to peak earlier on congruently primed trials might simply reflect the earlier point of recognition, on average, for these trials. In other words, the increase in fusiform activity on each trial may have arisen subsequent to object recognition, rather than reflecting pre-recognition priming processes, as the distribution of recognition points over objects (earlier on average for congruently primed) would mean that the average activation profile could still show an increase prior to the peak of that distribution (see Figure 4 - and Henson, 2003, for discussion). We therefore conducted further analyses that partitioned activity for each trial into pre- and post-recognition periods, based on trial-specific behavioural responses (Figure 4).
Figure 4.
In an analysis that averaged activity over congruently and incongruently primed trials with respect to stimulus onset (left part of figure), the tendency for activity to peak earlier on congruently primed trials might simply reflect the earlier average point of recognition for congruently primed trials (James et al. 2000, and initial analysis here). In other words, the increase in activation on each trial may have arisen only subsequent to object recognition, but due to the distribution of recognition times over objects (earlier on average for congruently primed) the average activation profile could still show an increase prior to the peak of that distribution. If activity for the two trial types was averaged with respect to the trial-by-trial recognition point (right part of figure, implemented in further analyses here), there would be no pre-recognition difference in this case.
Effects of prime condition in relation to trial-by-trial recognition point
In this analysis, we disambiguated effects due to congruent versus incongruent priming from effects due to absolute differences in degradation level, by modelling the effects due to degradation level per se (common to all trials) separately from the effects for congruently and incongruently primed conditions relative to the trial-by-trial recognition point (see Materials and Methods for details). This means that the present comparisons of activity between congruently and incongruently primed objects are deconfounded from the current level of visual information.
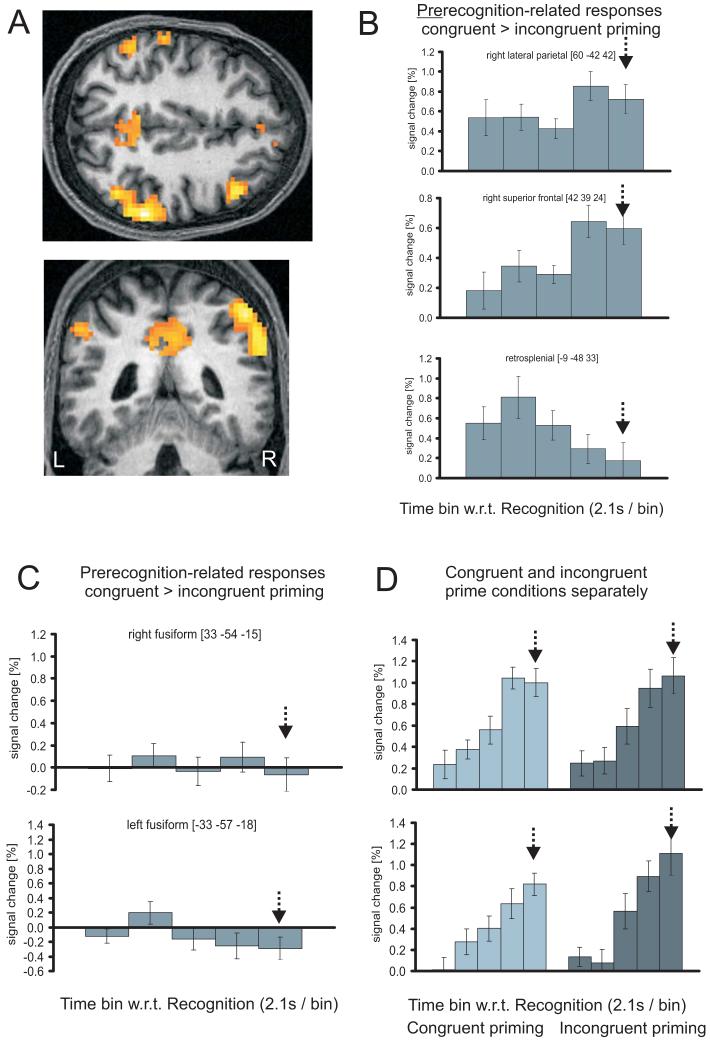
Top-down facilitation of object processing might be expected to have effects during build-up of stimulus evidence at degradation levels preceding recognition, as well as at the recognition point itself. We therefore examined whether activity relating to five time bins (comprising the four time-bins preceding the recognition point, plus the time-bin where recognition was indicated; see Materials and Methods) was affected by prime condition. Significant effects were observed in lateral parietal regions; medial parietal; and superior lateral prefrontal (Figure 5B, Table 2); but by contrast, not in ventral visual cortex. To rule out influences arising during explicit object recognition itself, we next restricted the analysis to four strictly defined pre-recognition bins. This analysis again revealed activations for congruently minus incongruently primed trials in lateral and medial parietal plus frontal regions (details in Supplemental Table 1), indicating that reliable effects of prime condition preceded recognition in these brain areas. However, ventral visual cortex was still unaffected. Thus, truly “pre-recognition” effects of congruent versus incongruent top-down priming, when accounting for trial-by-trial recognition points and factoring out any effects of degradation level per se, were found only in parietal and frontal cortex, but not in visual cortex.
