Abstract
Therapy-related myelodysplastic syndrome and acute myeloid leukemia (t-MDS/t-AML) are thought to be the direct consequence of mutational events induced by chemotherapy, radiation therapy, immunosuppressive therapy, or a combination of these modalities, given for a pre-existing condition. The outcomes for these patients have been poor historically compared to people who develop de novo AML. The spectrum of cytogenetic abnormalities in t-AML is similar to de novo AML, but the frequency of unfavorable cytogenetics, such as a complex karyotype or deletion or loss of chromosomes 5 and/or 7, is considerably higher in t-AML. Survival varies according to cytogenetic risk group in t-AML patients, with better outcomes being observed in those with favorable-risk karyotypes. Treatment recommendations should be based on performance status and karyotype. A deeper understanding of the factors that predispose patients to the development of therapy-related myeloid leukemia would help clinicians monitor patients more carefully after treatment for a primary condition. Ultimately, this knowledge could influence initial treatment strategies with the goal of decreasing the incidence of this serious complication.
Introduction
Therapy-related myeloid leukemia (t-MDS/t-AML) is a well recognized clinical syndrome occurring as a late complication following cytotoxic therapy 1–5. The term “therapy-related” leukemia is descriptive and is based on a patient’s history of exposure to cytotoxic agents. Although a causal relationship is implied, the mechanism remains to be proven. These neoplasms are thought to be the direct consequence of mutational events induced by the prior therapy. Table 1 shows the various primary diagnoses and primary cytotoxic therapies received by 306 patients with therapy-related myeloid leukemia studied at the University of Chicago 4.
Table 1.
Primary diagnoses and primary cytotoxic therapies in 306 patients who developed therapy-related myeloid leukemia: The University of Chicago series 4
| Primary Diagnosis | No. of Patients | Chemotherapy Only (%) | Radiotherapy Only (%) | Combined Modality Therapy (%) |
|---|---|---|---|---|
| No Prior Malignancy | 18 | 12 (67)a | 2 (11) | 4 (22) |
| Hematologic Malignancy | 171 | 69 (40) | 5 (3) | 97 (57) |
| Hodgkin lymphoma | 77 | 18 (23) | 4 (5) | 55 (71) |
| Non-Hodgkin lymphoma | 70 | 33 (47) | 1 (1) | 36 (51) |
| Myeloma | 23 | 17 (74) | 0 | 6 (26) |
| Other | 1 | 1 (100) | 0 | 0 |
| Solid Tumors | 117 | 40 (35) | 36 (32) | 38 (33) |
| Breast | 32b | 11 (35) | 5 (16) | 15 (49) |
| Ovary | 15 | 12 (80) | 1 (7) | 2 (13) |
| Prostate | 13b | 0 | 11 (100) | 0 |
| Lung | 9 | 5 (56) | 2 (22) | 2 (22) |
| Cervix | 7 | 0 | 4 (57) | 3 (43) |
| Other | 41 | 12 (29) | 13 (32) | 16 (39) |
| Totals | 306b | 121 (40) | 43 (14) | 139 (46) |
Numbers in parentheses are percentages for each row of data according to primary therapy.
In 3 patients, the entire chemotherapy regimen used as therapy for the primary tumor was not known.
Neither t-MDS nor t-AML is easily categorized according to the French-American- British (FAB) classification schema, but the World Health Organization (WHO) classification recognizes them as a distinct entity 6. Morphologically, t-MDS/t-AML most closely resembles acute myeloid leukemia with multilineage dysplasia, also a distinct form of de novo AML within the WHO classification. t-AML is distinguished from t-MDS solely based on the blast count being ≥20% in either the peripheral blood or the bone marrow, except for those cases associated with t(8;21) or inv(16) where a blast count <20% is sufficient for diagnosis of acute leukemia. Based on clinical, morphological, and genetic features, t-MDS and t-AML are a single disease, each representing different ends of the leukemic spectrum. The specific morphological and cytogenetic features of t-MDS/t-AML are related to the type of prior cytotoxic therapy that the patient received for his/her primary medical condition.
Subtypes of therapy-related myeloid leukemia
The characteristics of therapy-related leukemia and the timing of its development after a primary diagnosis depend on the exposure to specific agents as well as the cumulative dose and dose intensity of the preceding cytotoxic therapy.
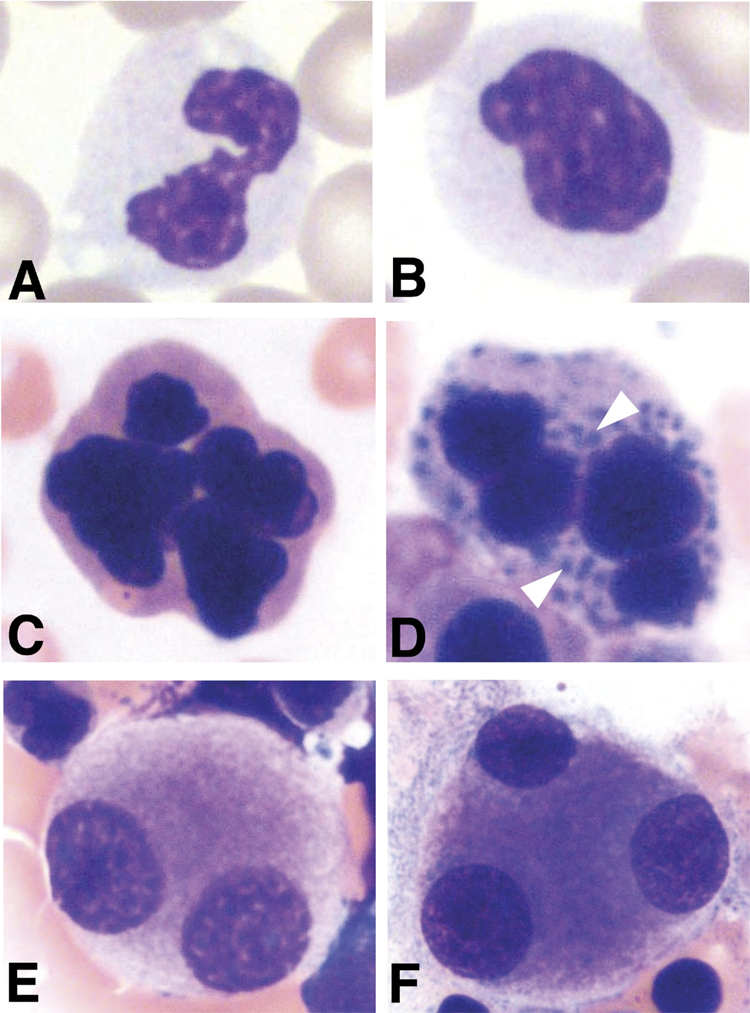
In the classic form of therapy-related leukemia that follows treatment with alkylating agents and/or radiation therapy, the blood and bone marrow findings resemble those seen in primary MDS, although the degree of dysgranulopoiesis and dysmegakaryocytopoiesis is typically greater. Fatigue, weakness, and occasionally fever are the most frequent patient complaints. Anemia, often macrocytic, and thrombocytopenia are extremely common, and an increased mean corpuscular volume (MCV) is often the first clue to the diagnosis. Leukopenia may also be present. Dysplastic changes are often observed in all three cell lines (Figure 1). Mild to marked reticulin fibrosis may be present. Auer rods are rarely seen, and myeloperoxidase and non-specific esterase reactivity are often only weakly expressed.
