Abstract
Although the postnatal development of opioid systems of mammalian brain has been well studied, little is known about the ontogeny of and relationship between embryonic (E) opioid peptides and their receptors. Moreover, a simultaneous assessment of levels of the 3 classes of opioid peptides and their putative receptors during embryonal development has not been made. To this end, the ontogeny of opioid peptides and receptors in mouse brain were examined during the period E11.5 to postnatal day 1 (P1). Met-enkephalin, dynorphin and β-endorphin immunoreactivity were detected before their putative opioid receptors. β-Endorphin can be discerned as early as E11.5, whereas μ binding was first observed at E12.5. Although dynorphin and Met-enkephalin were measurable at the same time as β-endorphin, κ-receptors were not detected until E14.5 and δ sites were not found at all prenatally. Differences in immunoreactivity levels of the 3 peptides occur with dynorphin being lower than Met-enkephalin and β-endorphin, consistent with a low Bmax for κ binding. Expression of the 3 opioid peptides as well as μ and κ opioid receptors rapidly increase in parallel from E14.5 to E18.5, Interestingly, levels of β-endorphin diminish by P1, the stage at which a sharp rise of μ receptors occurs. In a comparative study of the binding of β-endorphin1–31, its truncated form (1–27) and their N-acetyl derivatives to E14.5 brain membranes, β-endorphin1–31 exhibited the highest affinity.
Keywords: Ontogenesis, Opioid peptide, Opioid receptor, Phenotypic expression, Mouse, Central nervous system, Embryo
INTRODUCTION
The multiplicity of opioid receptors in mammalian brain raises the question of the mechanisms by which their type and subtype expression are regulated. There have been numerous investigations into the postnatal developmental pattern of opioid receptor expression in rodent brain3,4,8,11,22,23,25,37–45. Throughout brain ontogeny neuronal cells receive signals that operate as cues to the expression of cellular response. Among the signals are included growth factors, protooncogenes and neurotransmitters2,4,6,12,16,17,21,26,32,36,46,47. These stimuli direct the formation of brain structures, cell grouping and the expression of various receptors including the opioids. It is also established that during development, the activity Mate of a given brain structure can influence its anatomical Synaptic pattern and receptor profile16,17,26,30,47. For example depolarization of neuronal plasma membrane alters the Phenotypic expression of the opioid receptors4,40,41.
Is the expression of opioid receptor types another developmental feature that might be regulated by endogenous exogenous signals during normal development? It is well established that μ opioid receptors emerge in fetal rodent brain and their density when expressed in fmol/mg protein decreases during the postnatal period3,8,11,27,37,42. The κ receptor has also been found in both the embryonic and perinatal period3,42. In contrast, δ sites are detected postnatally and appear to increase in concentration with time in rat brain3,22,23,25,38,42,45. A similar developmental profile is exhibited in both human and mouse brain postnatally31,43. Similar to these perinatal developers, the prenatal developing guinea pig brain is characterized by the expression of κ opioid receptors at an early embryonic stage followed by progressive increases3. Interestingly, rat brain cell aggregates follow a similar developmental pattern in vitro as seen in vivo3. In contrast, whole brain guinea pig cultures alter the apparent programmed expression of opioid receptors and begin to express increased levels of κ receptor subtype. This observation indicates that by modifying the environmental milieu at a critical time, it is possible to change the opioid receptor profile.
What is the developmental pattern of the opioid peptides and their receptors during the ontogeny of the embryonic mouse brain? Is there any correlation between the appearance of various opioid peptides and the expression of opioid receptor subtypes at the embryonic and postnatal period? To begin to address these important questions, it is necessary to determine simultaneously the order of appearance of opioid peptides and their corresponding receptors during normal development. Here we describe our initial efforts to delineate the embryonic development of these molecules in mouse brain.
