Abstract
The elimination kinetics of polychlorinated biphenyls (PCBs) in humans is difficult to assess in observational studies, because PCB exposure is never completely abolished. In a community with high dietary PCB exposures from whale blubber, we examined two groups of children with increased body burdens from breast-feeding. Follow-up was from ages 4.5 years to 7.5 years (99 subjects) and 7 to 14 years (101 subjects). The calculations were performed by the use of structural equation models, with adjustment for body weight and dietary blubber intake as the main source of postnatal exposure. As a likely result of background exposures, apparent elimination half-lives were unexpectedly long when based on results from all cohort members. Subjects with exposures above the median and in the highest quartile showed half-lives of about 3-4 years for CB-138, and 4.5-5.5 years for CB-105 and CB-118; 6.5-7.5 years for CB-156, CB-170, and CB-187; and 7-9 years for CB-153 and CB-180. The longest half-lives correspond to elimination of the parent PCB solely with a daily fat excretion rate of 1-2 g, while shorter half-lives assume metabolic break-down.
Keywords: Biomonitoring, dietary intake, environmental exposure, polychlorinated biphenyls
Introduction
Human studies of exposure to polychlorinated biphenyls (PCBs) often include measurement of serum PCB concentrations as dose estimates. Because the stability of individual PCB congener concentrations over time depends upon the toxicokinetic fates, including elimination half-lives (1), the congener concentrations present in a serum sample at the time of clinical assessment may or may not represent the causative substances and their amounts at the time of toxic impact (2).
Elimination is thought to follow an approximate first-order process, and biological half-lives have been calculated for several congeners in laboratory animals (3-5). Food-mediated human exposures mainly involve persistent congeners, and the ‘weathered’ congener pattern differs substantially from the PCB products originally released into the environment. Thus, more highly chlorinated congeners, such as the hexachlorinated CB-153 and CB-180, are particularly prevalent congeners in food as well as in human serum.
Human data on PCB half-lives are based mainly on studies of small numbers of subjects with high exposures followed over time after cessation of exposure from a poisoning incident (6, 7) or after occupational exposure (8, 9). Reports of unusually short half-lives may reflect distribution processes, rather than elimination alone. Other studies have reported very long elimination half-lives, although assumptions may not be fulfilled that PCB exposure was negligible after cessation of a particular source, thereby disregarding continuing background exposures. The most carefully controlled study (10) was based on only three highly-exposed subjects.
PCB exposures during development are of particular concern, because accumulation of PCBs from prenatal exposure and breastfeeding can be substantial in comparison with subsequent dietary exposure levels during childhood and adolescence. Thus, a breastfed infant's daily dose on a body weight basis may be 100-fold higher than the mother's (11,12). An additional factor is that metabolic breakdown of PCBs may be less effective in infants (13). On the other hand, early growth will expand the lipid compartment volume, in which the PCBs are distributed, thereby leading to a decrease in serum concentrations (14). However, PCBs sequestered into fat tissue may be unavailable for metabolic elimination processes, thereby prolonging the elimination half-lives (15). For congeners not amenable to break-down, elimination primarily occurs via fecal lipid excretion (16). Elimination of fecal lipids may be greater in infants relative to their body weight, thus resulting in shorter half-lives of persistent lipophilic chemicals (16). The overall effect of these parameters on PCB half-lives in children is poorly known.
In the Faroe Islands, the main route of PCB exposure is the consumption of pilot whale blubber, which forms part of traditional diets. With average PCB concentrations in human milk fat of 1-2 μg/g lipid, the daily PCB intake of a Faroese infant has been calculated at about 8 μg/kg body weight (17). This exposure level is orders of magnitude higher than weaned Northern European children's average daily PCB intakes below 50 ng/kg (12,18). Hence, in Faroese adolescents aged 14 years, the serum concentrations of persistent PCB congeners remains significantly associated with the duration of breastfeeding (19). The rate of decrease in serum concentrations after cessation of breast-feeding may therefore allow calculation of elimination half-lives during childhood. For this purpose, we have used two sets of PCB data from Faroese birth cohorts, where serum PCB concentrations were determined on two occasions (19,20).