Figure 5.
Results of fMRI group analysis (N=13) testing for activity related to the time bins up to and including the point of recognition, as determined on a trial-by-trial basis, after factoring out any effects that can be explained by degradation level. Maps are displayed superimposed on the normalised structural scan of one participant. A - group activation map of an F-test for the difference between congruently and incongruently primed across the 5 time bins at p<.001, uncorrected, masked inclusively by the activation map for any difference between prime condition, as derived from our first analysis (Table 1 and Figure 2A). The plots in (B) illustrate differential activity (congruent minus incongruent priming) for the recognition time bin (arrows) and the 4 time bins prior to it (after accounting for the delay of the peak hemodynamic response) for right lateral parietal, right superior frontal, and medial parietal (retrosplenial) areas. These plots illustrate that differential effects due to primie condition precede recognition in these regions. C - Differential (congruent - incongruent priming) responses, and D - unsubtracted response profiles for the same 5 timebins, for left and right fusiform maxima. After accounting for individual times of recognition (arrows), these ventral visual regions importantly did not show enhanced pre-recognition activity for primed trials. The separate plots for congruently and incongruently primed in (D) illustrate that above and beyond what can be explained by changes in degradation level, these areas still did show a gradual rise of activity at levels leading up to recognition (see also Supplementary Table 2), reaching their peak at recognition; but did so in a similar way for congruently and incongruently primed trials.
Table 2.
Effects of prime condition up to and including the recognition point, with the latter determined on a trial-by-trial basis. (N = 13). Results of an F-test corresponding to any difference between congruently and incongruently primed conditions over the 5 timebins. Effects are reported at p<.001, uncorrected, inside the mask defined by the F-test for any difference between prime conditions as derived from our independent first analysis (see Table 1 and Figure 2) that did not consider individual recognition times across trials (at p<.001). Foci surving correction for the mask volume (p<.05) are indicated by *
| region | MNI coords | Z | ||
|---|---|---|---|---|
| Right IPL and SPL | 60 | -42 | 42 | 5.06 * |
| 51 | -48 | 51 | 4.40 * | |
| 57 | -51 | 39 | 4.30 * | |
| Left IPL | -54 | -51 | 42 | 3.62 |
| -54 | -57 | 36 | 3.56 | |
| -60 | -57 | 30 | 3.45 | |
| Left SPL | -42 | -36 | 60 | 3.83 |
| -42 | -51 | 57 | 3.67 | |
| Left parietocentral | -60 | -27 | 42 | 4.43 * |
| -60 | -18 | 27 | 4.27 | |
| Right superior LPF | 42 | 39 | 24 | 4.78 * |
| 48 | 21 | 42 | 4.16 | |
| Left superior LPF | -36 | 18 | 48 | 3.94 |
| Medial prefrontal | 3 | 51 | 21 | 3.98 |
| 9 | 54 | 33 | 3.47 | |
| 9 | 66 | 15 | 3.68 | |
| Ant. (para) cingulate | 0 | 39 | 42 | 3.67 |
| -6 | 36 | 36 | 3.57 | |
| Retrosplenial | -9 | -48 | 33 | 3.53 |
| 6 | -48 | 33 | 3.45 | |
| 15 | -48 | 33 | 3.43 | |
LPF - lateral prefrontal cortex, IPL - inferior parietal lobule, SPL - superior parietal lobule, IPS - intraparietal sulcus