MATERIALS AND METHODS
Animals
Adult male and female mice (NIH whites; NCI, Frederick, MD) were used. Tissue samples at E11.5 and E12.5 consisted of entire embryo heads, while whole brain was used from E14.5 to P1.
Radioimmunoassay
Brain samples were extracted using a solution containing 1 N HCl, 5% CH3COOH, 1% NaCl, and 1% CF3COOH. The homogenate was then concentrated and purified through an octadecasilyl-silica column as described5. The eluent was dried and resuspended in the appropriate buffers provided by the manufacturers of the radioimmunoassay kits. The peptide content was measured with the following commercially available kits; for Met-enkephalin and β-endorphin from Incstar (Stillwater, MN) and from Peninsula Laboratories (Belmont, CA) for dynorphin. The Met-enkephalin antibody displayed 2.8% cross-reactivity with Leu-enkephalin. The β-endorphin antibody cross-reacted fully with β-endorphin1–31, β-endorphin1–27, and their acetylated forms; and 5.6% with β-lipotropin. The dynorphin1–8 antibody cross-reacted 0.01% with larger or shorter forms of dynorphin.
Binding assay
Frozen mouse brains were homogenized in 20 vols of 50 mM Tris-HCl buffer, pH 7.4, using a polytron (Beckman, San Ramon, CA). The 20,000 g sedimented membranes to be used in binding assays were either prepared as described previously42 or, in some experiments, after the nuclear fraction was removed by centrifugation at 2–4 °C for 10 min at 1000 g. Pellets were resuspended in 50 mM Tris-HCl, pH 7.4. In binding experiments with β-endorphin derivatives, freshly prepared 0.1% bovine serum albumin and 0.01% bacitracin were also added, Duplicate samples were incubated in 50 mM Tris-HCl buffer, pH 7.4, with ligands for 40 min at 25 °C with shaking, and vacuum filtered through presoaked GF/B glass fiber filters on a Brandel harvester. The filters were washed 3 times with 5 ml ice-cold Tris-HCl buffer, dried and counted in Packard opti-fluor scintillation fluid. Binding parameters for the μ opioid receptor were derived from homologous competition curves generated with 1 nM [3H]DAMGE, (Tyr-D-Ala-Gly-NMe-Phe-Gly-ol) (55 Ci/mmol) (Amersham, Arlington Heights, IL)18,24. Specific κ receptor binding was monitored with 1 nM [3H](−)-ethylketocyclazocine (EKC, 25 Ci/mmol) (NEN-Dupont, Boston, MA) in the presence of 100 nM DAMGE and DPDPE (Tyr-D-Pen-Gly-Phe-D-Pen)33. ‘Non-specific’ binding values were determined from assays containing 5 μM etorphine. Additional characterization of μ binding sites was achieved by heterologous displacement of [3H]DAMGE with β-endorphin (β-lipotropin61–91, human), N-Ac-β-endorphin1–31 (human), N-Ac-β-endorphin1–27 (human) and β-endorphin1–27 (human) all purchased from Peninsula. Protein content was determined by the method of Lowry et al.29 with bovine serum albumin as standard. Binding data are presented as mean ± S.E.M., unless otherwise indicated. Bmax, Kd and IC50 values were calculated from 3–4 independent experiments with the LIGAND34 and ALLFIT13 programs.
Data analysis
All data points in Figs. 1–3 were fitted using a cubic spline curve with the Sigmaplot program (Jandel Scientific, Corte Madera, CA). For Fig. 4, the curves were generated using the ALLFIT program.