Materials and methods
Study populations
The first cohort of 1022 singleton births was assembled in the Faroe Islands during a 21-month period during 1986-1987 (21). When the cohort members were about 7 and 14 years old, they were invited for a thorough health examination with voluntary venipuncture. Height and weight were recorded as part of the physical examination. At both ages, questionnaire information was obtained about the frequency of whale meat dinners and blubber consumption. At age 7 years, 917 of the children completed the examinations, but sufficient serum for PCB analysis was available only for 124 children. Seven years later, a blood sample was obtained from 795 out of 878 subjects examined. Serum PCB analysis was conducted for a total of 101 subjects (53 boys and 48 girls) examined at both occasions (Table 1).
Table 1.
Characteristics of children from two birth cohorts (mean ± standard deviation, unless otherwise indicated).
| Variable | Cohort 1 (N = 101) | Cohort 2 (N = 99) |
|---|---|---|
| Obstetric and neonatal data | ||
| ΣPCBb concentration in pregnancy serum (μg/g lipid)c | ND | 1.22(0.71-1.87)a |
| Duration of exclusive breast-feeding (months) | 3.5 ± 1.9 | 3.5 ± 2.1 |
| Duration of total breast-feeding (months) | 7.0 ± 4.3 | 7.7 ± 5.5 |
| First examination | Year 1986/7 | 1994/5 |
| Age (years) | 6.7 ± 0.2 | 4.6 ± 0.1 |
| Weight (kg) | 24.8 ± 4.0 | 19.0 ± 2.4 |
| Height (cm) | 123 ± 5 | 107 ± 4 |
| Body mass index (kg/m2) | 16.4 ± 1.8 | 16.5 ± 1.4 |
| ΣPCB concentration in serum (μg/g lipid)c | 1.71 (1.06-2.66)a | 0.95 (0.50-1.79)a |
| Second examination | ||
| Age (years) | 13.8 ± 0.3 | 7.6 ± 0.1 |
| ΣPCB concentration in serum (μg/g lipid)c | 0.86 (0.47-1.62)a | 0.79 (0.46-1.43)a |
| Body weight (kg) | 54.0 ± 10.8 | 26.3 ± 4.3 |
| Height (cm) | 163 ± 7 | 126 ± 5 |
| Body mass index (kg/m2) | 20.2 ± 3.3 | 16.4 ± 1.8 |
Geometric mean with 50% range in parenthesis.
Sum of the major polychlorinated biphenyl congeners 138, 153, and 180 multiplied by 2.0.
A second cohort was recruited during a 12-month period in 1994-1995 and included 182 singleton term births from consecutive births at the Faroese National Hospital (20). Blood samples were obtained at 4.5 years and again at 7.5 years of age from 167 and 153 children, respectively, with sufficient serum for PCB analysis from 119 and 145. A total of 99 children had serum PCB results from both occasions. In cohort 2, the child's height and weight were recorded at 3.5 years, but not a year later. The values for age 4.5 years were therefore determined by interpolation between 3.5 and 7.5 years (Table 1).
Laboratory measurements
After collection of the blood samples, the blood was allowed to clot for approximately 30 minutes, spun at 10,000 rpm for 30 min to separate the serum portion of the blood, and transferred to clean cryovials. The frozen samples were hand-carried to the Centers for Disease Control and Prevention (CDC) laboratory in the United States, where they were stored at -70°C until analyzed for PCB congeners and lipid content as previously described (19). Samples were analyzed using gas chromatography-high resolution mass spectrometry with isotope dilution calibration. Ions representing the most abundant clorine-35 and chlorine-37 isotope peaks were measured for each congener and their ratios were within ± 20% of the theoretical ratios. Approximately 20% of the samples analyzed were quality control materials (positive or negative controls). Samples from runs in which a quality control material violated one of the Westgard multirules were repeated until a valid quality control result was obtained. Data which violated any quality control parameter (e.g., quality control material out-of-control, ion ratios outside 20% of theoretical ratio, retention times not matching with labeled standards, excessive background levels in negative control) were not reported. Relative recoveries for all congeners were between 90% and 111% and the relative standard deviations of the measurements were less than 20%.
For the present study, we selected individual congeners on the basis of their detectability in the chemical analyses. For congeners 118, 138, 153, 170, 180, and 187, all subjects had concentrations of at least 0.05 μg/g lipid, thus providing assurance that analytical variability was low. Further, because of the toxicological interest in mono-ortho congeners, such as CB-118, we also included congeners 105 and 156, with the caveat that added attention to results near the detection limit would be needed.
