Abstract
The main objective of this study was to determine whether elevated blood β-amyloid (Aβ) levels among the first-degree relatives of patients with Alzheimer’s Disease (AD) are associated with vascular risk factors of AD. Serum Aβ was measured in samples from 197 cognitively normal first-degree relatives of patients with AD-like dementia. Study participants were recruited as part of an ancillary study of the Alzheimer’s Disease Anti-inflammatory Prevention Trial (ADAPT subpopulation). The ADAPT subpopulation was found to be similar in age, sex, and ethnicity to another cognitively normal cohort (n = 98). Using cross-sectional analyses, we examined the association of Aβ with blood pressure, lipid levels, apolipoprotein E genotypes, and the use of prescribed medication to treat vascular risk factors in the ADAPT subpopulation. Aβ1–40 was positively associated with age, use of antihypertensives, and serum creatinine, and we observed a marginal negative interaction on Aβ1–40 associated with systolic blood pressure and use of antihypertensives. Serum Aβ1–42 was associated with statin use and a positive correlation of Aβ 1–42 with HDL was observed among statin nonusers. These findings suggest that high Aβ in the periphery among the family history–enriched cohorts may be due to enrichment of vascular risk factors and may reflect presymptomatic AD pathology. It remains to be determined whether the association of Aβ with medications used for treating vascular risk factors indicates prevention of AD. Longitudinal evaluation of blood Aβ in this cohort will provide a better understanding of the significance of this association in AD etiology.
INTRODUCTION
Alzheimer’s Disease (AD) is characterized clinically by progressive cognitive impairment and pathologically by the presence of β-amyloid (Aβ) plaques and neurofibrillary tangles. The etiology of the rare early-onset familial form of AD has been attributed to mutations in the genes for amyloid precursor protein (APP) and presenilin 1/2 (PS1/2). These mutations lead to aberrant proteolytic processing by the β- and γ-secretases, followed by increased production of Aβ1–42 and Aβ1–40 fragments (1). Pathogenesis of the common late-onset AD (LOAD) remains unclear, although it is postulated that either excessive Aβ production or reduced Aβ clearance may result in the accumulation and deposition of Aβ in the brain. Therefore, evaluation of the underlying mechanisms with respect to Aβ clearance from the brain into cerebrospinal fluid and blood remains an area of active investigation.
Despite uncertainty regarding the origin of Aβ in the blood, several studies provide strong evidence for the association of altered blood Aβ levels with AD risk, onset, and progression (2–6). Among AD patients, an initial increase in blood Aβ1–42 is followed by a decrease (2), and an increase in Aβ1–42 over time was shown to be a significant predictor of conversion to mild cognitive impairment or AD (5). More recently, Aβ1–42 was shown to be higher in patients with mild cognitive impairment compared with both AD patients and controls (7). These findings suggest that alterations in blood Aβ levels may be a reflection of brain Aβ pathology.
Evidence for a significant heredity component to blood Aβ1–42 and Aβ1–40 levels comes from family-based genetic linkage analyses pointing to markers on chromosome 10 (8) and an increase in plasma Aβ1–42 among the first-degree family members of patients with LOAD has also been observed (9). Mutations in PS1/2 genes are shown to influence blood Aβ1–42 levels in early-onset AD patients and their presymptomatic relatives (10,11). However, governance of elevated blood Aβ1–42 levels in the first-degree relatives of LOAD patients remains unexplained by these genes (9). Familial clustering in LOAD is attributed to an increased occurrence of the apolipoprotein E (APOE) ɛ4 allele, but it accounts for only 13.7% of the AD incidence (12–14), and no relationship of blood Aβ1–42 with ɛ4 carrier status has been observed in the first-degree relatives of LOAD patients (9). Thus, a possibility remains for presence of other factors that may impact blood Aβ in the first-degree relatives of LOAD patients.
