Abstract
Surplus or ‘wasteful’ killing of uneaten prey has been documented in the fourth larval instar of various species of the mosquito genus Toxorhynchites that occur in treeholes and other phytotelmata. Here we document surplus killing by the predatory midge Corethrella appendiculata, which in Florida cohabits treeholes and artificial containers with larvae of Toxorhynchites rutilus. Provided with a surfeit of larval mosquito prey, surplus killing was observed only in the fourth instar of C. appendiculata, peaking in intensity in the final 24 h prior to pupation, as observed for Toxorhynchites spp. Attack sites identified from videotaped encounters with mosquito prey were divided among head, thorax, abdomen, and siphon. Consumed mosquito larvae (n = 70) were attacked primarily on the head (46%) or siphon (34%), but surplus-killed prey (n = 30) were attacked predominantly on the thorax (83%). Despite its independent evolution among different insect species in aquatic container habitats, the functional significance of prepupal surplus killing remains unclear.
Keywords: Competition, midge, mosquito, predation, prepupae
Introduction
Wasteful killing, in which aquatic prey are killed but not eaten, has been identified in several genera and species of odonate nymphs and in larvae of many species of the mosquito genus Toxorhynchites (Corbet 1999, Russo 1986). Wasteful killing in odonates is density dependent (Johnson et al. 1975) and, in the treehole habitats of the giant damselfly Megaloprepus coerulatus, killing of conspecifics has been shown to reduce competition for larval food (Fincke 1994). Among Toxorhynchites spp., wasteful killing is most often observed in the terminal instar and is most intense among prepupae, peaking in incidence 1–2 days prior to pupation and coinciding with a decrease in prey consumption (Lounibos 1979, Russo 1986, Lounibos et al. 1998). Corbet and Griffiths (1963) proposed that killing by prepupae protected the vulnerable pupal stage of Toxorhynchites brevipalpis from predation by conspecifics. However, experiments to validate the vulnerable pupal hypothesis have yielded equivocal results (Corbet 1985). Russo (1986), who referred to the phenomenon as surplus killing after Kruuk (1972), suggested that this behavior might be a form of ‘inhibition’ competition which deprives competing Toxorhynchites spp. larvae of prey, thereby slowing development and increasing cannibalism of early instars.
Here we document surplus killing in the fourth larval instar of the predatory midge Corethrella appendiculata, which co-occurs in treeholes in the southern USA with Toxorhynchites rutilus, a species which is known to exhibit similar behavior (Russo 1986, Lounibos et al. 1998). Formerly regarded as highly derived chaoborids with blood-sucking mouthparts in adult females (McKeever 1986), the genus Corethrella is currently placed in a separate family (Corethrellidae) and includes four species in North America (McKeever and French 1991a, b). Although C. appendiculata is the only corethrellid known from treeholes in North America, many tropical species of this genus occur in phytotelmata (Borkent, personal communication).
In the Florida treehole aquatic community dominated by larval mosquitoes, T. rutilus and C. appendiculata partition prey by body size (Bradshaw and Holzapfel 1983, Lounibos 1985), the larger T. rutilus being more effective at regulating overall prey abundance and C. appendiculata controlling community diversity (Griswold and Lounibos 2006). Only in their third and fourth instars do C. appendiculata consume mosquito prey (Lounibos, unpublished) and, upon reaching the third instar, large culicids become relatively resistant to this size-selective predator (Kesavaraju et al. 2007). Both C. appendiculata and T. rutilus prey selectively on the invasive container mosquito Aedes albopictus in preference to the native treehole species Aedes triseriatus (Griswold and Lounibos 2005a, b), owing to predator-evasive behaviors of the native species (Kesavaraju and Juliano 2004, Kesavaraju et al. 2007). During the course of predator–prey studies on these species, we first encountered surplus killing by C. appendiculata.