All protocols were reviewed and approved by the Faroese ethical review committee and the institutional review board at Harvard School of Public Health; the analysis of coded samples at the CDC laboratory was determined not to require additional approval for human subjects research.
Data analysis
The two sets of paired serum PCB concentrations are thought to follow an exponential decay model:
where Yt is the concentration at time t and εt is a normally distributed error term. In this model, the half-life is given by
| [1] |
An estimate of the half-life can therefore be obtained by first estimating k. For the two sets of observations, the analysis is based on the log-transformed difference in concentrations between the two examinations, which is then given by
where t1 and t2 denote the child's age at the first and second examinations, respectively. Thus, k can be estimated as the coefficient of the age increase in a simple regression model without an intercept. For each PCB congener, we developed a joint regression model that included data from both examinations. As a starting point, the regression coefficient k and the variance of the error term εt1 - εt2 were allowed to depend on the cohort. The similarity of the regression coefficient k in the two cohorts was then tested. Assuming that they were identical, a joint estimate of the half-life was obtained. Confidence limits for the half-life was calculated from the confidence limits for k using the transformation given by equation [1].
These calculations were initially carried out without any cofactors. To adjust for possible confounding effects caused by age-related differences in distribution volumes, the body mass index (BMI) was taken into account by including the difference in log-transformed BMI as an additional predictor of the difference in log-transformed PCB concentrations, i.e.,
In this model, -ln(0.5)/k can be interpreted as the half-life for PCB concentrations in children with a constant BMI. Alternatively, this model can be interpreted as an exponential decay model in Xt = Yt ⋅ (BMIt)c were c is a constant independent of t. To test if BMI provided an adequate description of the changes in distribution volume, the difference in log-transformed weight was entered as an additional covariate, i.e.,
If this covariate had a negligible effect, the model only including BMI as a covariate was considered adequate.
Serum PCB concentrations are usually expressed in reference to the serum lipid concentration. While this tradition may be appropriate, the significance of the serum lipid concentration was tested by using the serum-lipid concentration as a covariate in calculations using the volume-based PCB concentrations. Thus, we fitted the model
where Zt1 and Zt2 are volume-based concentrations at the two time points and lipid is the serum-lipid concentration.
In regard to postnatal exposures, consumption of pilot whale could be a non-negligible PCB source in the children, depending on when they started eating this traditional food item. Two different approaches were taken to allow for this problem. First we stratified the analysis to include only those children who, according to the questionnaire, had not eaten whale. Then, to avoid problems with small numbers of subjects after the exclusions, we also adjusted for whale intake using a regression approach by including as a continuous covariate the number of monthly whale dinners that the child consumed at the time of the examination
In this model, -ln(0.5)/k can be interpreted as the half-life in children with no postnatal PCB intake from whale blubber.
To assess the robustness of these findings, we carried out additional calculations after exclusion of subjects with an initial PCB concentration below the median, where the impact of analytical imprecision may be relatively greater, and where dietary PCB exposures between the two examinations may have played a proportionately greater role. We also repeated the calculation for subjects with a total PCB concentration in the highest quartile.
Results
Descriptive results for the paired serum concentrations and their ratios are shown in Table 2. The lipid-based concentrations generally decreased between the two examinations, with substantial variability between subjects and among congeners. Two sets of paired results of PCB congener concentrations are shown graphically in Figure 1.
Table 2.
Geometric mean serum concentrations of six chlorinated biphenyl (CB) congeners in ng/g lipid (25th-75th percentiles in parenthesis) at two examinations of two birth cohorts.