Figure 1. Characteristic but non-specific cytologic changes are observed in therapy-related myeloid leukemias.
Trilineage dysplasia seen in the peripheral blood and bone marrow from a 38 year old male survivor of Hodgkin disease, who had been treated with chemotherapy, radiation, and an autologous stem cell transplant. The patient was asymptomatic until presentation with a macrocytic anemia and thrombocytopenia, five years after his diagnosis of Hodgkin lymphoma and three years after autologous stem cell transplant. A bone marrow biopsy revealed trilineage dysplasia and fewer than 5% myeloblasts, consistent with a diagnosis of therapy-related myelodysplasia. Karyotype analysis revealed a loss of chromosome 7, consistent with alkylating agent-induced disease. (A–C) Dysplasia in the peripheral blood. (A–B) Dysplastic neutrophils have hypolobated, hypercondensed chromatin and hypogranular cytoplasm. The cell shown in A has a pseudo-Pelger Huet nucleus. (C) A multinucleated abnormal erythroid precursor in the peripheral blood. (D–G) Dysplasia in the bone marrow. (D) An abnormal multinucleated erythroid cell with basophilic stippling (arrowheads). (E–F) Micromegakaryocytes with abnormal separation of nuclear lobes. Photomicrographs courtesy of Dr. Ozden Ozer, The University of Chicago Department of Pathology.
Clonal chromosome abnormalities, often of a complex nature, are identified in most cases of classical therapy-related leukemia 1, 3–5, 7, 8. Loss of part or all of chromosomes 5 and/or 7 are the characteristic findings, and have been reported in over 90% of cases in some series 4. The most common single abnormality is monosomy 7, followed in frequency by deletion of the long arm of chromosome 5 [del(5q)] and by monosomy 5. These same abnormalities are observed in primary MDS and AML de novo, especially in older patients and those with occupational exposure to potential carcinogens such as benzene. Alkylating agents vary in their likelihood of causing the development of therapy-related disease (melphalan>cyclophosphamide) 9, 10, and there is a dose-response relationship between the amount of alkylating agent received and the risk of disease development 1. This form of t-MDS/t-AML typically occurs within 5–7 years after chemotherapy and/or radiotherapy have been given, and confers a poor prognosis.
Therapy-related leukemia following chemotherapy with topoisomerase II inhibitors is characterized by translocations involving chromosome bands 11q23 or 21q22 11. Balanced translocations may involve the MLL gene at chromosome band 11q23, or the PML/RARA genes in the case of therapy-related acute promyelocytic leukemia. Rearrangements of the core binding factor genes AML1 (RUNX1/CBFA2) at chromosome band 21q22 and CBFB at chromosome band 16q22, as well as the NUP98 gene at chromosome band 11p15.5 have also been described. In contrast to alkylating agent-associated t-AML, these leukemias are rarely preceded by t-MDS. They occur with a shorter latency, often within 2–3 years of the first cytotoxic therapy and, in some cases, within 12 months. These t-AMLs often present with rapidly progressive leukemia and high white blood cell counts. Although they also have a poor prognosis overall, they are more responsive to initial remission induction chemotherapy.
Additional studies have indicated that patients treated with the nucleoside analog fludarabine are also at risk for t-AML 12. Of 521 patients treated for chronic lymphocytic lymphoma (CLL) with fludarabine alone or in combination with chlorambucil, six (1.2%) developed t-AML, many of whom also had characteristic abnormalities of chromosomes 5 and 7 13. In another study, eight of 202 CLL patients (4%) treated with fludarabine, mitoxantrone, and dexamethasone developed t-AML, commonly with abnormalities of chromosome 7, between one and five years after therapy 14. Another recent report describes a patient with grade 2 follicular lymphoma who was treated with fludarabine, cyclophosphamide, and rituximab who developed t-AML with a chromosomal translocation involving 11q23 40 months after the initial therapy 15. New compounds for the treatment of CLL include radioimmunotherapeutic agents, such as yttrium-90 ibritumomab tiuxetan and iodine-131 tositumomab, raising concerns about an increased incidence of t-AML after these agents, which add radiation to chemotherapy and might further damage DNA within bone marrow cells. Reassuringly, however, Czuczman and colleagues reported recently that the incidence of t-AML among non-Hodgkin lymphoma patients treated with yttrium-90 ibritumomab tiuxetan was 2.5% at 4.4 years, consistent with the expected incidence of t-AML in a patient population heavily pre-treated with chemotherapy 16.
In addition to cancer patients, t-AML has been seen in patients following treatment with immunosuppressive therapies not previously thought to cause DNA damage directly, particularly in patients who have received solid organ transplants 2. A mechanism for the development of t-AML has been proposed for azathioprine, an immunosuppressant widely used in recipients of organ transplantation, through selection of a mutator phenotype to allow the emergence of AML with abnormalities of chromosomes 5 and 7 2. Thus, a growing body of work suggests that the use of any compound that could damage DNA directly or suppress the immune system’s ability to detect malignant cells could lead to an increased risk of t-AML.
Risk factors for the development of therapy-related leukemias
The etiology and specific factors that predispose to the development of therapy-related leukemia have been difficult to study. It has not yet been possible to determine whether the development of t-MDS/t-AML is a stochastic event, occurring by chance, or whether certain individuals are at higher risk. However, more recent studies have employed newer technology that allows the rapid sequencing of a large number of single nucleotide polymorphisms (SNPs) and suggest that at least a fraction of patients probably have a heritable predisposition to the development of t-AML, such as altered drug metabolism or DNA repair- (reviewed in 17). Widespread identification of underlying preexisting conditions would help inform the choice of initial therapy as well as the screening and counseling of patients at the time of treatment for their primary disease.
We have previously reported that the frequency of the NQO1-187Ser SNP, an inactivating polymorphism, in the NQO1 gene (NAD(P)H:quinone oxidoreductase) is increased among individuals with t-AML 18. Homozygotes may be particularly vulnerable to leukemogenic changes induced by carcinogens, and heterozygotes are at risk for treatment-induced mutation or loss of the remaining wild-type allele in their hematopoietic stem cells. Other polymorphisms involving detoxifying enzymes have also been reported 19. A large Japanese study of patients with AML de novo and t-AML found that the NQO1 polymorphism was more strongly associated with t-AML than polymorphisms in GST-M1, GST-T1 and CYP3A4 20. Furthermore, patients carrying the NQO1-187Ser allele and who had been exposed to chemotherapy had significantly shorter telomeres in their neutrophils and lymphocytes and were more likely to develop clonal hematopoiesis than patients with wild-type NQO1 alleles 21. These findings provide a molecular link between NQO1 genotype and an increased risk of developing t-AML. Bolufer et al examined the prevalence of several genetic polymorphisms and found that the NQO1-187Ser polymorphism conferred a 2-fold increased risk of developing t-AML 22. This risk was increased when the NQO1-187Ser polymorphism was found in combination with CYP1A1*2A and del[GSTT1]. However, the risk of developing t-AML was 18-fold lower among patients lacking all three polymorphisms.