Methods
Husbandry of C. appendiculata
Larvae of C. appendiculata were obtained from a Florida colony originating from treehole and discarded tire collections of 200–300 immatures of this species during 2003. Larvae were reared until pupation in metal trays (5 × 25 × 35 cm) and offered a suffusion of cultured aquatic nematodes (Panagrellus sp.) or mosquito larvae as prey. Pupae were removed from trays two to three times per week and allowed to emerge from small cups with tap water into a 0.1 m3 fine-mesh square cage in an insectary maintained at 26 ± 1 (SD)°C and 85% rh. Adults in cages had continuous access to wicks of sugar water (10% sucrose) and laid fertile eggs autogenously in treehole water, even though females of this species have mouthparts for bloodsucking (McKeever 1986), are attracted to frog calls (McKeever and French 1991a), and have taken blood meals from hylid frogs in the laboratory (Kesavaraju, unpublished). First instar larvae which hatched in oviposition cups were transferred to rearing trays containing nematodes. The four larval instars of C. appendiculata were distinguished by comparative head capsule widths and used according to experimental designs, as described below.
Developmental Times and Prey Consumption of Third and Fourth Instars
A tray containing exclusively second instar C. appendiculata and nematode prey was isolated and checked daily for the appearance of third instars, which were removed and placed individually in cups with 10 ml distilled water. Predator larvae were provided with a surfeit of washed nematodes (n = 15 cups) or 20 first instar (<24 h old) A. albopictus larvae (n = 15 cups) from a Florida colony of this species maintained as described previously (Lounibos et al. 2001). Experiments were set in the same insectary described previously with a 16L:8D light:dark cycle. At daily checks, the numbers of mosquito prey missing and dead were enumerated, and fresh prey and water were provided to all cups every 24 h to maintain original conditions. Partially consumed prey were recovered infrequently and scored as a ‘missing’ prey, i.e., equivalent to complete consumption. Upon molting to the fourth instar, each C. appendiculata was offered 30, instead of 20, A. albopictus first instars daily. Each cup was checked daily and provided with fresh prey until the day of pupation, which was recorded.
In a modification of the previous design, 15 C. appendiculata larvae were isolated individually in cups on the first day of the fourth instar and offered 30 first instar A. albopictus daily. For both this and the previous experiment, five control cups containing only 10 ml water and 30 A. albopictus immatures were checked for mortality and replenished daily. Numbers of mosquito larvae missing or dead were recorded daily in both experimental and control cups until C. appendiculata pupated. Pupae were sexed to check for sex-dependent differences in developmental times and prey consumption.
Recording of Predatory Behaviors from Videotaped Encounters
Results from the previous experiments revealed that the intensity of killing increased as fourth instars neared pupation. Using the development of imaginal eyes as a marker for the approach of pupation, late-stage fourth instars were removed individually from trays and placed in 20 ml cups with 10 ml of tap water and 30 first instar A. albopictus. Each cup containing a predator and prey was videotaped under ambient light for 1 h with a Panasonic camera fitted with a 1:1 macro lens. Behavioral recordings of 13 individuals were saved on a personal computer in MPEG-2 format with a Winfast XP PCI card for observations and quantification of the following behaviors related to prey capture: (a) location of predators in cup at time of strike; (b) orientation of predators just prior to strike; (c) location on prey body of successful strike; (d) success rate, defined as the proportion of strikes leading to killing or consumption; (e) whether prey were consumed or not.
Results
Developmental Times and Prey Consumption
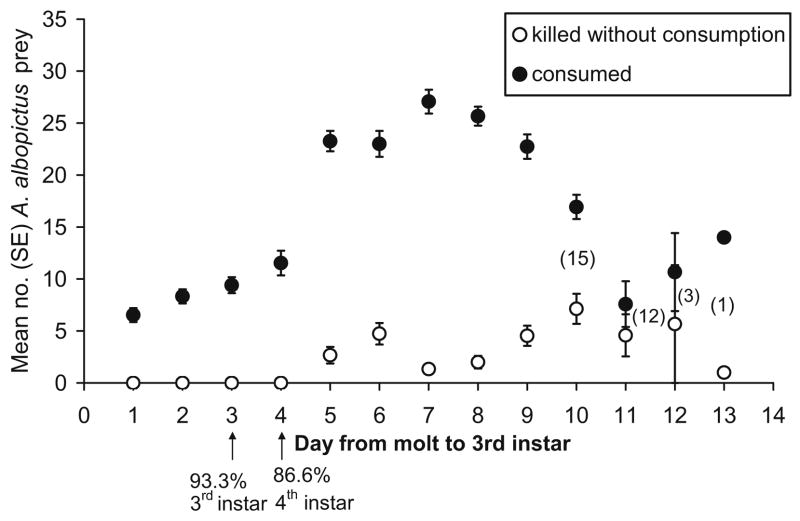
The mean times from first day of the third instar to pupation did not differ significantly (F1,23 = 2.25, P = 0.147) between diets of mosquito larvae (mean = 11.1d ± 0.2 SE) and nematodes (mean = 10.6d ± 0.7 SE). Approximately 3 days of this period were spent in the third instar, during which period an average of 6–9 mosquito larvae were consumed daily and no surplus killing was detected (Fig. 1). By the second day of the fourth instar, the daily mean number of prey consumed had more than doubled, and remained at 22–26 prey per day for the succeeding 4 days, decreasing before pupation (Fig. 1). During this same interval, surplus killing without consumption was detected at mean values ranging from two to seven larvae per day, peaking shortly before pupation.