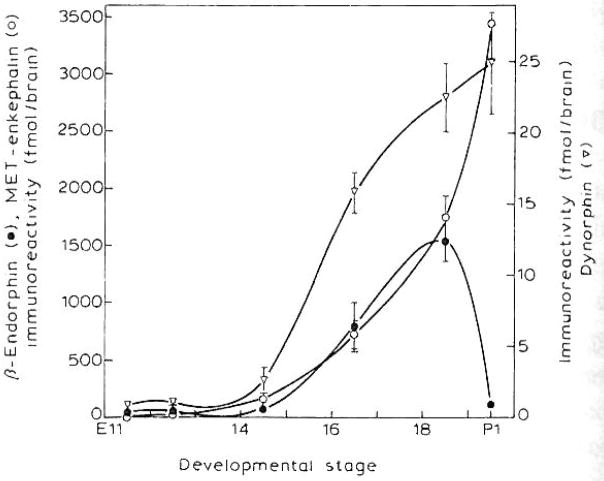
Fig. 1.
Developmental changes in Met-enkephalin, dynorphin, and β-endorphin in whole mouse brain. See Materials and Methods for details of the radioimmunoassay. Results represent the means ± S.E.M. of 2–5 independent determinations from 2–20 pooled brains for E11.5–E18.5. For P1 β-endorphin and Met-enkephalin values were derived from 1 determination of 20 brains pooled.
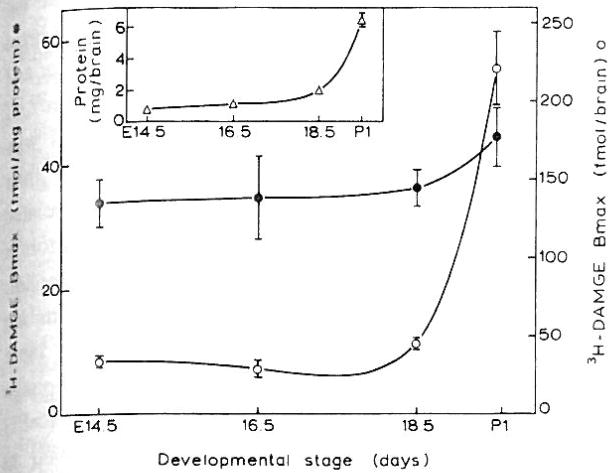
Fig. 3.
The developmental changes of [3H]DAMGE binding sites and the protein content of the receptor containing fraction (insert) of the mouse brain. Bmax and Kd values were obtained from homologous displacement curves that were analyzed with the LIGAND program. Data are the means ± S.E.M. of 3–6 experiments expressed as Bmax (fmol/mg protein) and as total receptor number per brain. The latter value was derived from the Bmax and the total protein content of the membrane fraction per brain.
Fig. 4.
Heterologous competition curves of [3H]DAMGE with β-endorphin and its derivatives. Fetal (E14.5) mouse brain membranes were incubated with 1 nM [3H]DAMGE and the indicated concentrations of β-endorphin (β-lipotropin61–91, human), NAc-β-endorphin1–27 (human), Nac-β-endorphin1–27 (human) or β-endorphin1–27 (human) as described in the Materials and Methods. IC50 values were 2.5 ± 0.2, 45.3 ± 2.7, 200 ± 25.2 and 786 ± 86.6 nM respectively.
RESULTS
As shown in Fig. 1, Met-enkephalin, dynorphin and β-endorphin immunoreactivity can be detected as early as E11.5 and rise rapidly until E18.5. Their increase parallels the gain in brain weight during the development of the mouse embryo (data not shown). At E11.5 Met-enkephalin, β-endorphin and dynorphin levels were found to be 17.7, 41.8 and 0.79 fmol/embryo, respectively. From E18.5 on immunoreactivity of Met-enkephalin and dynorphin continues to increase, whereas that of β-endorphin diminishes until by P1 it returns to levels seen at E14.5 and remains low even at P3 (Barg et al. manuscript in preparation). Interestingly, at E18.5 the immunoreactivity of Met-enkephalin and β-endorphin are higher than that of dynorphin. However, dynorphin levels in the adult mouse brain were almost 100-fold greater than the values reported for the neonate (data not shown).