Guillem et al identified a haplotype in MTHFR, the gene encoding methylene tetrahydrofolate reductase, which conferred an increased risk in particular patient populations 23. Two SNPs were included in the haplotype: 677C/T and 1298A/C. An increased risk of developing t-AML was associated with the 677T/1298A haplotype in breast cancer patients and the 677C/1298C haplotype in patients with a primary hematopoietic malignancy.
Several groups have examined the genes encoding components of DNA repair pathways. Although about half of t-AML cases show microsatellite instability, promoter hypermethylation and transcriptional silencing of the hMSH2 and hMLH1 genes are not common events 24. Instead, other genetic alterations of these genes seem to predispose to t-AML. Patients with t-AML who have been previously treated with O(6)-guanine alkylating agents, such as cyclophosphamide and procarbazine, have an increased frequency of a variant C SNP that occurs within an intron splice acceptor of the hMSH2 gene 25. Additionally, two of 13 cases that exhibited microsatellite instability were homozygous for the C allele, a frequency much higher than what was observed in a control population. Furthermore, a variant SNP at position –93 of the hMLH1 promoter was found in 75% of patients with t-AML who had received methylating chemotherapy as part of prior therapy for Hodgkin disease 26. In contrast, this variant SNP was found in only 30% of patients with t-AML who had not been treated previously with methylating agents. In patients who had been treated with a methylating agent, the presence of the variant –93 SNP conferred a significantly increased risk of developing t-AML, with an odds ratio of 5.31.
Genetic pathways and cooperating mutations in the etiology of therapy-related myeloid leukemia
Particular mechanisms of DNA damage that lead either to chromosomal deletions or balanced translocations may underlie the differences in latencies between the two main forms of therapy-related leukemia 1, 27. In the case of chromosomal deletions, one allele of a putative tumor suppressor gene may be inactivated. Before the affected cell would gain a proliferative advantage, however, the second allele might also have to be deleted or mutated. In some cases, the first allele may be inactivated in the germ-line DNA due to a heritable cause. More recent evidence suggests that haploinsufficiency of individual genes such as EGR1 on chromosome 5q may also allow for malignant transformation 28. However, even loss of both alleles of an individual tumor suppressor may not be sufficient to confer a malignant phenotype. As described in the model of colorectal tumorigenesis, multiple tumor suppressor genes or oncogenes may need to be mutated to ultimately transform a cell. This series of genetic changes may require an extended period of time, thus explaining the long latency of alkylator-induced t-AML. In contrast, balanced chromosome translocations result in the activation of cellular oncogenes in a dominant fashion. These rearrangements, such as those involving the MLL gene at 11q23, may yield a fusion gene that acts as a dominant oncogene. Whereas this fusion gene alone may not be sufficient to fully transform a hematopoietic progenitor cell, relatively fewer genetic events may be required to progress to the leukemic phenotype.
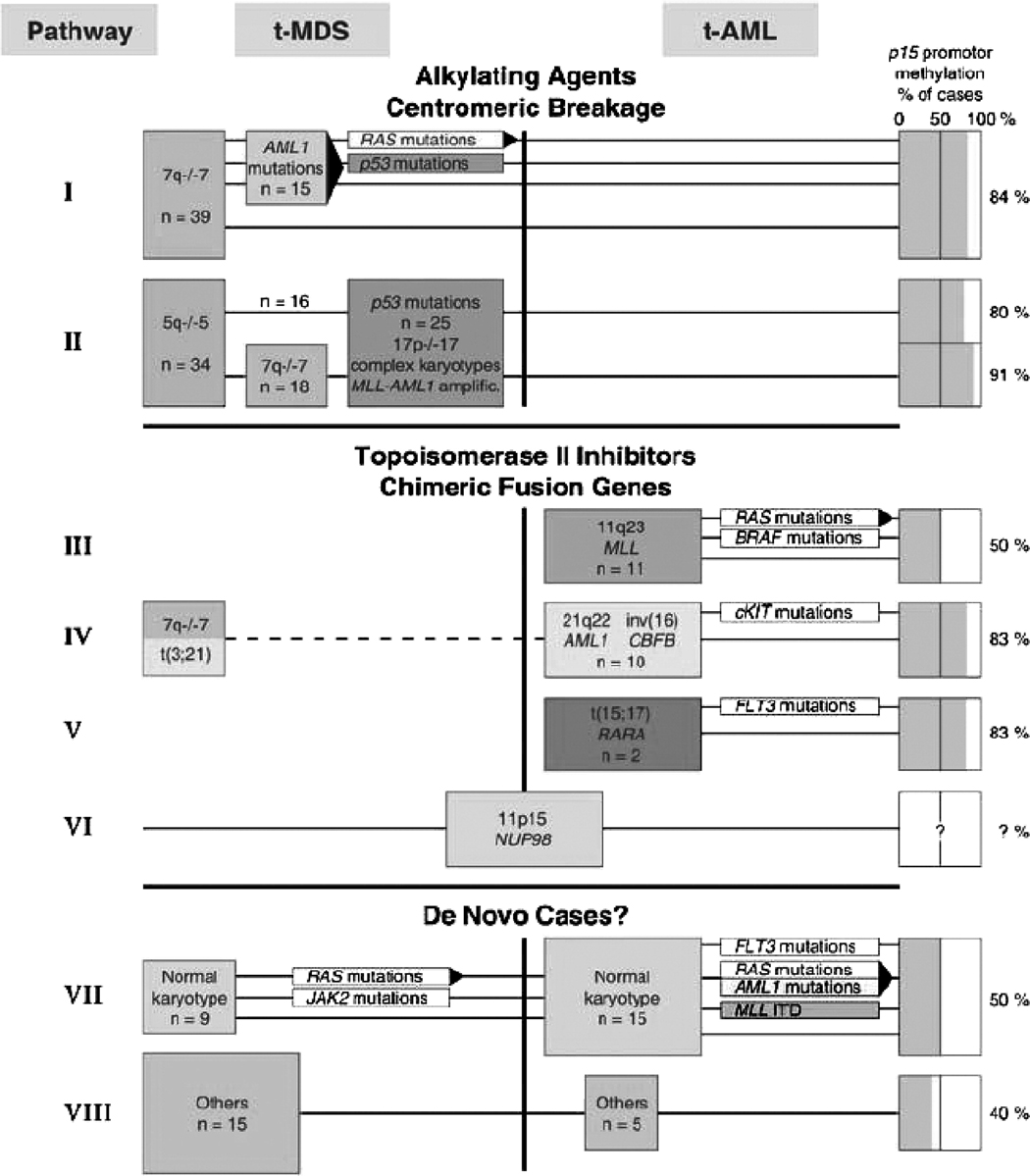
Pedersen-Bjergaard and his colleagues have proposed eight different genetic pathways for the multistep development of t-MDS/t-AML (Figure 2) 29. There is growing evidence that mutations in a limited number of molecular pathways may cooperate in the genesis of leukemia. Gilliland and colleagues have described AML as a disease arising from the cooperation between two types of alterations 30. Changes in signalling molecules deregulate proliferative and/or antiapoptotic activities, for example, through mutations that constitutively activate receptor tyrosine kinases like FLT3. In addition, fusion proteins encoded by recurring chromosomal translocations, such as MLL/AF9 that results from the t(9;11) impair normal differentiation pathways with little effect on cellular proliferation. Gene expression array experiments with CD34-positive t-AML cells have provided evidence to support this hypothesis 31. Loss of TAL1, GATA1, and EKLF expression has been observed, and these might result in impaired differentiation of hematopoietic cells, whereas overexpression of FLT3, PIK3C2B, and BCL2 result in a proliferative and survival advantage.