Fig. 1.
Mean numbers of A. albopictus first instars consumed (black circles) or surplus-killed (open circles) by 15 C. appendiculata offered 20 prey larvae daily during the third and 30 prey larvae daily during the fourth instar. Arrows indicate the timing of molting of most predators, and numbers in parentheses indicate numbers of predators remaining as larvae as the cohort approached metamorphosis. SEs were too small to show on some symbols.
An inverse temporal relationship between prey consumption and surplus killing in the fourth instar was clearly observed in the cohort monitored individually only in the fourth instar (Fig. 2). Mean number of prey killed without consumption peaked at 10.0 ± 1.1 (SE) 2 days before pupation, and mean number of prey consumed reached a maximum of 26.6 ± 0.8 (SE) 5 days before pupation (Fig. 2). In control cups without predators, the mean number of A. albopictus missing or dead per day was negligible (<0.2/day) in both this and the preceding experiment. Mean time in the fourth instar of males was shorter (mean = 6.33 ± 0.24 days SE, n = 9) than the mean duration for females (mean = 7.00 ± 0.26 days SE, n = 6), but this difference was not significant (t = 1.86, P = 0.09). Differences in the mean number of total prey killed by males (29.2 ± 2.3 SE, n = 9) and females (mean = 25.7 ± 4.2 SE, n = 6) were also non-significant (t = 0.80, P = 0.44).
Fig. 2.
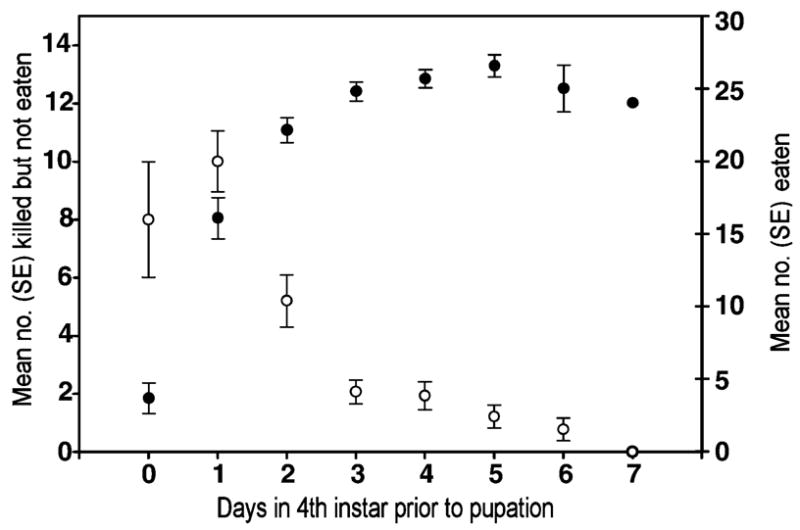
Mean numbers of prey consumed (black circles) or surplus-killed (open circles) by 15 C. appendiculata isolated individually on the first day of the fourth instar and offered 30 A. albopictus first instars daily until pupation. Data are from a different experiment than Fig. 1 and are plotted as days prior to pupation to synchronize individuals in relation to that event.
Attack Sites on A. albopictus Prey
Of 13 prepupal C. appendiculata videotaped in the presence of first instar A. albopictus prey, one individual attacked no prey during the 1-h observation interval, leaving 12 records for further behavioral analyses.
Of 119 strikes analyzed, 97 (81.5%) occurred while the predator was at the bottom of the cup, the remainder taking place at the middle (7), water surface (3), or wall (12) of the container. Eighty-nine (74.8%) of the strikes led to killing or consumption, i.e., were successful. Fifty-five (46.2%) of the 119 strikes were preceded by predator orientation, where the anterior segments were turned in the direction of the prey before striking. Pre-strike orientation was not detected for the remaining (53.8%) attacks.