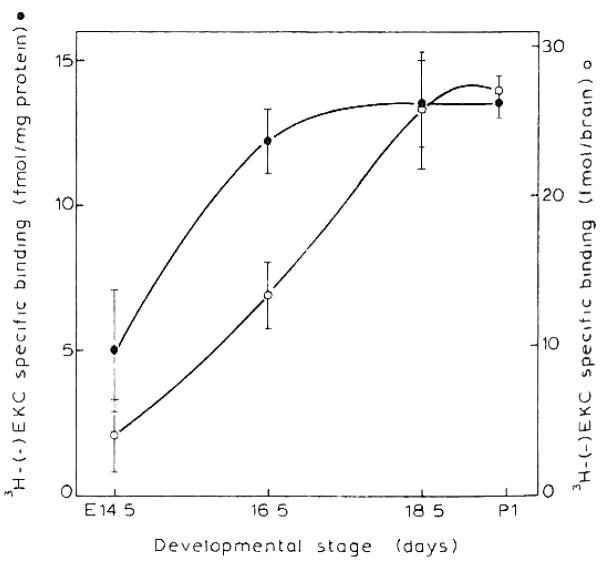
Specific binding of μ and κ opioid receptors (0.9 ± 0.1 and 5.0 ±2.1 fmol/mg protein) were first detected at E12.5 and E14.5, respectively (Figs. 2 and 3). Due to the paucity of sites, binding parameters for EKC were measured only with E16.5 whole brain. The κ receptor density at E16.5 (22.6 ±1.9 fmol/mg protein) was lower than μ levels (34.5 ± 6.6). The Kd for κ binding was 3.2 ± 1.0 nM while that of the μ sites ranged from 1.0–3.0 nM, ascertaining their authenticity as μ and κ sites. These Kd values did not change during ontogeny. Delta sites were not detected in the fetal period or at P1 (data not shown). The μ opioid receptor density (fmol/mg protein) undergoes little change from E14.5 to P1 whereas the total receptor number per brain increases in a similar developmental pattern as the rise in membrane protein per brain (Fig. 3 and insert).
Fig. 2.
Expression of [3H](−)EKC-specific binding during the development of mouse brain. Membranes were incubated with 1 nM [3H](−)EKC in the presence of 100 nM DAMGE and DPDPE to suppress μ and δ binding, respectively as described in Materials and Methods. Data are the means ± S.E.M. of 6 experiments performed in duplicate.
In an attempt to further characterize the μ opioid receptor expressed at E14.5, heterologous competition experiments were performed in which [3H]DAMGE was displaced by β-endorphin1–31, its truncated form (1–27) or their N-acetyl derivatives. As shown in Fig. 4 β-endorphin1–31 displaces [3H]DAMGE 18-, 80- and 312-fold more than N-Ac-β-endorphin1–31 (human), N-Ac-β-endorphin1–27 (human) and β-endorphin1–27, respectively. Like the adult form of the μ opioid receptor1 the feta1 counterpart exhibits decreased affinity for these β-endorphin derivatives.
DISCUSSION
In this study, we have focused simultaneously on the prenatal development profile of both opioid peptides and receptors, The ontogeny of β-endorphin, Met-enkephalin and dynorphin as well as μ, δ and κ binding sites have been investigated. It appears from the above data that all except δ binding emerge midway through gestation. This may represent one of the more important periods for mammalian brain development15.
Our assessment of the prenatal development of opioid peptides in mouse brain reveals differences in the immunoreactivity levels of the 3 peptides with dynorphin displaying the lowest content. In addition, the β-endorphin developmental pattern fluctuates while Met-enkephalin and dynorphin display a continuous increase. Interestingly, oscillations in β-endorphin content were also observed during the postnatal development of the rat brain44. In contrast, Met-enkephalin levels rise rapidly during the same time period. One interpretation of these observations argues that the developmental pattern of β-endorphin and Met-enkephalin appear to be independent of each other suggesting a differential process of gene expression14,27,44. Although this conclusion is consistent with the findings in the present study, there are alternative interpretations. It has been shown that the developing brain contains transient neurons which contain peptide immunoreactivity. Accordingly, we cannot rule out the possibility that the sharp decrease in β-endorphin immunoreactivity may reflect death of cells that produce this peptide9,10.