| Variable | Cohort 1 (N = 101) | Cohort 2 (N = 99) |
|---|---|---|
| First examination | ||
| CB-105 | 12.7 (7.9-20.9) | 7.9 (4.4-16.2) |
| CB-118 | 58.6 (36.1-95.8) | 47.6 (26.1-92.5) |
| CB-138 | 216 (141-360) | 148 (82.5-265) |
| CB-153 | 337 (196-518) | 213 (113-402) |
| CB-156 | 24.3 (14.9-37.9) | 13.8 (7.2-24.9) |
| CB-170 | 79.6 (49.1-130) | 45.1 (24.4-84.5) |
| CB-180 | 194 (110-315) | 113 (58.5-210) |
| CB-187 | 89.3 (55.7-157) | 51.5 (28.1-99.2) |
| Second examination | ||
| CB-105 | 5.4 (2.9-10.6) | 6.6 (4.3-11.0) |
| CB-118 | 30.6 (17.3-57.8) | 38.2 (24.5-63.7) |
| CB-138 | 74.1 (44.1-134) | 90.8 (58.3-161) |
| CB-153 | 210 (112-401) | 181 (107-333) |
| CB-156 | 13.8 (7.2-24.9) | 12.5 (7.3-24.4) |
| CB-170 | 42.1 (22.2-76.8) | 41.7 (24.8-80.9) |
| CB-180 | 128 (70.4-237) | 119 (69.0-225) |
| CB-187 | 52.6 (28.4-103) | 53.8 (33.8-98.8) |
Figure 1.
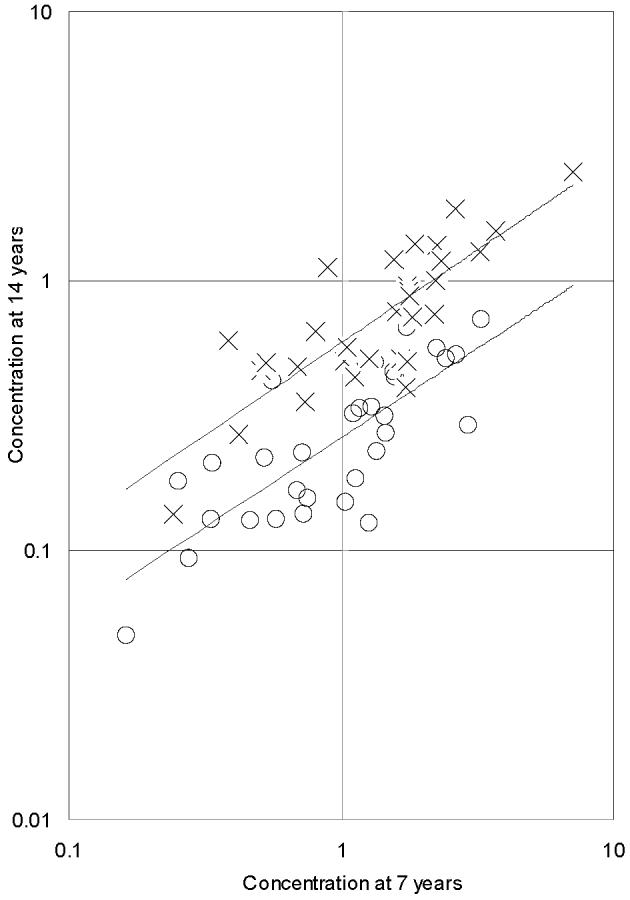
Paired serum concentrations (μg/g lipid) of congeners CB-138 (circles) and CB-153 (crosses) at ages 7 and 14 years in 32 Faroese subjects not consuming whale blubber. The two regression lines are parallel and suggest a faster elimination of CB-138 than of CB-153. Apparent elimination at lower concentrations appears to be relatively slower, but is likely affected by background exposures between the two examinations.
Half-lives were first calculated in structural equation models without any adjustment for covariates. However, with overall averages approaching 10 years, these results were considered unreliable and possibly affected by confounders. The BMI increased between the examinations; e.g., in Cohort 1 by 23% from age 7 to 14 years. Increases in BMI were associated with decreased serum congener concentrations; the negative association between BMI and PCB concentrations was clearer at age 14 than at age 7 years (data not shown). BMI was therefore included in the model, assuming that the BMI reflects the distribution volume (addition of body weight to the equation did not cause any changes). In addition, as previously reported (19), some cohort subjects had started eating whale blubber, and dietary intake during the observation period could therefore have added to their serum PCB concentrations. However, these adjustments changed the estimates only marginally (data not shown).
Stratification was therefore used to rule out possible impact of background exposures that might cause an apparent increase of half-lives at lower concentration ranges. Calculations based on subjects with a total PCB concentration above the median are shown in Table 3. All elimination half-lives now averaged less than 10 years. A repeated calculation for the subjects in the highest quartile (Table 4) resulted in a further, though less certain, decrease in half-lives.