From 89 analyzed successful attacks, 30 (33.7%) resulted in killing without consumption, nine (10.1%) in partial consumption, and the remaining 50 (56.2%) in complete consumption. Eleven individuals contributed to the killing, and ten of these contributed to the attacks leading to complete consumption. Most (83.3%) of the attacks leading to killing without consumption were directed at the prey thorax, but only 6.0% of the attacks that led to prey consumption were on the thorax (Table 1). The prey’s larval head was the preferred site for attacks leading to consumption (46%) but was never attacked in surplus killing (Table 1). The frequencies of attack sites in surplus killing, predation followed by complete consumption, and predation followed by partial consumption were significantly heterogeneous ( , P = 0.016) when tested by a maximum likelihood (ML) ANOVA using PROC CATMOD in SAS (SAS Institute 1989). Contrasts of ML estimates showed that most of this variation was attributable to the contrast between attack sites of surplus killing vs predation with complete consumption ( , P = 0.003). The other two contrasts were not significantly different , P > 0.70).
Table 1.
Attack Sites on First Instar A. albopictus by 12 Prepupal C. appendiculata Isolated Individually and Videotaped with Prey
| Number of strikes
|
||||
|---|---|---|---|---|
| Head | Thorax | Abdomen | Siphon | |
| Surplus killing | 0 | 25 (10) | 0 | 5 (4) |
| Partial prey consumption | 3 (2) | 0 | 1 | 5 (5) |
| Complete prey consumption | 23 (10) | 3 (3) | 7 (2) | 17 (7) |
Numbers in parentheses are numbers of C. appendiculata which accounted for attacks.
Discussion
Several experimental studies have recently demonstrated that selective predation by C. appendiculata may influence the relative abundances and community structure of larval mosquitoes in treeholes or other container habitats (Griswold and Lounibos 2005a, b, 2006). Both prey size and species contribute to a preference for A. albopictus over A. triseriatus, which is influenced by predator-avoidance behaviors in the latter prey species (Kesavaraju et al. 2007). The current study is the first to quantify consumption rates of small mosquito prey by C. appendiculata, which were shown here to consume approximately 200 first-instar A. albopictus larvae during the third and, especially, fourth instar.
The prepupal timing of surplus killing behavior by C. appendiculata and inverse relationships between numbers of prey consumed and surplus-killed parallels similar patterns in all species of Toxorhynchites so far quantified (Lounibos 1979, Russo 1986). Surplus killing has been studied primarily in the fourth instar of Toxorhynchites spp., in which stage diapausing T. rutilus exhibit surplus killing that is not associated with pupation (Lounibos et al. 1998). Only in T. splendens from the Oriental Region has surplus killing been observed in other instars (Chan 1968).
An attack site relatively specific to surplus killing, by C. appendiculata on the thorax of larval A. albopictus, was not observed in any of five studied species of Toxorhynchites (Russo 1986). However, surplus killing in Toxorhynchites spp. was associated with post-attack wiping of the predator’s mandibles across the aciculae (Russo 1986), a behavior not observable in our recordings of C. appendiculata. The orientation towards motile prey preceding most attacks by C. appendiculata has been examined in detail for two species of Toxorhynchites (Linley 1990), and the incidence of successful attacks was very similar between C. appendiculata (74.8%) and the mean for five Toxorhynchites spp. (74.6%; Russo 1986). However, C. appendiculata appears more likely to strike from a benthic location than Toxorhynchites spp. (Steffan and Evenhuis 1981), and Kitching (2000) regards corethrellids in phytotelmata as ‘detritus-based’ predators in contrast to ‘free-swimming’ Toxorhynchites spp.
All species of Toxorhynchites use treeholes or other phytotelmata as larval habitats (Steffan and Evenhuis 1981), and we believe that the occurrence of surplus killing by container-inhabiting damselfly (Fincke 1994), predatory midge, and mosquito larvae is not merely coincidental. Predation and competition can be especially intense in container habitats (Kitching 2000), and killing without consumption has also been identified among facultative predators of the phyto-telm-inhabiting mosquito tribe Sabethini (e.g., Marti et al. 2007). In the Corethrellidae, phytotelm- and ground water-dwelling clades are divergent (Borkent, personal communication), and we predict that the latter does not surplus-kill.