Vascular risk factors are shown to play a significant role in the etiology of LOAD (15). Several studies provide strong evidence that high blood pressure and hypercholesterolemia are associated with an increased risk of dementia, including AD (16–19). Therefore, we hypothesize that these vascular risk factors may impact blood Aβ levels. Support for this hypothesis comes from a study of two independent mouse models of hypertension, which demonstrated that chronic hypertension results in increased brain Aβ deposition in the cortex and hippocampus attributable to increased permeability of the blood-brain barrier. This finding suggests that chronic hypertension may be responsible for brain Aβ deposition by either reducing brain Aβ clearance or allowing entrance of peripheral Aβ into the brain (20). Cholesterol clearance pathways are also fundamental to AD pathophysiology, and a key receptor for lipoprotein, low-density lipoprotein (LDL)-receptor–related protein 1, has been shown to mediate Aβ clearance from the brain via its transport across the blood-brain barrier. Alteration in expression of LDL-receptor–related protein 1 and a receptor for advanced glycation end products in AD mouse models is shown to result in dysregulation of Aβ efflux/influx across the blood-brain barrier, further enhancing brain Aβ deposition (21). These findings suggest that chronic hypertension and a dysfunction in cholesterol efflux pathways may disrupt brain Aβ homeostasis.
It is widely accepted that the AD process begins decades before the presence of clinical symptoms, and the relevant peripheral Aβ levels associated with AD pathology may be those observed several years prior to the appearance of cognitive symptoms. The examination of vascular risk factors for AD and Aβ levels among cognitively normal individuals may provide insight into the potentially pathological processes that may predispose individuals to an increased risk. The cognitively normal cohort with an enriched family history of AD-like dementia may be a high-risk population for development of this disease and an ideal group for evaluation of vascular risk factors of AD and blood Aβ levels. Therefore, in this study, we examined the potential association between serum Aβ1–40 and Aβ1–42 levels and vascular risk factors among cognitively normal first-degree relatives of patients with AD or related dementia.
MATERIAL AND METHODS
Study Population and Data Collection
The Alzheimer’s Disease Antiinflammatory Prevention Trial (ADAPT) was a multicenter trial funded by the National Institutes of Health to test the effects of nonsteroidal antiinflammatory drugs (NSAIDs) on the prevention of AD. The participants in ADAPT were selected based on the presence of a first-degree relative with AD-like dementia. The study intervention (celecoxib, 200 mg twice a day; naproxen sodium, 220 mg twice a day; or placebo) was randomly assigned to each participant. At eligibility determination a study-specific standard questionnaire was administered, which included questions pertaining to the presence of cardiovascular disease and associated vascular risk factors. Data were collected on history of angina, congestive heart disease, occurrence and number of strokes, and current and/or past use of medications for hypertension, hypercholesterolemia, and diabetes. Vital signs, such as blood pressure and weight, were also measured. To determine eligibility a physical exam was conducted by a medical doctor, and a brief neuropsychological battery was administered by trained psychometricians. A similar questionnaire aimed at gathering new occurrences of the above data during the length of the trial was administered at each semiannual in-person visit (physical exam and cognitive testing was performed at each annual visit). Blood samples were collected for safety monitoring at these visits, which included measurement (performed by Covance) of serum creatinine, total cholesterol, LDL, and high-density lipoprotein (HDL). Additional information on eligibility and methods is available at http://www.jhucct.com/adapt/manall43.pdf.
For this ancillary study, 197 cognitively normal participants (ADAPT subpopulation) were recruited from the Tampa Bay area and Sarasota (Florida site), and additional blood samples were collected at the semiannual visits to measure Aβ levels. Within the ADAPT subpopulation, we examined the association of Aβ with total cholesterol, LDL, HDL, systolic blood pressure (SBP), diastolic blood pressure (DBP), serum creatinine, and use of prescribed medications to treat vascular risk factors. Self reports of current use (at the time of the blood draw for this ancillary study) of angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, dihydropyridines, and nondihydropyridine calcium-channel blockers were combined into one single binary variable as antihypertensives use, and code was indicated by 1 for yes. Similarly, current use of statins was also coded as 1 into a separate binary variable. Aβ levels were also examined with the study treatment assignments and this was coded into a single categorical variable for which placebo was coded as 0, celecoxib as 1, and naproxen as 2.