Table 3.
Elimination half-life (years) of serum concentrations of eight major PCB congeners in two birth cohorts each examined at two occasions, adjusted for changes in body size and for dietary blubber intake, as calculated for subjects with an initial serum total PCB concentration above the median.
| Congener | Cohort 1 | Cohort 2 | p-value* | Both cohorts | 95% CI |
|---|---|---|---|---|---|
| CB-105 | 5.5 | 5.2 | 0.77 | 5.4 | 4.7 ; 6.4 |
| CB-118 | 6.1 | 4.9 | 0.13 | 5.7 | 5.0 ; 6.5 |
| CB-138 | 3.9 | 3.1 | 0.022 | 3.7 | 3.4 ; 4.0 |
| CB-153 | 8.9 | 6.3 | 0.091 | 8.4 | 7.1 ; 10.1 |
| CB-156 | 7.1 | 11.3 | 0.073 | 7.5 | 6.5 ; 8.9 |
| CB-170 | 7.3 | 9.2 | 0.32 | 7.6 | 6.6 ; 9.0 |
| CB-180 | 8.4 | 17.9 | 0.043 | 9.1 | 7.6 ; 11.2 |
| CB-187 | 8.3 | 5.9 | 0.15 | 8.0 | 6.8 ; 9.6 |
for difference between the two data sets
Table 4.
Elimination half-life (years) of serum concentrations of eight major PCB congeners in two birth cohorts each examined at two occasions, adjusted for changes in body size and for dietary blubber intake, as calculated for subjects with an initial serum total PCB concentration in the highest quartile.
| Congener | Cohort 1 | Cohort 2 | p-value* | Both cohorts | 95% CI |
|---|---|---|---|---|---|
| CB-105 | 4.5 | 4.3 | 0.78 | 4.4 | 3.8 ; 5.4 |
| CB-118 | 5.3 | 3.9 | 0.049 | 4.5 | 3.9 ; 5.4 |
| CB-138 | 3.7 | 2.7 | 0.0088 | 3.3 | 2.9 ; 3.7 |
| CB-153 | 7.7 | 4.7 | 0.034 | 6.6 | 5.4 ; 8.5 |
| CB-156 | 6.8 | 7.6 | 0.70 | 7.0 | 5.6 ; 9.1 |
| CB-170 | 6.7 | 6.4 | 0.87 | 6.6 | 5.4 ; 8.6 |
| CB-180 | 8.5 | 9.4 | 0.79 | 8.7 | 6.7 ; 12.4 |
| CB-187 | 7.4 | 4.7 | 0.10 | 6.7 | 5.3 ; 9.0 |
for difference between the two data sets
Because postnatal PCB intake may be inaccurately reflected by the dietary questionnaire response, additional analyses were carried out on data only from subjects who did not eat whale blubber at all (Figure 1). This restriction did not affect the findings (data not shown). Expression of PCB concentrations in relation to the lipid concentration may have some limitations; therefore, the same calculations were repeated using the volume-based serum concentrations, while the lipid concentration was entered as a covariate. This adjustment also rendered very similar results (not shown). Stratification by sex showed no differences in half-lives between boys and girls.
Overall, the half-lives for CB-138 were about 3-4 years; for CB-105 and CB-118, 4.5-5.5 years; for CB-156, CB-170, and CB-187, 6.5-7.5 years; and for CB-153 and CB-180, the half-lives were 7-9 years.
Discussion
The present study extends the data on temporal decay of children's serum PCB concentrations. The material is unique, because a high PCB accumulation by the time of weaning resulted from the occurrence of highly increased maternal PCB body burdens from the traditional habit of eating pilot whale blubber. As calculated by the European Food Safety Authority (12), daily intakes from breast-feeding may be two orders of magnitude higher than background dietary exposures. Under most circumstances, the exposure during the post-weaning period will be close to negligible. In agreement with this notion, Barr et al. found that the duration of the breast-feeding period remained a significant predictor for serum-PCB concentrations in Faroese adolescents at age 14 years, although this association was not statistically significant for some less chlorinated congeners (19). This study also reported that postnatal blubber intake was associated with increases of serum PCB concentrations. Accordingly, adjustment or stratification for dietary PCB exposure from traditional food was necessary in the calculation of elimination half-lives. Further, adjustment for body size as an indicator of the lipid compartment size was also included. The impact of continuous background exposures was controlled for by relying on the results obtained at higher average PCB exposures that would be less amenable to bias from comparatively low background exposures.