At the fusiform maxima from our first unselective analysis of basic effects of prime condition across all ten degradation levels (Figure 3 and Table 1), activity over successive time-bins up to and including the trial-by-trial recognition point showed no significant difference between congruently and incongruently primed objects. Z-values corresponding to F-tests for any difference across these five time bins were: 0.12 for the right maximum (33 -54 -15) from our first analysis; and 1.68 for the left maximum (-33 -57 -18), with the latter trend going against increased activation for congruently primed objects. Subtracted response profiles for these two fusiform regions of interest are displayed in Figure 5C. These confirm that there was no evidence to support previous claims (James et al. 2000) of increased pre-recognition activity for congruently primed objects in ventral visual cortex, once trial-by-trial recognition point is taken into account. On the other hand, pre-recognition increases due to congruent priming were observed in lateral parietal, lateral frontal, and retrospenial cortex (Figure 5A).
Figure 5D displays activation profiles separately for congruently and incongruently primed trials in right and left fusiform, for the five successive time-bins leading up to the recognition point. These show a gradual rise of activity across the time bins leading to the point of recognition (see Supplementary Table for detailed results of (pre)-recognition activity combined for both prime conditions). Thus, activity in ventral visual cortex does rise prior to the recognition point (over and above any rise due merely to less degraded images, which was accounted for separately in our model), but does so equivalently for congruently and incongruently primed objects.
Further statistical comparisons confirmed significant differences in the effect of prime congruency across regions. The region-by-prime congruency interaction (for data averaged across the five time bins included in the analysis above) reached significance for comparisons between lateral parietal and fusiform cortex, F(1,12) = 26.0, p<.0001, reflecting stronger increase due to prime congruency in parietal cortex, and similarly for the comparison between lateral prefrontal and fusiform cortex, F(1,12) = 24.1, p<.0001. In addition, the same comparison involving the lateral parietal and lateral prefrontal maxima showed a reliable effect, although at a lower level of significance, F(1,12) = 8.7, p<.05, reflecting stronger effects of prime congruency in parietal than prefrontal cortex.
Functional coupling analyses
Differential activity due to prime condition at and preceding the recognition point was observed in parietal (and to a lesser degree frontal) cortex; but not in ventral visual cortex. On the other hand, ventral visual cortex did show increased activity as the recognition point approached (equivalently so for congruently and incongruently primed objects). Moreover, this point was reached earlier for congruently primed objects. One potential mechanism that might explain all these findings is that higher-level regions affected by prime condition (e.g. in parietal cortex) may engage in top-down modulatory interactions with ventral visual cortex, that leads to an earlier recognition point. We tested this possibility with an analysis of functional coupling (“psychophysiological interaction”- PPI). This analysis takes a single “seed” area in the brain and tests for other areas where BOLD signal shows stronger coupling with this region as a function of psychological context, in this instance for congruent more than for incongruent prime conditions (i.e. stronger covariation with the signal timecourse of the seed region, over and above those effects explicable by direct effects of prime condition on activation levels - see Materials and Methods for details).