Figure 2. Genetic pathways identified in 140 cases of t-MDS and t-AML by Pedersen-Bjergaard et al29.
At least eight genetic pathways could be distinguished among 140 cases of therapy-related myeloid malignancies. Pathways I and II are characteristic of patients who had been treated previously with alkylating agents. Mutations of AML1, p53, and RAS are common in patients with abnormalities of 7q or –7. Pathways III–VI are often seen in patients who had received topoisomerase II inhibitors. By definition, any myeloid leukemia arising after prior chemo- or radiotherapy is considered therapy-related. However, because there are few characteristic chromosomal changes or genetic mutations seen in leukemias described by Pathways VII and VIII, these may actually represent de novo cases, or cases in which the underlying genetic defect has not yet been elucidated. RAS mutations are commonly seen in the transition to t-AML within Pathway I, in t-AMLs with MLL rearrangements (Pathway III), and in t-AMLs with a normal karyotype (Pathway VII). Reprinted with permission.29
Additional evidence for the notion that t-AML arises from a combination of mutations comes from studies that identify point mutations plus recurring chromosomal rearrangements. One study examined three cases of t-AML with t(8;21), and two of the patients were also found to be positive for the JAK2 V617F mutation 32. Both patients had received chemotherapy with anthracyclines and topoisomerase inhibitors, one for breast cancer and one for testicular seminoma. Another group examined 140 unselected patients with t-MDS/t-AML and found that two of the 89 t-MDS patients had the JAK2 V617F mutation 33. In these cases, however, both patients had received radiotherapy, presented with myeloproliferative features including splenomegaly, and had a normal karyotype. Notably, these features are unusual for t-MDS. These investigators went on to look for PTPN11 mutations in this patient cohort and found four patients with such mutations; three had deletions or loss of chromosome 7q 34. In addition, all four of them had rare balanced chromosomal translocations: two had breakpoints at 3q26 with rearrangement of EVI1, and two had breakpoints at 21q22 and RUNX1 rearrangements. Such cases provide additional evidence that t-AML arises through multiple genetic events.
Therapy-related leukemia after breast cancer
Although long-term survivors of Hodgkin disease were among the first patients to suffer the development of t-AML, this complication is now increasingly observed in those treated for solid tumors. Several large studies have examined the risks for women receiving adjuvant chemoradiotherapy for breast cancer. Several groups have addressed the question as to whether the addition of G-CSF to routine therapy regimens contributes to the risk of developing t-AML out of the concern that G-CSF could promote the proliferation of damaged cells, which might otherwise undergo apoptosis, and drive the development of t-AML. In six trials completed by the National Surgical Adjuvant Breast and Bowel Project, the incidence of therapy-related leukemia was sharply elevated among patients receiving intensified doses of adriamycin and cyclophosphamide that required G-CSF support; the relative risk (RR) was 6.16 (P=0.0001) 35. Breast radiotherapy alone increased the RR to 2.38 (P=0.006). In a second study, 5510 women over 65 years old who received chemotherapy for stage I–III breast cancer were analyzed 36. Sixteen (1.77%) of the 906 patients who also received G-CSF or GM-CSF developed t-AML compared to 1.04% of those who did not receive myeloid growth factors. The incidence of t-MDS was not evaluated. The hazard rate was 2.59 (95% CI, 1.30 – 5.15) for the development of leukemia within 4 years. Finally, a large case-control study from France compared 182 patients who developed therapy-related leukemia after breast cancer treatment and 534 matched controls 37. The risk of leukemia was markedly increased after chemotherapy that included a topoisomerase-II inhibitor (P<0.0001), and was higher after mitoxantrone (RR=15.6) than after anthracyclines. The risk was increased 3.9–fold after breast radiotherapy (P=0.003). The risk was also increased among those who received G-CSF (RR=6.3, P=0.0009), even when controlling for chemotherapy doses.
Therapy-related leukemia after autologous hematopoietic cell transplantation (HCT)
Several thousand autotransplants are performed each year for patients with relapsed lymphoma and other diseases. Estimates of the incidence of therapy-related leukemia among these lymphoma and Hodgkin disease patients range between 1–14% at 3–15 years 38. The risk appears lower in patients undergoing autologous HCT for breast or germ cell cancers or for myeloma. Important risk factors include age, extent of prior therapy, and exposure to certain agents before and during the transplant procedure. Genotoxic damage and stresses imposed on hematopoietic stem cells during the priming or mobilizing chemotherapy and engraftment are also likely to play a role. As noted above, inherited polymorphisms in genes governing drug metabolism and DNA repair may also contribute to leukemogenesis. Studies of the latency periods between first cytotoxic exposure, the autologous HCT itself, and the emergence of therapy-related leukemia suggest that the initial malignant event occurs prior to HCT in most cases 4, 38. However, the cytotoxic therapy delivered during the multi-step HCT procedure is likely additive to previous genomic damage and contributes to the etiology by cooperating mutations and selective pressure.
Factors that influence outcome in t-AML
Therapy-related myeloid leukemia is generally a fatal disease. The life-threatening complications of this disorder are the result of persistent and profound cytopenias due to the failure of normal hematopoiesis regardless of the fraction of myeloblasts accumulating in the bone marrow or blood. There has been general agreement that patients with t-AML have shorter survivals than patients with de novo AML. Supportive care is still considered by many to be the standard management.
A number of potential factors explain the poor outcome of patients with therapy-related leukemia. The persistence of the primary malignant disease, particularly metastatic cancer or lymphoma, causes morbidity and mortality independent of the bone marrow failure caused by leukemia. Injury to organs and their vascular supply from prior treatment may compromise the ability of these patients to receive intensive remission induction chemotherapy or bone marrow transplantation. There may be depletion of normal hematopoietic stem cells as a consequence of previous therapy, so that these patients suffer prolonged cytopenias after induction chemotherapy. The bone marrow stroma may have been damaged, especially by therapeutic radiation to fields that include the pelvis or lumbosacral spine, so that it will not support regeneration of normal hematopoiesis. Patients with t-AML are often chronically immunosuppressed from prior disease or on-going therapy or may have dysfunctional phagocytes, and thus are often colonized with pathogenic or antibiotic-resistant bacteria and fungi. Following prior supportive care, patients may be refractory to additional transfusion support, and therefore, not good candidates for intensive myelosuppressive chemotherapy. Finally, the high frequency of unfavorable cytogenetic aberrations arising during or after chemoradiotherapy appears to result in the rapid emergence of chemotherapy resistance in t-AML stem cells.