During embryonic development the total number of μ binding sites increases, whereas the density is almost unchanged (Fig. 3). This finding suggests that the increase in μ receptor number is due to addition of cells bearing opioid receptor and/or an increase in the number of receptors per cell. This must be accompanied by augmentation in number and/or growth of cells that may or may not express opioid receptors.
Another observation that is in agreement with previous reports27,39,44 is that opioid peptides can be detected before opioid receptors and there is a sequential expression of the peptides with β-endorphin being at least 2–3 fold greater than the others when first detected. Moreover, μ sites were detected before κ These findings raise the question of a close relationship between the peptides and the receptor which they precede. In addition, at P1, when there is a decline of β-endorphin immunoreactivity levels, there is a sharp increase in total μ receptors per brain. Although this may be mere coincidence, the timing of the two events raises the possibility that there is an interaction between the two systems to influence the phenotypic expression of the opioid receptors. Consistent with this hypothesis dynorphin immunoreactivity continues to rise while κ receptors plateau in the perinatal period (E16-P1). The basis for such speculation would be strengthened by replication of these results with more sensitive techniques of receptor detection and brain regional analysis. One such approach would exploit receptor mRNA hybridization probes. This method awaits the successful cloning of genes which express opioid receptors28.
There is evidence to support the notion that the transient appearance of opioid peptides in defined brain regions precede the emergence of the opioid receptor and regulate dendritic growth in the developing brain20. If this hypothesis is correct, a complication arises with regard to the emergence of enkephalins and their putative receptors of the δ class. It has been postulate that the preferred endogenous ligand for μ receptors is unknown19, whereas that of δ sites are the enkephalins. Less controversial is the evidence that dynorphins are κ-specific7. Here we find a considerable time lapse between the onset of the appearance of Met-enkephalin and δ binding which did not occur until postnatally (>P1) similar to previous findings on mouse43 as well as rat3,22,23,25,38,42,45 and human31 brain. The developmental profile of Leu-enkephalin remains to be determined.
Our results show that at E14.5, the μ receptor exhibits the highest affinity to β-endorphin1–31 as compared with its truncated form (1–27) and their N-acetyl derivatives. This finding is in agreement with previous reports indicating that the adult μ receptor exhibits decreased affinity for these β-endorphin derivatives1. Nevertheless, the β-endorphin truncated form (1–27) has been found in embryonic mouse brain tissue (Rius and Loh, unpublished data). Another possible explanation for the occurrence of β-endoiphm1–27 exists. The peptide GlyGln, released upon synthesis of the truncated form from β-endorphin, has been shown to be a putative neurotrans-mitter and this peptide may have a function during CNS development35. Investigations to test these theories are underway.
Acknowledgments
This research was supported in part by NIDA Grant DA 05412. We thank Drs. David Rodbard and Peter Munson, NICHD for the ALLFIT program.