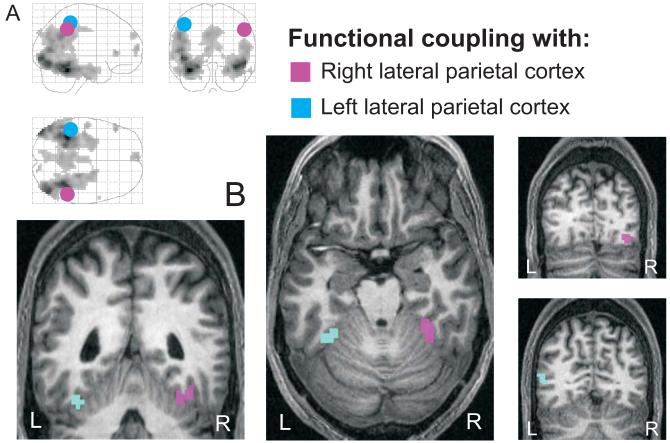
As outlined above, the hypothesis we sought to test was that the parietal regions showing pre-recognition increases for congruently primed objects showed greater functional coupling with ventral visual cortex in this condition. Accordingly, we first seeded the coupling analysis with the right lateral parietal peak (57 -48 42) from our first analysis (Figure 3). PPI analysis seeded here did indeed reveal significantly greater functional coupling for congruently than incongruently primed conditions, between this region and right posterior and mid-fusiform cortex (see Figure 6 and Table 3). Moreover, seeding with the timecourse of the left lateral parietal peak instead (-45 -39 54) revealed an analogous pattern of coupling, but now with maxima in left fusiform and superior occipital cortex (Figure 6 and Table 3). Although the results for the left parietal seed did not reach full significance at p<.001, we report them for completeness, since they provide a conceptual replication of the right-hemisphere coupling results, that was very close to significance (p=.002).
Figure 6.
Results of an fMRI functional-coupling group analysis (N=13) (“psychophysiological interaction”, PPI). B - Areas in right fusiform cortex (purple) showed stronger coupling with the timecourse of the right lateral parietal activation maximum (purple circle in A) during the congruently than incongruently primed condition (averaged across the 4 pre-recognition bins plus the recognition bin, as considered in our previous analyses). Areas in left fusiform and lateral occipital cortex (blue) were found to show coupling in an equivalent analysis with left lateral parietal cortex (blue circle in A), at a slightly lower significance value, and are reported for completeness. For display purposes, maps were thresholded at p<.005 (right parietal seed), and p<.01 (left parietal seed), uncorrected, extent threshold 10 voxels, and superimposed onto the structural scan of one participating subject. Since we were interested in effects of functional coupling within object-responsive visual regions, we restricted interrogation of the data to the group LOC-localiser results at p<.001, uncorrected (mask volume shown in A which also indicates the average locations of the left and right seed regions across subjects).
Table 3.
Results of functional coupling analyses (psychophysiological interactions, PPI) with lateral parietal cortex, at p<.001, uncorrected, masked with the localising contrast for object responsive areas at p <.001 uncorrected. Functional coupling in the analysis seeding with left lateral parietal cortex was found at a slightly lower significance threshold (as indicated by z-values in brackets), but is reported for completeness
| region | Stereotactic coordinates | Z | ||
|---|---|---|---|---|
| Functional coupling with right lateral parietal cortex | ||||
| Right inf. occipital | 36 | -81 | -12 | 3.51 |
| Right fusiform | 33 | -42 | -27 | 3.27 |
| Functional coupling with left lateral parietal cortex | ||||
| Left fusiform | -33 | -48 | -27 | (2.88) |
| Left sup. occipital | -54 | -69 | 9 | (2.87) |
In addition, connectivity analyses were seeded with the lateral prefrontal maxima observed here (-33 24 -6 and 39 21 -6). The analysis with the left prefrontal region as seed resulted in significant coupling in bilateral parietal regions (left parietal cortex: -39 -30 57, z = 3.52; right parietal cortex: 63 -21 48, z = 3.43), but not in ventral visual cortex. The equivalent analysis with the right prefrontal maximum found here as seed region produced no significant effects1.
Discussion
We used fMRI to investigate changes in processing of degraded objects elicited by top-down knowledge about their potential identity, provided via verbal primes. To our knowledge, the present fMRI study is the first to show the neural consequences of purely top-down priming of degraded visual objects. Several previous fMRI studies have examined priming of objects degraded by partial occluders or pixel noise (James et al., 2000); by using two-tone versions (Dolan et al., 1997; George et al., 1999); or with letters gradually revealed by varying figure-ground contrast (Kleinschmidt et al., 2002). But in all these studies, corresponding visual stimuli were used as pictorial primes, leaving unanswered whether purely bottom-up visual-repetition mechanisms, rather than conceptual top-down influences account for the findings. Our study shows that top-down conceptual priming is sufficient to produce lower identification thresholds and to engender activity differences in e.g., fusiform and parietal regions.