Treatment of therapy-related myeloid leukemia
The survival of patients with therapy-related leukemia is often poor despite prompt diagnosis and treatment. However, there is a paucity of prospective treatment data since these patients are most often excluded from frontline clinical trials. There are no randomized studies comparing standard AML therapy to other forms of treatment. In a nationwide Japanese study of 256 patients with t-MDS (41%) or t-AML (59%), a poor prognosis was associated with abnormalities of chromosome 5, hypoproteinemia, high C-reactive protein, thrombocytopenia, and persistence of the primary malignancy 39. The majority of the Japanese patients (72%) received antileukemia chemotherapy, either a standard combination using an anthracycline plus cytarabine, or low dose cytarabine, or tretinoin (ATRA) in the case of 7 patients with therapy-related acute promyelocytic leukemia (t-APL). The median age was 61 years, and the median survival was only 9.7 months. A complete remission (CR) was seen in 85 patients (46%). The median remission duration was 8.2 months.
Poor hematopoietic reserves make the administration of standard AML therapy difficult. Many patients have poor tolerance for the acute toxicity of treatment. Because therapy-related leukemia evolves in the milieu of chemotherapy, the malignant cells are relatively drug-resistant. Expression of the multidrug resistance phenotype is common. In a review of 644 t-AML patients treated with a variety of standard AML chemotherapy regimens, only 182 (28%) achieved a CR 40. Individual small series report CR rates of 40–50%. This is considerably lower than the 55–80% CR rate observed in patients with de novo AML. In addition, remissions are often short even when confirmed cytogenetically and consolidated intensively 41.
Hematopoietic cell transplantation for t-AML
The treatment most likely to cure t-AML is allogeneic HCT. Several small case series have described the outcomes of these patients, and the survival appears to be about 20–30% 1,42. However, chronic and cumulative toxicities from prior chemoradiotherapy impact on the ability to perform HCT and adversely affect survival. Early deaths from regimen-related toxicity are more common after HCT for therapy-related leukemia than for primary AML.
In an analysis of 70 patients (31 with t-MDS and 39 with t-AML) who underwent allogeneic HCT between 1980–1998 in France, poor outcomes were associated with age greater than 37 years, male sex, positive cytomegalovirus serology in the recipient, absence of CR at the time of HCT, and the use of intensive conditioning chemotherapy 43. The treatments given were heterogeneous, and the donors were varied. The estimated 2-year survival rate was 30%, event-free survival rate 28%, relapse rate 42%, and transplant-related mortality 49%. Thus, for patients who have chemotherapy-responsive t-AML, allogeneic HCT can be curative, but it is unfortunately not often successful. Nonmyeloablative, reduced intensity allogeneic HCT is under investigation for those who are not eligible for standard myeloablative HCT.
Similar results have been seen in children who have undergone allogeneic HCT for t-AML developing after therapy for acute lymphoblastic leukemia (ALL). Hale et al reported the outcomes of 21 children who had received epipodophyllotoxin-containing regimens for ALL and subsequently developed t-AML 44. Thirteen received induction chemotherapy prior to HCT, whereas seven underwent HCT immediately after diagnosis. One patient received an autologous HCT in first CR from t-AML, but later relapsed, and was subsequently treated at second relapse with an allogeneic HCT. Eleven patients received bone marrow cells from HLA-matched siblings, eight received bone marrow cells from matched unrelated donors, and 2 received haploidentical marrow from family members. Three years after HCT, only 4 patients (19%) were alive. Seven patients died from transplant-related causes, and 10 patients died from relapsed t-AML after a median of 5 months.
The European Bone Marrow Transplant registry has reported on 65 t-AML patients who underwent autologous HCT 45. The median age was 39 years (range, 3–69). Estimates of overall and disease-free survival at 3 years were 35% and 32%, respectively. The relapse rate was lower for patients transplanted in first CR (48% vs 89%, P=0.05). Age over 40 years resulted in higher transplant-related mortality (47% vs 7%, P=0.01).
Treatment of t-AML with balanced chromosomal rearrangements
In marked contrast to the poor outcome overall for t-AML, those patients who develop t-APL with t(15;17) or those with t(8;21) or inv(16) have response rates that are similar to patients with de novo AML with the same chromosomal rearrangements, although overall survival is less. In a report on 106 cases of t-APL identified between 1982–2001 in France, Spain, and Belgium, the characteristics of the t-APL patients were similar to those of de novo APL 46. In addition, more than 80% of those treated with anthracycline-based chemotherapy and/or ATRA achieved a CR. Ten of the complete responders relapsed, and 7 others died from persistent primary tumor. The actuarial survival was 58% at 8 years, and did not differ between patient groups analyzed by primary treatment (chemotherapy, radiotherapy, or both) or prior exposure to particular drugs (alklyating agents, topoisomerase-II inhibitors, or both).
Among patients analyzed at the International Workshop in Chicago in 2000, 33 of 39 intensively treated patients (85%) with t-AML and inv(16) achieved a CR 47. Twelve (36%) subsequently relapsed. Five underwent HCT in first CR (4 allogeneic; 1 autologous), and all of them were alive and leukemia-free at last follow-up. The responding patients were significantly younger than those who did not achieve CR (median, 44 years vs 62 years, P=0.012). Patients younger than 55 years of age had improved survival when compared to older patients. The median survival in the young patient group (n=26) was not reached and was longer than 3 years, but was only 12 months for the 13 older patients (P=0.006). Similarly, 24 of 35 (69%) patients treated aggressively for therapy-related APL achieved a CR. Development of both inv(16) t- AML and t-APL was associated with prior exposure to topoisomerase-II inhibitors. However, 21% of the inv(16) patients and 29% of the t(15;17) patients had received only radiotherapy previously. The median overall survival for t-AML patients with either inv(16) or t(15;17) was 29 months after receiving intensive AML therapy.
Seventy-two t-AML patients with any t(21q22) were also studied at the International Workshop 48. Their median survival was 14 months, and 18% were alive after 5 years. Patients with t(8;21) had a more favorable outcome than those with other 21q22 rearrangements (p=0.014). Survival was similar in t-AML patients with only t(8;21) (n=11) and those (n=3) with t(8;21) plus other abnormalities (P=0.6). Fifty-three patients with t(21q22) received intensive AML therapy; the median survival for the seven who underwent HCT was 31 months compared to 17 months for those who did not. Mutations in C-KIT were not studied in these patients.
The importance of cytogenetic abnormalities in predicting patient outcomes
The most informative data on the prognostic impact of karyotype on outcome in t-AML were reported by the German AML Cooperative Group (AMLCG) 49. This group compared karyotype analysis and survival between 93 patients with t-AML and 1091 with de novo AML, all of whom received intensive treatment. Favorable, intermediate, and unfavorable karyotypes were observed in 26%, 28%, and 46% of t-AML patients, and in 22%, 57%, and 20% of de novo AML patients. Overall, the median survival was 10 months for patients with t-AML compared to 15 months for patients with de novo AML (P=0.0007).