These findings should be considered in the light of what is known about PCB elimination mechanisms. PCB excretion with lipids in the stool is thought to represent the major non-metabolic excretion route for persistent, lipophilic substances (16). Based on this elimination route only, and given the average fecal lipid excretion rate, a maximum half-life of 9.5 years can be calculated for adults (16). Assuming that proportional lipid excretions levels are similar in children, calculated half-lives substantially in excess of this level would therefore likely be biased. Half-lives of this magnitude would correspond to a fecal fat excretion of about 1-2 g/day in children.
More rapid elimination may occur if metabolic conversion takes place. Phase-1 conversion by P450 enzymes may constitute the major rate-limiting step for certain PCB congeners (1). The present study shows that elimination half-lives in the subjects with PCB levels above the median were similar to those observed in the subjects from the highest quartile. These findings do not support a deviation from first-order kinetics, whereby increased elimination would occur at higher exposure level.
The results obtained coincide with data from previously published studies of adults. Follow-up of occupational cohorts have resulted in CB-118 half-lives of 5.8 years (9) and 9.6 years (8), although overestimation due to continued background exposures cannot be ruled out. Similarly, Masuda (6) reported a half-life above 9 years for this congener in a recent follow-up study of poisoning victims, while Ryan et al. (10) had calculated a shorter half-life of 1.2 years in highly exposed Yu-Cheng patients during the first decade after the incident. Using measured intakes in the diet as an indicator of daily exposure, and assuming steady-state conditions, the half-life for CB-118 was calculated at between 3.8 years and 6.3 years (7). Our calculated half-life of about 5 years concurs with this magnitude.
Half-lives for PCB congeners 138, 153, and 180 were estimated to be of 3.4 years, 3.8 years, and 4.3 years in Yu-Cheng patients during the first decade after the toxic exposure (10). Somewhat greater values of 6-7 years for CB-138 and 12.4 years for CB-153 were based on follow-up of workers after cessation of occupational exposure (9). Long-term half-lives of at least 9 years for CB-138, CB-153, and CB-180 were reported by Masuda (6) and Wolff et al. (8). As previously indicated, these results may have been biased by continued background exposures. Our data on children suggest that CB-138 is less persistent than CB-153, which has a half-life about twice as long. Our results for CB-180 are affected by the less robust findings from Cohort 2 likely from analytic variation, but suggest that its half-life is similar to the one of CB-153.
For related lipophilic substances, such as tetrachlorodibenzo-p-dioxin, young children reportedly exhibit shorter elimination half-lives than adults (16). An age dependency was also supported by the findings in an 11-month follow-up study of exposed children (22), where serum-PCB concentrations decreased by half, thus suggesting half-lives of about one year. However, the first serum sample could perhaps reflect PCB concentrations in more superficial compartments before complete redistribution within the lipid phase, and the lipid phase may have expanded due to growth during the observation period. The elimination rate may therefore have been overestimated. In the present study, the calculated half-lives of PCB congeners did not differ between the two cohorts, despite the difference in age. Also, the results obtained are in better agreement with findings from exposed adults than with the limited short-term studies in children. This finding suggests that fecal lipid excretion is of the same proportional importance in children and adolescents as in adults.
Metabolic elimination of PCBs involves conversion into polar hydroxylated metabolites, which are more easily excreted in urine and feces. P450 enzymes contribute to this Phase I metabolism. For the most relevant enzyme P450 1A2, small children below about 6 months of age have a reduced activity in comparison with adults, while the activity is relatively increased during later childhood and early adolescence (13). A recent study from the Faroe Islands showed that, in comparison with their mothers, 7-year-old children had higher blood concentrations of OH-PCB-107, which is a metabolite formed from either PCB-105 or PCB-118; the three other main OH-PCB congeners were of comparable magnitude (23). This evidence would suggest that the half-life of these two congeners could be shorter in children than in adults. Although this possibility cannot be decided from the present data, both PCB-105 and PCB-118 had half-lives of 4.5-5.5 years that clearly were lower than those observed for PCB-153 and PCB-180.