When a written word, preceding a sequence of progressively less degraded images of an object, matched the name of that object (congruently primed condition), identification was reported earlier in the sequence than with a non-matching word (incongruently primed condition). Activation profiles in fusiform, lateral and medial parietal, and frontal cortices differed between congruently and incongruently primed objects, showing on average an earlier peak of activity (Figure 3C) for congruently primed objects, in an initial fMRI analysis that did not take into account trial-by-trial recognition points.
To test whether this difference in activation profiles was due to the earlier recognition success revealed in behaviour, or instead might reflect neural processes arising prior to and leading up to recognition, we examined activity for time-bins defined relative to the trial-by-trial recognition point. When time relative to recognition success was equated in this way across congruently and incongruently primed trials, with any differences in degradation level factored out, pre-recognition activity was found to be equivalent for congruently and incongruently primed trials in ventral visual areas such as the fusiform (Figure 5C). Although activity here rose across successive time-bins leading up to the recognition point (Figure 5D), it did so equivalently for both prime conditions. By contrast, regions in parietal and frontal cortex showed significantly elevated activity specifically for congruently primed objects, prior to actual recognition.
While the absence of activity increases due to prime congruency in ventral visual cortex, after accounting for the trial-by-trial recognition point, might be seen as a “null result”, we believe that result is actually informative in the present context. Firstly, an absence of any pre-recognition increase is unlikely to be explained by a lack of sensitivity of the analysis, induced by covarying out the effect of degradation level. Strong activation effects independent of degradation level were observed in ventral visual cortex in the same time period (see Supplementary Table 2, and Figure 5D), but this was common to congruently and incongruently primed trials. Furthermore, reliable differences between the two trial types were still observed in other regions, in the parietal and frontal lobes, and priming effects differed significantly between regions (parietal versus fusiform, frontal versus fusiform). In fact, if anything, effects of prime congruency in the fusiform appeared to be of opposite sign (a tendency for reduced activation for congruently than incongruently primed in the left fusiform, see Figure 5). This would agree with the priming-related activity reductions that commonly occur with undegraded stimuli (e.g, Schacter and Buckner, 1998; Henson, 2003; van Turennout et al., 2000; Dehaene et al., 2001; Koutstaal et al., 2001; James et al., 2002; Vuilleumier et al., 2002, Simons et al., 2003), however in the present context this effect did not reach significance and awaits further replication. Important for present purposes is that activity increases related to prime congruence in the fusiform, which were obvious in the first analysis timed with respect to sequence onset, were clearly no longer found when accounting for the recognition point of individual trials.
Thus, our results do not support the idea that activation enhancements due to prime condition in ventral visual areas arise prior to (or at) the trial-specific recognition point. Although James et al. (2000) found an earlier peak of activity in ventral visual cortex for (pictorially) primed objects relative to unprimed when averaged, as noted earlier (see also Figure 4), that result might simply have reflected earlier recognition success on average for the primed objects. More broadly, our findings suggest an explanation for other cases of priming-induced activity increases in ventral visual cortex (Dolan et al., 1997; George et al., 1999): In all such cases, identification of the impoverished pictures was more likely after congruent priming. Therefore the ‘repetition increases’ in visual cortex attributed to priming in such studies probably reflected the difference in recognition success between trials (as shown here) rather than facilitatory mechanisms per se. This also accords with previous proposals (Grill-Spector et al., 2000; Bar et al., 2001; Kleinschmidt et al., 2002; Logothetis, 1998) that activity in ventral visual cortex can reflect perception and recognition success, rather than mere stimulus quality. Once the recognition point of individual trials was taken into account (and differences in degradation level factored out), (pre)-recognition related activity was no longer increased for congruently primed objects.