Armand et al also analyzed the outcomes of 80 patients with therapy-related disease treated at the Dana Farber Cancer Institute 50. They found that cytogenetic abnormalities were the strongest predictors for overall survival. When adjusted for patients' cytogenetic changes, the patients with t-AML did as well as patients with de novo AML, further emphasizing the importance of cytogenetic abnormalities in predicting severity of disease and outcomes.
At the University of Chicago, 306 consecutive patients with t-AML were analyzed for clinical outcome according to cytogenetic subset as well as other clinical features, including disease latency 4. In contrast to the German series, not all of our patients underwent intensive remission induction chemotherapy. Many received only supportive care. Survival times are shown in Table 2. Only 24 patients (8%) were alive 3 years after diagnosis. Patients with t-AML who responded to remission induction therapy but subsequently died from their primary malignancy were included in the survival analysis. Even patients with a normal karyotype or with a balanced chromosomal rearrangement did poorly overall. The incidence of unfavorable karyotypes was greater than 70%. The patients who had the worst overall survival were those patients with abnormalities of both chromosomes 5 and 7 (P=0.005).
Table 2.
Survival of 306 patients with therapy-related myeloid leukemia according to clinical and cytogenetic features: The University of Chicago series 4
| Clinical/cytogenetic subset* | No. of patients (%) | Median Survival, months (95% confidence interval) |
|---|---|---|
| Total group | 306 | 8 (7–9) |
| Presenting as t-MDS | 224 (73) | 8.6 (7.6–9.9) |
| Presenting as t-AML | 82 (27) | 6.9 (4.0–8.5) |
| Abnormal chromosome 5 | 63 (21) | 7 |
| Abnormal chromosome 7 | 85 (28) | 9 |
| Abnormalities of both chromosomes 5 and 7 | 66 (22) | 5 |
| Recurring balanced rearrangement | 31 (10) | 11 |
| Other clonal abnormality | 39 (13) | 9 |
| Normal karyotype | 24 (8) | 11 |
139/306 patients (45%) had complex karyotypes, i.e. 3 or more unrelated chromosomal abnormalities.
In an updated analysis of the German AMLCG study, the survival of 121 patients with t-AML was compared to 1511 patients with de novo AML according to karyotype 51. All received intensive AML therapy. The median survival for the t-AML patients ranged from 27 months for those with a favorable karyotype to 6 months for those with an unfavorable karyotype (Table 3, data taken from 51). Importantly, almost half of the patients with t-AML (58/121) had an unfavorable karyotype, whereas only about 20% (302/1511) of the de novo AML patients had an unfavorable karyotype. For those with a favorable karyotype, the median survival was not yet reached after 5 years for the 306 de novo AML patients compared to 27 months for the 29 t-AML patients (P=0.02). Some of these t-AML patients appeared to be cured. Within the large intermediate risk cytogenetic group, no significant difference in survival was observed between the t-AML and de novo AML patients. An unfavorable karyotype predicated a very short survival in both groups of AML patients.
Table 3.
Survival according to cytogenetic risk group for patients with t-AML or de novo AML treated by the German AML Cooperative Group (AMLCG)51
| Karyotype | No. of patients (%) | Median survival (months) | |||
|---|---|---|---|---|---|
| t-AML (n=121) | de novo AML (n=1511) | t-AML | de novo AML | P | |
| Favorable | 29 (24) | 306 (20) | 27 | Not reached | 0.02 |
| Intermediate | 34 (28) | 903 (60) | 12 | 16 | 0.19 |
| Unfavorable | 58 (48) | 302 (20) | 6 | 7 | 0.006 |
Recommendations for treatment of t-AML
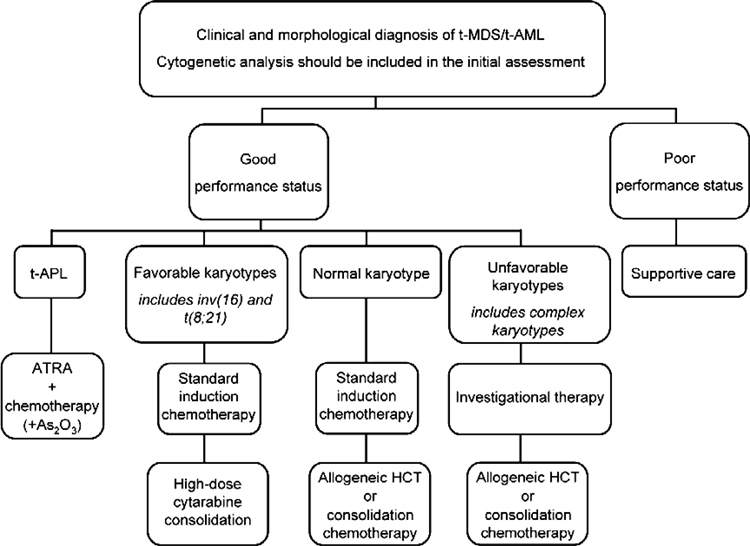
Figure 3 shows a treatment algorithm for the management of patients who develop therapy-related myeloid leukemia. Primary considerations are the patient’s performance status, which reflects age, co-morbidities, the status of the primary disease, and the presence of complications from primary therapy, as well as the clonal abnormalities detected in the t-AML cells. In general, t-AML patients should be encouraged to participate in prospective clinical trials that are appropriately designed for other AML patients with similar cytogenetic abnormalities. Patients who have an HLA-matched donor should be considered for allogeneic HCT, although patients with favorable karyotypes may do well with conventional intensive chemotherapy.
Figure 3. Decision tree for the management of t-AML.
Acknowledgments
Supported in part by grants CA40046 and CA14599 from the National Cancer Institute, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There no relevant conflicts of interest for either author.