The predominant attack site for surplus killing on the prey thorax suggests that killing without consumption is adaptive and in C. appendiculata did not evolve directly from ordinary predatory behavior. In his study of predation by five Toxorhynchites spp., Russo (1986) indicated that no prey body region was preferentially attacked and that, behaviorally, surplus killing resembled normal predation. An attack on the thorax, the center of mass of a culicid prey, makes logical sense if the prey is not to be ingested. On the other hand, if prey are to be eaten intact, as corethrellids ordinarily do, attack sites at either the anterior or posterior end would be more efficient, as observed for most consumed prey of C. appendiculata (Table 1). None of the nine partially consumed prey observed on video recordings was attacked on the thorax (Table 1), which confirms the interpretation that partial prey consumption is ordinary predatory behavior in which a portion of the prey item is dropped and, hence, not eaten.
The vulnerable pupal hypothesis of Corbet and Griffiths (1963) attempted to explain surplus killing as a mechanism for destroying potential predators of Toxorhynchites spp. pupae. However, potential predators of Toxorhynchites spp. pupae are most likely to be fourth-instar conspecifics, which were usually not killed in experiments to test this hypothesis (Corbet 1985). Although experiments to determine the vulnerability of C. appendiculata pupae to predation by conspecifics have not been done, the sedentary nature of this stage (Lounibos, unpublished) would seem to make C. appendiculata pupae relatively resistant to attacks by ambush predators.
An alternative rationale for surplus killing, invoked to explain ‘obligate’ killing of conspecifics by damselfly larvae in treeholes (Fincke 1994), is reduction of competition for prey. However, the peak intensity of surplus killing by prepupal C. appendiculata and Toxorhynchites spp. does not fit this hypothesis, since pupae of these dipterous predators do not feed.
A final hypothesis of ‘inhibition’ competition invokes kin selection (Hamilton 1970) and suggests that surplus killing deprives competing predators of food, thus slowing development and increasing cannibalism of earlier instars (Russo 1986). This hypothesis might have merit if individuals in a cohort are related and develop relatively synchronously, e.g., larvae from the same mother reach the prepupal stage about the same time, and their surplus killing deprives a younger cohort (progeny of a different mother) of excess prey. Too little is known at present of the natural history of C. appendiculata to evaluate the merit of this hypothesis.
Acknowledgments
This research was supported in part by NIH grant AI-044793 to LPL and NSF doctoral dissertation improvement grants to BWA (DEB-0407689) and BK (DEB-0507015). We thank R. Escher and N. Nishimura for technical support, A. Borkent for sharing his knowledge of the Corethrellidae, and M. Griswold and S. Yanoviak for critical comments on a previous draft of this paper.
References
- Bradshaw WE, Holzapfel CM. Predator-mediated, non-equilibrium coexistence of tree-hole mosquitoes in southeastern North America. Oecologia. 1983;57:239–256. doi: 10.1007/BF00379586. [DOI] [PubMed] [Google Scholar]
- Chan KL. Observations on Toxorhynchites splendens (Wiedemann) (Diptera:Culicidae) in Singapore. Mosq News. 1968;28:91–95. [Google Scholar]
- Corbet PS. Prepupal killing behavior in Toxorhynchites brevipalpis: a status report. In: Lounibos LP, Rey JR, Frank JH, editors. Ecology of mosquitoes: proceedings of a workshop. Florida Medical Entomology Laboratory; Vero Beach: 1985. pp. 407–417. [Google Scholar]
- Corbet PS. Dragonflies. Behavior and ecology of Odonata. Cornell University Press; Ithaca: 1999. [Google Scholar]
- Corbet PS, Griffiths A. Observations on the aquatic stages of two species of Toxorhynchites (Diptera:Culicidae) in Uganda. Proc R Ent Soc Lond. 1963;38:125–135. [Google Scholar]
- Fincke OM. Population regulation of a tropical damselfly in the larval stage by food limitation, cannibalism, intraguild predation and habitat drying. Oecologia. 1994;100:118–127. doi: 10.1007/BF00317138. [DOI] [PubMed] [Google Scholar]