References
- 1.Akil H, Young E, Watson SJ. Opiate binding properties of naturally occurring N- and C-Terminus modified β-endorphins. Peptides. 1981;2:289–292. doi: 10.1016/s0196-9781(81)80121-0. [DOI] [PubMed] [Google Scholar]
- 2.Black IB, Adler JE, Dreyfus CF, Jonakait GM, Katz DM, LaGamma EF, Markey KM. Neuratransmitter plasticity at the molecular level. Science. 1984;225:1266–1270. doi: 10.1126/science.6147894. [DOI] [PubMed] [Google Scholar]
- 3.Barg J, Levy R, Simantov R. Expression of three opioid receptor subtypes μ, δ and κ in guinea pig and rat brain cell cultures and in vivo. Int J Dev Neurosci. 1989;7:173–179. doi: 10.1016/0736-5748(89)90067-1. [DOI] [PubMed] [Google Scholar]
- 4.Barg J, Simantov R. Depolarization regulates selectively the expression of different opioid receptors: a decreased number of κ receptors in chronically activated guinea pig brain cultures. Neuroses Lett. 1990;111:222–227. doi: 10.1016/0304-3940(90)90372-g. [DOI] [PubMed] [Google Scholar]
- 5.Bennett HPJ, Hudson AM, Kelly L, McMartin C, Purdon GE. A rapid method, using octadecasilyl-silica, for the extraction of certain peptides from tissue. Biochem J. 1978;175:1139–1141. doi: 10.1042/bj1751139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cartwright CA, Simantov R, Cowan WM, Hunter T, Eckhardt W. pp60c-src Expression in the developing rat brain. Proc Natl Acad Sci USA. 1988;85:3348–3352. doi: 10.1073/pnas.85.10.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chavkin C, James IF, Goldstein A. Dynorphfo is a specific endogenous ligand of the κ opioid receptor. Science. 1982;215:413–415. doi: 10.1126/science.6120570. [DOI] [PubMed] [Google Scholar]
- 8.Clendennin NJ, Petraitis M, Simon EJ. Ontological development of opiate receptors in rodent brain. Brain Res. 1976;118:157–160. doi: 10.1016/0006-8993(76)90852-0. [DOI] [PubMed] [Google Scholar]
- 9.Chun JJM, Nakamura MJ, Shatz CJ. Transient cells of the developing mammalian telencephalon are peptide-immunoreactive neurons. Nature. 1987;325:617–620. doi: 10.1038/325617a0. [DOI] [PubMed] [Google Scholar]
- 10.Cowan WM, Fawcett JW, O’Leary DD, Stanfield BB. Regressive events in neurogenesis. Science. 1984;225:1258–1265. doi: 10.1126/science.6474175. [DOI] [PubMed] [Google Scholar]
- 11.Coyte JT, Pert CB. Ontogenetic development of [3H] naloxone binding in rat brain. Neuropharmacology. 1976;15:555–560. doi: 10.1016/0028-3908(76)90107-6. [DOI] [PubMed] [Google Scholar]
- 12.Davies AM, Bandtlow C, Heumann R, Korsching S, Roher H, Thoenen H. Timing and site of nerve growth factor synthesis in developing skin in relation to innervation and expression of the receptor. Nature. 1987;326:353–358. doi: 10.1038/326353a0. [DOI] [PubMed] [Google Scholar]
- 13.DeLean A, Munson PJ, Rodbard D. A User’s Guide to ALLFIT. Natl. Inst. Hlth; Maryland: 1988. Simultaneous analysis of families of sigmoidal curves: application to bioassay radioligand assay, and physiological dose–response curves; pp. 97–102. [DOI] [PubMed] [Google Scholar]
- 14.Elkabes S, Loh YP, Niebergs A, Wray S. Prenatal ontogenesis of proopiomelanocortin in the mouse central nervous system and pituitary gland: an in situ hybridization. Dev Brain Res. 1989;46:85–95. doi: 10.1016/0165-3806(89)90145-4. [DOI] [PubMed] [Google Scholar]
- 15.Fentress JC, Stanfield BB, Cowan WM. Observations on the development of the striatum in mice and rats. Anal Embryol. 1981;163:275–298. doi: 10.1007/BF00315705. [DOI] [PubMed] [Google Scholar]