References
- 1.Godley LA, Larson RA. The syndrome of therapy-related myelodysplasia and myeloid leukemia. In: Bennett JM, editor. The myelodysplastic syndromes: pathobiology and clinical management. New York: Marcel Dekker, Inc.; 2002. pp. 139–176. [Google Scholar]
- 2.Offman J, Opelz G, Doehler B, et al. Defective DNA mismatch repair in acute myeloid leukemia/myelodysplastic syndrome after organ transplantation. Blood. 2004;104:822–828. doi: 10.1182/blood-2003-11-3938. [DOI] [PubMed] [Google Scholar]
- 3.Rowley JD, Olney HJ. International workshop on the relationship of prior therapy to balanced chromosome aberrations in therapy-related myelodysplastic syndromes and acute leukemia: overview report. Genes Chromosomes Cancer. 2002;33:331–345. doi: 10.1002/gcc.10040. [DOI] [PubMed] [Google Scholar]
- 4.Smith SM, Le Beau MM, Huo D, et al. Clinical-cytogenetic associations in 306 patients with therapy-related myelodysplasia and myeloid leukemia: the University of Chicago series. Blood. 2003;102:43–52. doi: 10.1182/blood-2002-11-3343. [DOI] [PubMed] [Google Scholar]
- 5.Brunning RD, Matutes E, Flandrin G, et al. Acute myeloid leukaemias and myelodysplastic syndromes, therapy related. In: Jaffe ES, Harris NL, Stein H, et al., editors. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2001. pp. 89–91. [Google Scholar]
- 6.Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292–2302. doi: 10.1182/blood-2002-04-1199. [DOI] [PubMed] [Google Scholar]
- 7.Le Beau MM, Espinosa R, 3rd, Davis EM, et al. Cytogenetic and molecular delineation of a region of chromosome 7 commonly deleted in malignant myeloid diseases. Blood. 1996;88:1930–1935. [PubMed] [Google Scholar]
- 8.Zhao N, Stoffel A, Wang PW, et al. Molecular delineation of the smallest commonly deleted region of chromosome 5 in malignant myeloid diseases to 1–1.5 Mb and preparation of a PAC-based physical map. Proc Natl Acad Sci U S A. 1997;94:6948–6953. doi: 10.1073/pnas.94.13.6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curtis RE, Boice JD, Jr, Stovall M, et al. Risk of leukemia after chemotherapy and radiation treatment for breast cancer. N Engl J Med. 1992;326:1745–1751. doi: 10.1056/NEJM199206253262605. [DOI] [PubMed] [Google Scholar]
- 10.Greene MH, Harris EL, Gershenson DM, et al. Melphalan may be a more potent leukemogen than cyclophosphamide. Ann Intern Med. 1986;105:360–367. doi: 10.7326/0003-4819-105-3-360. [DOI] [PubMed] [Google Scholar]
- 11.Olney HJ, Mitelman F, Johansson B, et al. Unique balanced chromosome abnormalities in treatment-related myelodysplastic syndromes and acute myeloid leukemia: report from an international workshop. Genes Chromosomes Cancer. 2002;33:413–423. doi: 10.1002/gcc.10045. [DOI] [PubMed] [Google Scholar]
- 12.Coso D, Costello R, Cohen-Valensi R, et al. Acute myeloid leukemia and myelodysplasia in patients with chronic lymphocytic leukemia receiving fludarabine as initial therapy. Ann Oncol. 1999;10:362–363. doi: 10.1023/a:1008397226387. [DOI] [PubMed] [Google Scholar]
- 13.Morrison VA, Rai KR, Peterson BL, et al. Therapy-related myeloid leukemias are observed in patients with chronic lymphocytic leukemia after treatment with fludarabine and chlorambucil: results of an intergroup study, cancer and leukemia group B 9011. J Clin Oncol. 2002;20:3878–3884. doi: 10.1200/JCO.2002.08.128. [DOI] [PubMed] [Google Scholar]
- 14.McLaughlin P, Estey E, Glassman A, et al. Myelodysplasia and acute myeloid leukemia following therapy for indolent lymphoma with fludarabine, mitoxantrone, and dexamethasone (FND) plus rituximab and interferon alpha. Blood. 2005;105:4573–4575. doi: 10.1182/blood-2004-08-3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin-Salces M, Canales MA, de Paz R, et al. Treatment-related acute myeloid leukemia with 11q23 translocation following treatment with fludarabine, cyclophosphamide and rituximab. Leuk Res. 2007 doi: 10.1016/j.leukres.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 16.Czuczman MS, Emmanouilides C, Darif M, et al. Treatment-related myelodysplastic syndrome and acute myelogenous leukemia in patients treated with ibritumomab tiuxetan radioimmunotherapy. J Clin Oncol. 2007;25:4285–4292. doi: 10.1200/JCO.2006.09.2882. [DOI] [PubMed] [Google Scholar]
- 17.Seedhouse C, Russell N. Advances in the understanding of susceptibility to treatment-related acute myeloid leukaemia. Br J Haematol. 2007;137:513–529. doi: 10.1111/j.1365-2141.2007.06613.x. [DOI] [PubMed] [Google Scholar]
- 18.Larson RA, Wang Y, Banerjee M, et al. Prevalence of the inactivating 609C--> T polymorphism in the NAD(P)H:quinone oxidoreductase (NQO1) gene in patients with primary and therapy-related myeloid leukemia. Blood. 1999;94:803–807. [PubMed] [Google Scholar]
- 19.Allan JM, Wild CP, Rollinson S, et al. Polymorphism in glutathione S-transferase P1 is associated with susceptibility to chemotherapy-induced leukemia. Proc Natl Acad Sci U S A. 2001;98:11592–11597. doi: 10.1073/pnas.191211198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naoe T, Takeyama K, Yokozawa T, et al. Analysis of genetic polymorphism in NQO1, GST-M1, GST-T1, and CYP3A4 in 469 Japanese patients with therapy-related leukemia/myelodysplastic syndrome and de novo acute myeloid leukemia. Clin Cancer Res. 2000;6:4091–4095. [PubMed] [Google Scholar]
- 21.Fern L, Pallis M, Ian Carter G, et al. Clonal haemopoiesis may occur after conventional chemotherapy and is associated with accelerated telomere shortening and defects in the NQO1 pathway; possible mechanisms leading to an increased risk of t-AML/MDS. Br J Haematol. 2004;126:63–71. doi: 10.1111/j.1365-2141.2004.05006.x. [DOI] [PubMed] [Google Scholar]
- 22.Bolufer P, Collado M, Barragan E, et al. Profile of polymorphisms of drug-metabolising enzymes and the risk of therapy-related leukaemia. Br J Haematol. 2007;136:590–596. doi: 10.1111/j.1365-2141.2006.06469.x. [DOI] [PubMed] [Google Scholar]
- 23.Guillem VM, Collado M, Terol MJ, et al. Role of MTHFR (677, 1298) haplotype in the risk of developing secondary leukemia after treatment of breast cancer and hematological malignancies. Leukemia. 2007;21:1413–1422. doi: 10.1038/sj.leu.2404709. [DOI] [PubMed] [Google Scholar]
- 24.Sheikhha MH, Tobal K, Liu Yin JA. High level of microsatellite instability but not hypermethylation of mismatch repair genes in therapy-related and secondary acute myeloid leukaemia and myelodysplastic syndrome. Br J Haematol. 2002;117:359–365. doi: 10.1046/j.1365-2141.2002.03458.x. [DOI] [PubMed] [Google Scholar]
- 25.Worrillow LJ, Travis LB, Smith AG, et al. An intron splice acceptor polymorphism in hMSH2 and risk of leukemia after treatment with chemotherapeutic alkylating agents. Clin Cancer Res. 2003;9:3012–3020. [PubMed] [Google Scholar]