- Griswold MW, Lounibos LP. Competitive outcomes of aquatic container Diptera depend on predation and resource levels. Ann Entomol Soc Am. 2005a;98:673–681. doi: 10.1603/0013-8746(2005)098[0673:COOACD]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold MW, Lounibos LP. Does differential predation permit invasive and native mosquito larvae to coexist in Florida? Ecol Entomol. 2005b;30:122–127. doi: 10.1111/j.0307-6946.2005.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold MW, Lounibos LP. Predator identity and additive effects in a treehole community. Ecology. 2006;87:987–995. doi: 10.1890/0012-9658(2006)87[987:piaaei]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton WD. Selfish and spiteful behaviour in an evolutionary model. Nature. 1970;228:1218–1220. doi: 10.1038/2281218a0. [DOI] [PubMed] [Google Scholar]
- Johnson DM, Akre BG, Crowley PH. Modeling arthropod predation—wasteful killing by damselfly naiads. Ecology. 1975;56:1081–1093. [Google Scholar]
- Kesavaraju B, Juliano SA. Differential behavioral responses to water-borne cues to predation in two container-dwelling mosquitoes. Ann Entomol Soc Am. 2004;97:194–201. doi: 10.1603/0013-8746(2004)097[0194:dbrtwc]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavaraju B, Alto BW, Lounibos LP, Juliano SA. Behavioural responses of larval container mosquitoes to a size-selective predator. Ecol Entomol. 2007;32:262–272. doi: 10.1111/j.1365-2311.2006.00846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitching RL. Food webs and container habitats. The natural history and ecology of phytotelmata. Cambridge University Press; Cambridge: 2000. [Google Scholar]
- Kruuk H. Surplus killing by carnivores. J Zool. 1972;166:233–244. [Google Scholar]
- Linley JR. The predatory behavior of Toxorhynchites amboinensis and Tx. brevipalpis larvae (Diptera: Culicidae) in response to subsurface prey. Fla Entomol. 1990;73:9–50. [Google Scholar]
- Lounibos LP. Temporal and spatial distribution, growth and predatory behaviour of Toxorhynchites brevipalpis (Diptera: Culicidae) on the Kenya coast. J Anim Ecol. 1979;48:213–236. [Google Scholar]
- Lounibos LP. Interactions influencing production of treehole mosquitoes in south Florida. In: Lounibos LP, Rey JR, Frank JH, editors. Ecology of mosquitoes: proceedings of a workshop. Florida Medical Entomology Laboratory; Vero Beach: 1985. pp. 65–77. [Google Scholar]
- Lounibos LP, Martin EA, Duzak D, Escher RL. Daylength and temperature control of predation, body size, and rate of increase in Toxorhynchites rutilus (Diptera: Culicidae) Ann Entomol Soc Am. 1998;91:308–314. [Google Scholar]
- Lounibos LPO, Meara GF, Escher RL, Nishimura N, Cutwa MM, Nelson T, Campos RE, Juliano SA. Testing predictions of displacement of native Aedes by the invasive Asian Tiger Mosquito Aedes albopictus in Florida, USA. Biol Invas. 2001;3:151–166. [Google Scholar]
- McKeever S. Mouthparts of the four North American Corethrella species (Diptera: Chaoboridae), with detailed study of Corethrella appendiculata. J Med Entomol. 1986;23:502–512. [Google Scholar]
- McKeever S, French FE. Corethrella (Diptera: Corethrellidae) of eastern North-America: laboratory life history and field responses to anuran calls. Ann Entomol Soc Am. 1991a;84:493–497. [Google Scholar]
- McKeever S, French FE. Corethrella (Diptera: Corethrellidae) of North America north of Mexico—distribution and morphology of immature stages. Ann Entomol Soc Am. 1991b;84:522–530. [Google Scholar]
- Marti GA, Micieli MV, Maciá] A, Lounibos LP, Garcia JJ. Seasonality and abundance of the mosquito Isostomyia paraensis from phytotelmata in temperate Argentina. J Am Mosq Cont Assoc. 2007;23:252–258. doi: 10.2987/8756-971X(2007)23[252:SAAOTM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Russo R. Comparison of predatory behavior in five species of Toxorhynchites (Diptera: Culicidae) Ann Entomol Soc Am. 1986;79:715–722. [Google Scholar]
- SAS Institute. SAS/STAT User’s Guide. SAS Institute; Cary, NC, USA: 1989. [Google Scholar]
- Steffan WA, Evenhuis NL. Biology of Toxorhynchites. Ann Rev Entomol. 1981;26:159–181. [Google Scholar]