If congruently and incongruently primed objects are not differentiated within ventral object-selective cortex prior to (or at) the recognition point, as our results for those object-processing areas indicate, then additional brain regions beyond these may be necessary to explain the facilitated recognition for the congruently primed objects. Here, we found the strongest effects of prime condition during the pre-recognition period in lateral parietal regions, with further significant effects in medial parietal areas, as well as frontal regions. Parietal cortex has previously been implicated in some aspects of object perception, in addition to ventral visual cortex, albeit mostly with an emphasis on mechanisms related to spatial transformation or object-related action (Goodale and Milner, 1992; though see Sereno and Maunsell, 1998). Lesions of human parietal cortex can also impair integration of multiple items/objects into a coherent whole (Humphreys and Riddoch, 1992). Moreover, although object agnosias are traditionally associated with ventral lesions, parietal lesions can in fact lead to specific difficulties in identifying, naming or matching objects, when shown in degraded or “unusual views” (Warrington and James, 1967). This deficit can occur even when the patient has successfully named a standard view of the same object, and hence possesses top-down cues about the possible identity of the degraded view that they cannot subsequently identify (Warrington and Taylor, 1973, 1978; Warrington and James, 1988, Layman and Greene, 1988). Thus, while severe agnosic deficits in object recognition are classically associated with ventral occipito-temporal damage, deficits specifically in recognising unusual or degraded views in the context of matching top-down knowledge (as for the congruently primed trials here), have been associated with parietal damage, consistent with the particular functional role for parietal cortex suggested by the present fMRI data.
Moreover, in our data lateral parietal cortex showed stronger functional coupling with fusiform cortex (see Figure 6) for congruently than incongruently primed objects, in the period leading up to recognition. One previous PET study (Dolan et al., 1997) also reported functional coupling between parietal and ventral visual cortex, in the context of degraded (two-tone) objects and faces. However, in addition to the problems regarding interpretation of previous priming-related activation increases outlined above, the coupling analyses for that study were not related to the effect of prime condition (but contrasted faces to objects instead). Here, we were able to provide a more direct test for the hypothesis that the mechanism by which top-down priming facilitates object recognition involves modulatory interactions between lateral parietal and ventral visual cortex, in the time period leading up to the trial-by-trial recognition point. While these results point to coupling between the two structures as one critical part of the mechanisms of top-down knowledge-based facilitation, our data are also compatible with the view that these effects may arise within a broader network of areas, probably further involving left lateral prefrontal cortex. The latter region showed stronger functional coupling with bilateral parietal cortex here in the congruently primed condition, and is in close spatial correspondence with areas implicated by previous work in priming effects for undegraded objects (e.g., van Turennout, 2000), in addition to ventral visual regions. We suggest that top-down interactions between left lateral prefrontal, lateral parietal, and ventral visual regions may normally allow the earlier recognition produced by matching top-down knowledge, but could be disrupted by parietal lesions, producing the object processing deficits described above.
Conclusions
We found that the threshold for identification of gradually revealed visual objects can be lowered by congruent top-down knowledge in the form of strictly verbal primes, paralleled by an earlier rise of mean fMRI activity in ventral visual cortex, plus in parietal and frontal regions. However, activity leading up to the trial-by-trial recognition point was not increased for congruently compared with incongruently primed objects (when factoring out degradation level) within ventral visual cortex. This suggests that activity increases attributed to prime condition in previous work may have related to differences in recognition success, rather than the facilitatory mechanisms per se, for ventral visual cortex. Our findings suggest that the mechanisms by which top-down knowledge facilitates object recognition may not arise within ventral visual cortex alone (which, since it mirrors recognition success, may not in isolation explain why this occurs earlier for congruently primed objects). Instead, our results support an account according to which top-down facilitation also involves higher-order areas, such as parietal and prefrontal cortex, and their functional interactions, which may subsequently result in the observed earlier rise of activity in visual cortex and earlier recognition.
Supplementary Material
Acknowledgements
This research was supported by programme grants from the Wellcome Trust to RJD and JD. We thank Philippe Schyns for help with stimulus computation.