- 26.Worrillow L, Smith A, Scott K, et al. Polymorphic MLH1 and risk of cancer after methylating chemotherapy for Hodgkin lymphoma. J Med Genet. 2007 doi: 10.1136/jmg.2007.053850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fenske TS, McMahon C, Edwin D, et al. Identification of candidate alkylator-induced cancer susceptibility genes by whole genome scanning in mice. Cancer Res. 2006;66:5029–5038. doi: 10.1158/0008-5472.CAN-05-3404. [DOI] [PubMed] [Google Scholar]
- 28.Joslin JM, Fernald AA, Tennant TR, et al. Haploinsufficiency of EGR1, a candidate gene in the del(5q), leads to the development of myeloid disorders. Blood. 2007;110:719–726. doi: 10.1182/blood-2007-01-068809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pedersen-Bjergaard J, Christiansen DH, Desta F, et al. Alternative genetic pathways and cooperating genetic abnormalities in the pathogenesis of therapy-related myelodysplasia and acute myeloid leukemia. Leukemia. 2006;20:1943–1949. doi: 10.1038/sj.leu.2404381. [DOI] [PubMed] [Google Scholar]
- 30.Gilliland DG, Jordan CT, Felix CA. The molecular basis of leukemia. Hematology Am Soc Hematol Educ Program. 2004:80–97. doi: 10.1182/asheducation-2004.1.80. [DOI] [PubMed] [Google Scholar]
- 31.Qian Z, Fernald AA, Godley LA, et al. Expression profiling of CD34+ hematopoietic stem/ progenitor cells reveals distinct subtypes of therapy-related acute myeloid leukemia. Proc Natl Acad Sci U S A. 2002;99:14925–14930. doi: 10.1073/pnas.222491799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schnittger S, Bacher U, Kern W, et al. JAK2 seems to be a typical cooperating mutation in therapy-related t(8;21)/ AML1-ETO-positive AML. Leukemia. 2007;21:183–184. doi: 10.1038/sj.leu.2404465. [DOI] [PubMed] [Google Scholar]
- 33.Desta F, Christiansen DH, Andersen MK, et al. Activating mutations of JAK2V617F are uncommon in t-MDS and t-AML and are only observed in atypic cases. Leukemia. 2006;20:547–548. doi: 10.1038/sj.leu.2404072. [DOI] [PubMed] [Google Scholar]
- 34.Christiansen DH, Desta F, Andersen MK, et al. Mutations of the PTPN11 gene in therapy-related MDS and AML with rare balanced chromosome translocations. Genes Chromosomes Cancer. 2007;46:517–521. doi: 10.1002/gcc.20426. [DOI] [PubMed] [Google Scholar]
- 35.Smith RE, Bryant J, DeCillis A, et al. Acute myeloid leukemia and myelodysplastic syndrome after doxorubicin-cyclophosphamide adjuvant therapy for operable breast cancer: the National Surgical Adjuvant Breast and Bowel Project Experience. J Clin Oncol. 2003;21:1195–1204. doi: 10.1200/JCO.2003.03.114. [DOI] [PubMed] [Google Scholar]
- 36.Hershman D, Neugut AI, Jacobson JS, et al. Acute myeloid leukemia or myelodysplastic syndrome following use of granulocyte colony-stimulating factors during breast cancer adjuvant chemotherapy. J Natl Cancer Inst. 2007;99:196–205. doi: 10.1093/jnci/djk028. [DOI] [PubMed] [Google Scholar]
- 37.Le Deley MC, Suzan F, Cutuli B, et al. Anthracyclines, mitoxantrone, radiotherapy, and granulocyte colony-stimulating factor: risk factors for leukemia and myelodysplastic syndrome after breast cancer. J Clin Oncol. 2007;25:292–300. doi: 10.1200/JCO.2006.05.9048. [DOI] [PubMed] [Google Scholar]
- 38.Hake CR, Graubert TA, Fenske TS. Does autologous transplantation directly increase the risk of secondary leukemia in lymphoma patients? Bone Marrow Transplant. 2007;39:59–70. doi: 10.1038/sj.bmt.1705547. [DOI] [PubMed] [Google Scholar]
- 39.Takeyama K, Seto M, Uike N, et al. Therapy-related leukemia and myelodysplastic syndrome: a large-scale Japanese study of clinical and cytogenetic features as well as prognostic factors. Int J Hematol. 2000;71:144–152. [PubMed] [Google Scholar]
- 40.Kantarjian HM, Estey EH, Keating MJ. Treatment of therapy-related leukemia and myelodysplastic syndrome. Hematol Oncol Clin North Am. 1993;7:81–107. [PubMed] [Google Scholar]
- 41.Larson RA, Wernli M, Le Beau MM, et al. Short remission durations in therapy-related leukemia despite cytogenetic complete responses to high-dose cytarabine. Blood. 1988;72:1333–1339. [PubMed] [Google Scholar]
- 42.Anderson JE, Gooley TA, Schoch G, et al. Stem cell transplantation for secondary acute myeloid leukemia: evaluation of transplantation as initial therapy or following induction chemotherapy. Blood. 1997;89:2578–2585. [PubMed] [Google Scholar]
- 43.Yakoub-Agha I, de La Salmoniere P, Ribaud P, et al. Allogeneic bone marrow transplantation for therapy-related myelodysplastic syndrome and acute myeloid leukemia: a long-term study of 70 patients-report of the French society of bone marrow transplantation. J Clin Oncol. 2000;18:963–971. doi: 10.1200/JCO.2000.18.5.963. [DOI] [PubMed] [Google Scholar]
- 44.Hale GA, Heslop HE, Bowman LC, et al. Bone marrow transplantation for therapy-induced acute myeloid leukemia in children with previous lymphoid malignancies. Bone Marrow Transplant. 1999;24:735–739. doi: 10.1038/sj.bmt.1701962. [DOI] [PubMed] [Google Scholar]
- 45.Kroger N, Brand R, van Biezen A, et al. Autologous stem cell transplantation for therapy-related acute myeloid leukemia and myelodysplastic syndrome. Bone Marrow Transplant. 2006;37:183–189. doi: 10.1038/sj.bmt.1705226. [DOI] [PubMed] [Google Scholar]
- 46.Beaumont M, Sanz M, Carli PM, et al. Therapy-related acute promyelocytic leukemia. J Clin Oncol. 2003;21:2123–2137. doi: 10.1200/JCO.2003.09.072. [DOI] [PubMed] [Google Scholar]
- 47.Andersen MK, Larson RA, Mauritzson N, et al. Balanced chromosome abnormalities inv(16) and t(15;17) in therapy-related myelodysplastic syndromes and acute leukemia: report from an international workshop. Genes Chromosomes Cancer. 2002;33:395–400. doi: 10.1002/gcc.10043. [DOI] [PubMed] [Google Scholar]
- 48.Slovak ML, Bedell V, Popplewell L, et al. 21q22 balanced chromosome aberrations in therapy-related hematopoietic disorders: report from an international workshop. Genes Chromosomes Cancer. 2002;33:379–394. doi: 10.1002/gcc.10042. [DOI] [PubMed] [Google Scholar]
- 49.Schoch C, Kern W, Schnittger S, et al. Karyotype is an independent prognostic parameter in therapy-related acute myeloid leukemia (t-AML): an analysis of 93 patients with t-AML in comparison to 1091 patients with de novo AML. Leukemia. 2004;18:120–125. doi: 10.1038/sj.leu.2403187. [DOI] [PubMed] [Google Scholar]
- 50.Armand P, Kim HT, DeAngelo DJ, et al. Impact of cytogenetics on outcome of de novo and therapy-related AML and MDS after allogeneic transplantation. Biol Blood Marrow Transplant. 2007;13:655–664. doi: 10.1016/j.bbmt.2007.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kern W, Haferlach T, Schnittger S, et al. Prognosis in therapy-related acute myeloid leukemia and impact of karyotype. J Clin Oncol. 2004;22:2510–2511. doi: 10.1200/JCO.2004.99.301. [DOI] [PubMed] [Google Scholar]