Another cognitively normal group (non-ADAPT) was used only for comparison of general demographics, hypertension, hypercholesterolemia, diabetes, APOE genotypes, and serum Aβ with the ADAPT subpopulation. This non-ADAPT group consisted of 98 participants recruited from the local retirement centers in Tampa and Sarasota, Florida. Family history data were available for 82 of the non-ADAPT group, and 37 of those had a positive family history. For this non-ADAPT cohort, history of past or present occurrence of conditions such as hypertension, hypercholesterolemia, diabetes, and cardiovascular diseases, and current use of prescribed medications, was also collected by self-report via answers to an intake questionnaire. A mini–mental status examination was administered to determine cognitive status. In addition, these individuals maintained independent activities of daily living and were free of active neurological illness, psychiatric disorders, or other medical conditions that would potentially interfere with their cognitive performance. For both study groups, past or present occurrence of hypertension, hypercholesterolemia, and diabetes was coded as 1 in separate binary variables.
Ethical Considerations
The Western Institutional Review Board approved this study.
Sample Collection and Preparation and Aβ Measurements
Venous blood was collected in serum separator tubes (BD Diagnostics, Oxford, UK), allowed to clot for 30 min, centrifuged for 15 min at 1380g, aliquoted, and immediately frozen at −80°C. The Aβ content of serum was determined, according to manufacturer’s instructions, by use of the human Aβ1–40 and highsensitivity Aβ1–42 ELISA kits (Invitrogen, Carlsbad, CA, USA). As per the manufacturer, for Aβ1–40, the interassay coefficient of variation (CV) was ≤2.8% and the intraassay CV was about 4%. For Aβ1–42 both interassay and intraassay CVs were reported to be about 10%. The detection limits for Aβ1–40 and Aβ1–42 were approximately 8 pg/mL and 1 pg/mL, respectively. We were unable to obtain readings for Aβ on 18 individuals.
APOE Genotyping
We extracted DNA from whole blood by using Pure Gene Kits (Gentra Systems, Minneapolis, MN, USA) and performed APOE genotyping by using previously established methods (22).
Statistical Analyses
Data were analyzed with a cross-sectional design. All statistical analyses were performed using Stata software (version 9.0 and 9.2). Group differences were determined with either the Student t test or χ2 statistics, based on the type of variable. Upon evaluation of normality, Aβ1–42, and Aβ1–42/Aβ1–40 ratios were determined to be positively skewed. Therefore we used the Mann-Whitney test to determine group differences and the Spearman’s correlation coefficient (rho) to assess possible linear associations. The means and standard deviations were used to summarize symmetric continuous variables; and the medians and interquartile ranges (IQR) were used to summarize data with nonsymmetric distributions. Regression modeling was employed to examine the multivariate relationship of vascular risk factors with Aβ1–40, and given the lack of normality of the Aβ1–42 data, the bootstrap resampling approach was used to estimate standard errors. These analyses were exploratory; no adjustments were made for multiple testing, and therefore the results are interpreted cautiously.
RESULTS
Difference in hypertension, diabetes, hypercholesterolemia, APOE ɛ4 carrier status, and basic demographics were evaluated between the ADAPT subpopulation and the non-ADAPT group. Both groups were volunteer based and similar in age, sex, and ethnicity (Table 1). Comparison of Aβ1–40 and Aβ1–42 levels between these two groups revealed that Aβ1–40, Aβ1–42, and Aβ1–42/Aβ1–40 ratios were higher in the ADAPT subpopulation than the non-ADAPT group (Table 1). Age was associated with Aβ1–40 in both of these groups; a correction for age was applied, and the difference in Aβ1–40 was no longer significant (Table 1). Age was not associated with Aβ1–42, and sex was not associated with Aβ1–42 or Aβ1–40 levels. Though not significant, the proportion of individuals with APOE ɛ4 genotypes, hypertension, and diabetes was higher in the ADAPT subpopulation compared with non-ADAPT (Table 1). Frequency of individuals with hypercholesterolemia was significantly higher in the ADAPT subpopulation compared with nonADAPT (Table 1).
Table 1.