These data therefore point to the degree of chlorination as an important predictor of biological persistence, with a tendency of longer retention in heptachlorinated congeners (CB-170, CB-180, and CB-187) than in less chlorinated substances, especially the pentachlorinated CB-105 and CB-118. The retention of CB-138, CB-156 and CB-170 would likely be intermediate between these two groups. The latter five congeners share the property of possessing two vicinal, unsubstituted carbon atoms, thereby providing easier access for metabolizing enzymes. However, the hexachlorinated CB-153 does not share that property and also has a fairly long half-life. Thus, prediction of PCB congener persistence seems insufficient from these two aspects of chemical structure.
In conclusion, the present study utilized a large data set on serum-PCB concentrations from prospective studies of Faroese birth cohorts. Highly chlorinated congeners CB-153 and CB-180 persist in the body and show an apparent half-life averaging about 7-9 years, which can be explained by elimination with fecal lipids. CB-138 (3-4 years) and CB-105 and CB-118 (about 5 years) clearly had shorter half-lives. No indication was found that half-lives in children aged 4-14 years are shorter than in adults, thus supporting the notion that fecal fat elimination may represent a fairly constant proportion in regard to body size.
Acknowledgements
This study was supported by grants from the US National Institute of Environmental Health Sciences (ES06112 and ES09797). The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention, the NIEHS, NIH or any other funding agency.
References
- (1).Shirai JH, Kissel JC. Uncertainty in estimated half-lives of PCBS in humans: impact on exposure assessment. Sci. Total Environ. 1996;187:199–210. doi: 10.1016/0048-9697(96)05142-x. [DOI] [PubMed] [Google Scholar]
- (2).Lotti M. Pharmacokinetics and blood levels of polychlorinated biphenyls. Toxicol. Rev. 2003;22:203–215. doi: 10.2165/00139709-200322040-00003. [DOI] [PubMed] [Google Scholar]
- (3).Matthews HB, Anderson MW. The distribution and excretion of 2,4,5,2',5'-pentachlorobiphenyl in the rat. Drug Metab. Dispos. 1975;3:211–219. [PubMed] [Google Scholar]
- (4).Mes J, Arnold DL, Bryce F. The elimination and estimated half-lives of specific polychlorinated biphenyl congeners from the blood of female monkeys after discontinuation of daily dosing with Aroclor 1254. Chemosphere. 1995;30:789–800. doi: 10.1016/0045-6535(94)00408-m. [DOI] [PubMed] [Google Scholar]
- (5).Öberg M, Sjödin A, Casabona H, Nordgren I, Klasson-Wehler E, Hakansson H. Tissue distribution and half-lives of individual polychlorinated biphenyls and serum levels of 4-hydroxy-2,3,3',4',5-pentachlorobiphenyl in the rat. Toxicol. Sci. 2002;70:171–182. doi: 10.1093/toxsci/70.2.171. [DOI] [PubMed] [Google Scholar]
- (6).Masuda Y. Fate of PCDF/PCB congeners and change of clinical symptoms in patients with Yusho PCB poisoning for 30 years. Chemosphere. 2001;43:925–930. doi: 10.1016/s0045-6535(00)00452-5. [DOI] [PubMed] [Google Scholar]
- (7).Ogura I. Half-life of each dioxin and PCB congener in the human body. Organohalogen Comp. 2004;66:3376–3384. [Google Scholar]
- (8).Wolff MS, Fischbein A, Selikoff IJ. Changes in PCB serum concentrations among capacitor manufacturing workers. Environ. Res. 1992;59:202–216. doi: 10.1016/s0013-9351(05)80240-3. [DOI] [PubMed] [Google Scholar]
- (9).Brown JF, Lawton RW, Ross MR. Persistence of PCB congeners in capacitor workers and Yusho patients. Chemosphere. 1989;19:829–834. [Google Scholar]
- (10).Ryan JJ, Levesque D, Panopio LG, Panopio LG, Sun WF, Masuda Y, Kuroki H. Elimination of polychlorinated dibenzofurans (PCDFs) and polychlorinated biphenyls (PCBs) from human blood in the Yusho and Yu-Cheng rice oil poisonings. Arch. Environ. Contam. Toxicol. 1993;24:504–512. doi: 10.1007/BF01146170. [DOI] [PubMed] [Google Scholar]
- (11).Jensen AA, Slorach S, editors. Chemical contaminants in human milk. CRC Press; Boca Raton: 1991. [Google Scholar]
- (12).European Food Safety Authority . Opinion of the Scientific Panel on Contaminants in the Food Chain on a request from the commission related to the presence of non dioxin-like polychlorinated biphenyls (PCBs) in feed and food (question N° EFSA-Q-2003-114) EFSA; Brussels: 2006. URL: http://www.efsa.europa.eu/science/contam/contam_opinions/1229_en.html (accessed, 3 March, 2008) [Google Scholar]
- (13).Ginsberg G, Hattis D, Sonawane B. Incorporating pharmacokinetic differences between children and adults in assessing children's risks to environmental toxicants. Toxicol. Appl. Pharmacol. 2004;198:164–183. doi: 10.1016/j.taap.2003.10.010. [DOI] [PubMed] [Google Scholar]
- (14).Grandjean P, Kimbrough RD, Rantanen J, Tarkowski S, Yrjänheikki E. Assessment of health risks in infants associated with exposure to PCBs, PCDDs and PCDFs in breast milk. Environmental Health No. 29. Copenhagen: World Health Organization, Regional Office for Europe, 1988.