- 16.Frank E. The influence of neuronal activity on patterns of synaptic connections. Trends Neurosci. 1987;5:188–190. [Google Scholar]
- 17.Friedman WJ, Dreyfus CF, McEwen B, Black IB. Presynaptic transmitters and depolarizing influences regulate development of the substantia nigra in culture. J Neurosci. 1988;8:3616–3623. doi: 10.1523/JNEUROSCI.08-10-03616.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gillan MGC, Kosterlitz HW. Spectrum of the μ-, δ-, and κ-binding sites in homogenates of rat brain. Br J Pharmacol. 1982;77:461–469. doi: 10.1111/j.1476-5381.1982.tb09319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldstein A. Binding selectivity profile for ligands of multiple receptor types: focus on opioid receptors. Trends Pharmacol Sci. 1987;8:456–459. [Google Scholar]
- 20.Hauser KF, McLaughlin PJ, Zagon I. Endogenous opioids regulate dendritic growth and spine formation in developing brain. Brain Res. 1987;416:157–161. doi: 10.1016/0006-8993(87)91509-5. [DOI] [PubMed] [Google Scholar]
- 21.Jaramillo F, Vicine S, Schuetze SM. Embryonic acetylcholine receptors guarantee spontaneous contractions in rat developing muscle. Nature. 1988;335:66–68. doi: 10.1038/335066a0. [DOI] [PubMed] [Google Scholar]
- 22.Kent JL, Pert CB, Herkenham M. Ontogeny of opiate receptors in rat forebrain visualization by in vitro autoradiography. Dev Brain Res. 1982;2:487–504. doi: 10.1016/0165-3806(81)90018-3. [DOI] [PubMed] [Google Scholar]
- 23.Kornblum HI, Hurlbut DE, Leslie PM. Postnatal development of multiple opioid receptors in rat brain. Dev Brain Res. 1987;37:21–41. doi: 10.1016/0165-3806(87)90226-4. [DOI] [PubMed] [Google Scholar]
- 24.Kosterlitz HW, Paterson SJ, Robson LE. Characterization of the κ-subtype of opiate receptor in the guinea-pig brain. Br J Pharmacol. 1981;73:939–949. doi: 10.1111/j.1476-5381.1981.tb08749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leslie PM, Tso S, Hurlbut DE. Differential appearance of opiate receptor subtypes in neonatal rat brain. Life Set. 1982;31:1393–1396. doi: 10.1016/0024-3205(82)90389-7. [DOI] [PubMed] [Google Scholar]
- 26.Liles WC, Nathanson NM. Regulation of muscarinic acetylcholine receptor number in cultured neuronal cells by chronic membrane depolarization. J Neurosci. 1987;6:2208–2214. [PMC free article] [PubMed] [Google Scholar]
- 27.Loh YP, Rius RA, Elkabes S, Bem WT, Coscia CJ. NIDA Monograph on “Molecular Approaches to Drug Abuse Research”. Prenatal expression of proopiomelanocortin (POMC) mRNA, POMC-derived peptides and μ opiate receptors in the mouse embryo. in press. [PubMed] [Google Scholar]
- 28.Loh HH, Smith AP. Molecular characterization of opioid receptors. Annu Rev Pharmacol Toxicol. 1990;30:123–147. doi: 10.1146/annurev.pa.30.040190.001011. [DOI] [PubMed] [Google Scholar]
- 29.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 30.Luqmani YA, Bradford AF, Birdsall NJM, Hulme EG. Depolarization-induced changes in muscarinic cholinergic receptors in synaptosomes. Nature. 1979;277:481. doi: 10.1038/277481a0. [DOI] [PubMed] [Google Scholar]
- 31.Magnam J, Tiberi M. Evidence for the presence of μ- and κ-opioid sites in the human fetal brain. Dev Brain Res. 1989;45:275–281. doi: 10.1016/0165-3806(89)90045-x. [DOI] [PubMed] [Google Scholar]
- 32.Mattson MP. Neurotransmitters in the regulation of neuronal cytoarchitecture. Brain Res Rev. 1988;13:179–212. doi: 10.1016/0165-0173(88)90020-3. [DOI] [PubMed] [Google Scholar]