Comparison of the non-ADAPT and ADAPT subpopulations.a
| Non-ADAPT subjects (n = 98) | ADAPT subpopulation subjects (n = 197) | P-value | |
|---|---|---|---|
| n (%) | |||
| Female (%) | 54 (55) | 91 (47) | 0.17 |
| White (%) | 96 (98) | 193 (98) | 0.68 |
| Diabetes (%) | 8 (8) | 19 (10) | 0.74 |
| Hypertension (%) | 55 (57) | 122 (62) | 0.39 |
| Hypercholesterolemia (%) | 38 (39) | 103 (52) | 0.03 |
| APOE ɛ4 allele (%) | 27 (28) | 63 (32) | 0.44 |
| Mean ± SD | |||
| Age (years) | 75.2 ± 8.21 | 76.8 ± 3.93 | 0.07 |
| Serum Aβ1–40 (pg/mL)b | 132.6 ± 49.65 | 146.3 ± 54.59 | 0.13 |
| Median [IQR] | |||
| Serum Aβ1–42 (pg/mL) | 4.9 [2.9, 9.1] | 12.0 [5.0, 22.9] | P < 0.001 |
| Serum Aβ1–42/Aβ1–40 | 0.04 [0.02, 0.07] | 0.08 [0.05, 0.15] | P < 0.001 |
Aβ1–40 below detection limit for 9 individuals and Aβ 1–42 below detection limit for 18 individuals.
Differences in Aβ1–40 between the two groups are corrected for age.
The following analyses were performed only within the ADAPT subpopulation to determine possible relationships between the use of medications to treat vascular risk factors, blood pressure, lipid levels, serum creatinine, and assignment of the study medications with Aβ1–42 and Aβ1–40 (Table 2). No differences in Aβ1–40 and Aβ1–42 between the placebo, celecoxib, or naproxen were detected, although median Aβ1–42 levels were slightly lower in the celecoxib group compared with the other groups (placebo, median 14.61 pg/mL [IQR 6.49–23.24 pg/mL]; celecoxib, median 9.73 pg/mL [IQR 5.34–18.13 pg/mL]; naproxen, median 11.14 pg/mL [IQR 4.67–24.16 pg/mL]). Aβ1–40 was positively associated with Aβ1–42 (rho = 0.314, P <0.001), SBP (r = 0.150, P = 0.035), age (r = 0.212, P = 0.003), and serum creatinine (r = 0.361, P <0.001). Serum creatinine was associated with SBP (r = 0.216, P = 0.002). No correlation was observed for either Aβ1–40 or Aβ1–42 with weight (r =0.037, P = 0.60 and rho = 0.022, P = 0.76 respectively). No relationship between SBP and Aβ1–42 (rho = 0.007, P = 0.93) or between diastolic blood pressure with Aβ1–40 or Aβ1–42 was observed (r = −0.004, P = 0.96 and rho = 0.01, P = 0.88, respectively). Aβ1–40 levels were found to be higher among users of antihypertensives compared with nonusers (155.15 pg/mL ± 52.70 SD and 141.1 pg/mL ± 56.95 SD, respectively).
Table 2.
Vascular factors and medication use in the ADAPT subpopulation.a
| Baseline measurements | Mean ± SD |
|---|---|
| SBP (mmHg) | 133.47 ± 11.65 |
| DBP (mmHg) | 75.30 ± 6.50 |
| Creatinine (mg/dL) | 0.95 ± 0.22 |
| Cholesterol (mg/dL) | 191.92 ± 39.34 |
| LDL (mg/dL) | 105.27 ± 34.07 |
| HDL (mg/dL) | 48.70 ± 12.51 |
| Medication use (yes) | Number (%) |
| Dihydropyridine | 18 (9) |
| Nondihydropyridine | 2 (1) |
| Angiotensin-converting enzyme inhibitor | 46 (23) |
| Angiotensin II receptor blockers | 21 (11) |
| Statin | 84 (43) |
| Placebo | 83 (42) |
| Celecoxib | 52 (26) |
| Naproxen | 62 (32) |
SBP and DBP were unavailable for one individual, and creatinine was unavailable for two individuals.Total cholesterol was available for 195 subjects, HDL was available for 102 subjects, and LDL was available for 100 subjects.