- (15).Wolff MS, Anderson HA. Polybrominated biphenyls: Sources and disposition of exposure among Michigan farm residents, 1976-1980. Eur. J. Oncol. 1999;4:645–651. [Google Scholar]
- (16).Kreuzer PE, Csanády GA, Baur C, Kessler W, Päpke O, Greim H, Filser JG. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) and congeners in infants. A toxicokinetic model of human lifetime body burden by TCDD with special emphasis on its uptake by nutrition. Arch. Toxicol. 1997;71:383–400. doi: 10.1007/s002040050402. [DOI] [PubMed] [Google Scholar]
- (17).Grandjean P, Weihe P, Needham LL, Burse VW, Patterson DG, Jr., Sampson EJ, Jørgensen PJ, Vahter M. Effect of a seafood diet on mercury, selenium, arsenic, and PCBs and other organochlorines in human milk. Environ. Res. 1995;71:29–38. doi: 10.1006/enrs.1995.1064. [DOI] [PubMed] [Google Scholar]
- (18).Petzold G, Schäfer M, Benthe C, Ostendorp G, Schade G, Wilhelm M, Heinzow B. Dietary exposure and human body burden to organochlorine pesticides and PCBs in children and women in northern Germany. Organohalogen. Comp. 1999;44:119–122. [Google Scholar]
- (19).Barr DB, Weihe P, Davis MD, Needham LL, Grandjean P. Serum polychlorinated biphenyl and organochlorine insecticide concentrations in a Faroese birth cohort. Chemosphere. 2006;62:1167–1182. doi: 10.1016/j.chemosphere.2005.06.063. [DOI] [PubMed] [Google Scholar]
- (20).Grandjean P, Bjerve KS, Weihe P, Steuerwald U. Birth weight in a fishing community: significance of essential fatty acids and marine food contaminants. Int. J. Epidemiol. 2001;30:1272–1278. doi: 10.1093/ije/30.6.1272. [DOI] [PubMed] [Google Scholar]
- (21).Grandjean P, Weihe P, Jørgensen PJ, Clarkson T, Cernichiari E, Viderø T. Impact of maternal seafood diet on fetal exposure to mercury, selenium, and lead. Arch. Environ. Health. 1992;47:185–195. doi: 10.1080/00039896.1992.9938348. [DOI] [PubMed] [Google Scholar]
- (22).Wolff MS, Schecter A. Accidental exposure of children to polychlorinated biphenyls. Arch. Environ. Contam. Toxicol. 1991;20:449–453. doi: 10.1007/BF01065832. [DOI] [PubMed] [Google Scholar]
- (23).Fängström B, Hovander L, Bignert A, Athanassiadis I, Linderholm L, Grandjean P, Weihe P, Bergman Å. Concentrations of PBDEs, PCBs, and OH-PCBs in serum from seven-year-old children and their mothers during pregnancy. Environ. Sci. Technol. 2005;39:9457–9463. doi: 10.1021/es0513032. [DOI] [PubMed] [Google Scholar]