- 33.Mosberg HI, Hurst R, Hruby VJ, Gee K, Yamamura HJ, Galligan JJ, Burks TF. Bis-penicillamine enkephalins possess highly improved specificity towards δ-opioid receptors. Proc Natl Acad Sci USA. 1983;80:5871–5874. doi: 10.1073/pnas.80.19.5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munson PJ, Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980;107:220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- 35.Parish DC, Smyth DG, Normanton JR, Wolstencroft JT. Glycyl glutamine, an inhibitory neuropeptide from β-endorphin. Nature. 1983;306:267–270. doi: 10.1038/306267a0. [DOI] [PubMed] [Google Scholar]
- 36.Patterson P. Environmental determination of autonomic neurotransmitter functions. Annu Rev Neurosci. 1978;1:1–17. doi: 10.1146/annurev.ne.01.030178.000245. [DOI] [PubMed] [Google Scholar]
- 37.Pert CB, Kuhar MJ, Snyder SH. Opiate receptor: autoradiographic localization in rat brain. Proc Nad Acad Sci USA. 1976;73:3729–3733. doi: 10.1073/pnas.73.10.3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petrillo P, Tavani A, Verotta D, Robson LE, Kosterlitz HW. Differential postnatal development of μ-, δ- and κ opioid binding sites in rat brain. Dev Brain Res. 1987;31:53–58. doi: 10.1016/0165-3806(87)90082-4. [DOI] [PubMed] [Google Scholar]
- 39.Pittius CW, Ellendorff F, Hollt V, Parvizi N. Ontogenetic development of proenkephalin A and proenkephalin B messenger RNA in fetal pigs. Exp Brain Res. 1987;69:208–212. doi: 10.1007/BF00247043. [DOI] [PubMed] [Google Scholar]
- 40.Simantov R, Levy R. Neuronal activation regulates the expression of opioid receptors: possible role of glial-derived factors and voltage-depended ion channels. J Neurochem. 1989;52:305–309. doi: 10.1111/j.1471-4159.1989.tb10931.x. [DOI] [PubMed] [Google Scholar]
- 41.Simantov R, Levy R. Plasticity in the phenotypic expression of brain opioid receptors: differential response of forebrain and hindbrain cultures to chemical depolarization. Dev Brain Res. 1986;26:301–304. doi: 10.1016/0165-3806(86)90297-x. [DOI] [PubMed] [Google Scholar]
- 42.Spain JW, Roth BL, Coscia CJ. Differential ontogeny of multiple opioid receptors (μ, δ and κ) J Neurosci. 1985;5:584–588. doi: 10.1523/JNEUROSCI.05-03-00584.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tavani A, Robson LE, Kosterlitz HW. Differential postnatal development of μ-, δ- and κ-opioid binding sites in mouse brain. Dev Brain Res. 1985;23:306–309. doi: 10.1016/0165-3806(85)90056-2. [DOI] [PubMed] [Google Scholar]
- 44.Tsang D, Ng SC, Ho KP, Ho WKK. Ontogenesis of opiate binding sites and radioimmunoassayable β-endorphin and enkephalin in regions of rat brain. Dev Brain Res. 1982;5:257–261. doi: 10.1016/0165-3806(82)90124-9. [DOI] [PubMed] [Google Scholar]
- 45.Wohltmann M, Roth BL, Coscia CJ. Differential postnatal development of μ and δ receptors. Dev Brain Res. 1982;3:679–684. doi: 10.1016/0165-3806(82)90066-9. [DOI] [PubMed] [Google Scholar]
- 46.Zagon IS, McLaughlin PJ. Opioid antagonist-induced modulation of cerebral and hippocampal development: histological and morphometric studies. Dev Brain Res. 1986;28:233–246. doi: 10.1016/0165-3806(86)90025-8. [DOI] [PubMed] [Google Scholar]
- 47.Zigmond R, Bowers C. Influence of nerve activity on the macromolecular content of neurons and their effector organs. Annu Rev Physiol. 1981;43:673–687. doi: 10.1146/annurev.ph.43.030181.003325. [DOI] [PubMed] [Google Scholar]