Footnotes
Effects within this analysis were restricted by a combined mask of object responsive areas (localiser) and priming-related regions as determined by our first analysis of overall priming effects, to reduce the number of multiple comparisons.
References
- Bar M, Tootell RBH, Schacter DL, Greve DN, Fischl B, Mendola JD, Rosen BR, Dale AM. Cortical mechanisms specific to explicit visual object recognition. Neuron. 2001;29:529–535. doi: 10.1016/s0896-6273(01)00224-0. [DOI] [PubMed] [Google Scholar]
- Biederman I. Perceiving real-world scenes. Science. 1972;177:77–80. doi: 10.1126/science.177.4043.77. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Naccache L, Cohen L, Bihan DL, Mangin JF, Poline JB, Riviere D. Cerebral mechanisms of word masking and unconscious repetition priming. Nat. Neurosci. 2001;4:52–758. doi: 10.1038/89551. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Fink GR, Rolls E, Booth M, Holmes A, Frackowiak RSJ, Friston KJ. How the brain learns to see objects and faces in an impoverished context. Nature. 1997;389:96–599. doi: 10.1038/39309. [DOI] [PubMed] [Google Scholar]
- Doniger GM, Foxe JJ, Schroeder CE, Murray MM, Higgins BA, Javitt DC. Visual perceptual learning in human object recognition areas, a repetition priming study using high-density electrical mapping. Neuroimage. 2001;13:305–313. doi: 10.1006/nimg.2000.0684. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- George N, Dolan RJ, Fink GR, Baylis GC, Russell C, Driver J. Contrast polarity and face recognition in the human fusiform gyrus. Nat. Neurosci. 1999;2:574–580. doi: 10.1038/9230. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Gosselin F, Schyns PG. Bubbles: a technique to reveal the use of information in recognition tasks. Vision Res. 2001;41:2261–2271. doi: 10.1016/s0042-6989(01)00097-9. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kushnir T, Hendler T, Malach R. The dynamics of object-selective activation correlate with recognition performance in humans. Nat. Neurosci. 2000;3:837–843. doi: 10.1038/77754. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K. The neural basis of object perception. Curr. Opin. Neurobiol. 2003;13:159–166. doi: 10.1016/s0959-4388(03)00040-0. [DOI] [PubMed] [Google Scholar]
- Henson RNA. Neuroimaging studies of priming. Prog. Neurobiol. 2003;70:53–81. doi: 10.1016/s0301-0082(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Henson RNA. Analysis of fMRI timeseries: Linear Time-Invariant models, event-related fMRI and optimal experimental design. In: Frackowiak, Friston, Frith, Dolan, Price, editors. Human Brain Function. 2nd ed. London: Elsevier; 2004. pp. 793–822. [Google Scholar]
- Hirshman E, Snodgrass JG, Mindes J, Feenan K. Conceptual priming in fragment completion. J. Exp. Psychol. Learn. Mem. Cognit. 1990;16:634–647. [Google Scholar]
- Humphreys GW, Riddoch MJ. Interactions between objects and space-vision revealed through neuropsychology. In: Meyers DE, Kornblum S, editors. Attention and Performance XIV. Hillsdale: Lawrence Erlbaum Associates; 1992. pp. 143–162. [Google Scholar]
- James TW, Humphrey GK, Gati JS, Menon RS, Goodale MA. The effects of visual object priming on brain activation before and after recognition. Curr. Biol. 2000;10:1017–1024. doi: 10.1016/s0960-9822(00)00655-2. [DOI] [PubMed] [Google Scholar]
- James TW, Humphreys GK, Gati JS, Menon RS, Goodale MA. Differential effect of viewpoint on object-driven activation in dorsal and ventral streams. Neuron. 2002;35:793–801. doi: 10.1016/s0896-6273(02)00803-6. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt A, Buechel C, Hutton C, Friston KJ, Frackowiak RSJ. The neural structures expressing perceptual hysteresis in visual letter recognition. Neuron. 2002;34:659–666. doi: 10.1016/s0896-6273(02)00694-3. [DOI] [PubMed] [Google Scholar]
- Koutstaal W, Wagner AD, Rotte M, Maril A, Buckner RL, Schacter DL. Perceptual specificity in visual object priming: functional magnetic resonance imaging evidence for a laterality difference in fusiform cortex. Neuropsychologia. 2001;39:184–199. doi: 10.1016/s0028-3932(00)00087-7. [DOI] [PubMed] [Google Scholar]
- Layman S, Greene E. The effect of stroke on object recognition. Brain. Cognit. 1988;7:87–114. doi: 10.1016/0278-2626(88)90022-x. [DOI] [PubMed] [Google Scholar]
- Logothetis N. Object vision and visual awareness. Curr. Opin. Neurobiol. 1998;8:536–544. doi: 10.1016/s0959-4388(98)80043-3. [DOI] [PubMed] [Google Scholar]
- Palmer SE. The effects of contextual scenes on the identification of objects. Mem. Cognit. 1975;3:519–526. doi: 10.3758/BF03197524. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS. In: In The Artful Eye. Gregory RL, Harris J, editors. Oxford: Oxford University Press; 1994. pp. 249–267. [Google Scholar]
- Sadr J, Sinha P. Object recognition and random image structure evolution. Cog. Sci. 2004;28:259–287. [Google Scholar]
- Schacter DL, Buckner RL. Priming and the brain. Neuron. 1998;20:185–195. doi: 10.1016/s0896-6273(00)80448-1. [DOI] [PubMed] [Google Scholar]
- Sereno AB, Maunsell JH. Shape selectivity in primate lateral parietal cortex. Nature. 1998;395:500–503. doi: 10.1038/26752. [DOI] [PubMed] [Google Scholar]
- Simons JS, Koutstaal W, Prince S, Wagner AD, Schacter DL. Neural mechanisms of visual object priming: evidence for perceptual and semantic distinctions in fusiform cortex. Neuroimage. 2003;19:613–626. doi: 10.1016/s1053-8119(03)00096-x. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Feenan K. Priming effects in picture fragment completion: support for the perceptual closure hypothesis. J. Exp. Psychol. Gen. 1990;119:276–296. doi: 10.1037//0096-3445.119.3.276. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Marshall JC, Friston KJ, Rowe JB, Ritzl A, Zilles K, Fink GR. Lateralized cognitive processes and lateralized task control in the human brain. Science. 2003;301:384–386. doi: 10.1126/science.1086025. [DOI] [PubMed] [Google Scholar]
- Tovee MJ, Rolls ET, Ramachandran VS. Visual learning in neurons of the primate temporal visual cortex. Neuroreport. 1996;7:2757–2760. doi: 10.1097/00001756-199611040-00070. [DOI] [PubMed] [Google Scholar]
- van Turennout M, Ellmore T, Martin A. Long-lasting cortical plasticity in the object naming system. Nat. Neurosci. 2000;3:1329–1334. doi: 10.1038/81873. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Henson RN, Driver J, Dolan RJ. Multiple levels of visual object constancy revealed by event-related fMRI of repetition priming. Nat. Neurosci. 2002;5:491–494. doi: 10.1038/nn839. [DOI] [PubMed] [Google Scholar]
- Warrington EK, James M. Disorders of visual perception in patients with localised cerebral lesions. Neuropsychologia. 1967;5:253–266. [Google Scholar]
- Warrington EK, Taylor AM. Contribution of the right parietal lobe to object recognition. Cortex. 1973;9:152–164. doi: 10.1016/s0010-9452(73)80024-3. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Taylor AM. Two categorical stages of object recognition. Perception. 1978;7:695–705. doi: 10.1068/p070695. [DOI] [PubMed] [Google Scholar]
- Warrington EK, James M. Visual apperceptive agnosia: A clinico-anatomical study of three cases. Cortex. 1988;24:13–32. doi: 10.1016/s0010-9452(88)80014-5. [DOI] [PubMed] [Google Scholar]
- Wiggs CL, Martin A. Properties and mechanisms of perceptual priming. Curr. Opin. Neurobiol. 1998;8:227–233. doi: 10.1016/s0959-4388(98)80144-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.