Multivariate regression analyses revealed a linear relationship of Aβ1–40 with age, serum creatinine, and hypertension medication use (Table 3). A marginal negative interactive association was observed between SBP, use of antihypertensives, and Aβ1–40 levels (β = −1.28, 95% CI [−2.57 to 0.014], P = 0.052, Table 3). Stratification of the relationship between SBP and Aβ1–40 by use of antihypertensives showed a positive correlation among the nonusers (r = 0.25, P = 0.006), but no such correlation was evident among users (r = −0.06, P = 0.61)
Table 3.
Predictors of Aβ1–40 levels among the ADAPT subpopulation
| Predictorsa | β | [95% CI] | P-value |
|---|---|---|---|
| Age (1-year increase) | 2.41 | [0.57 to 4.26] | 0.011 |
| Creatinine (mg/dL) | 81.52 | [47.29 to 115.75] | <0.001 |
| Antihypertensive use (yes) | 176.43 | [1.36 to 351.50] | 0.048 |
| SBP (mmHg) | 0.75 | [−0.04 to 1.54] | 0.063 |
| Antihypertensive use × SPP | −1.28 | [−2.57 to 0.014] | 0.052 |
Analyses were performed using multivariate regression modeling, in which age, creatinine, antihypertensive use, SBP, and the interactive term were in the same linear model.
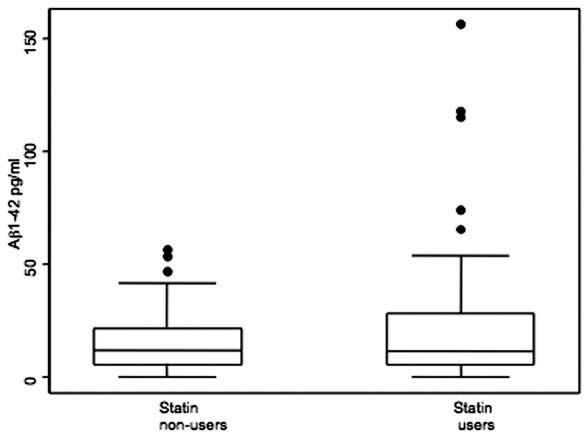
As expected, significantly lower cholesterol and LDL levels were observed among statin users, and although 5% higher HDL was also observed, these differences were not significant (data not shown). An association was observed between statin use and Aβ1–42 (β = 6.93, bias corrected 95% CI [1.26–13.59], P = 0.02, Figure 1). In this model, no adjustment was required for age, serum creatinine, or blood pressure because these variables were not associated with Aβ1–42. Given the impact of statins on cholesterol levels and the observed association of Aβ1–42 levels with statin use, we stratified the study sample by statin use to determine the relationship of Aβ1–42 with lipid levels. A positive correlation of HDL levels was observed with Aβ1–42 in nonusers (rho = 0.309, P = 0.02), but no such association was observed in the statin users (rho = −0.091, P > 0.05). No relationship of Aβ1–42 with LDL and total cholesterol was observed. We also examined the potential influence of the ɛ4 allele of APOE on Aβ1–42 levels. APOE genotypes were determined to be in Hardy-Weinberg equilibrium. No statistically significant differences with APOE genotype were observed, although median Aβ1–42 levels were generally lower in the ɛ4 carriers (non–ɛ4 carriers, median 12.14 pg/mL [IQR 4.85–24.16 pg/mL]; ɛ4 heterozygotes, median 10.56 pg/mL [IQR 6.49–22.53 pg/mL]; and ɛ4 homogygotes, median 10.11 pg/mL, [IQR 3.72–16.90 pg/mL]).
Figure 1.
Median Aβ1–42 levels among statin nonusers is 12.03 pg/mL (interquartile range [IQR] 5.90–21.75 pg/mL), and median among statin users is 11.75 pg/mL (IQR 5.52–28.76 pg/mL).
DISCUSSION
Findings reported here show significantly higher Aβ1–42 among the ADAPT subpopulation (selected based on the presence of first-degree relatives with AD) compared with the non-ADAPT group. These findings are consistent with those of Ertekin-Taner and colleagues showing elevated Aβ1–42 in another family history–enriched cohort (9). Although several studies have shown differences between APOE ɛ4 carriers and noncarriers with cognitive decline and disease progression, a clear association of APOE ɛ4 status with plasma Aβ has not yet been demonstrated (23,24). Therefore, we speculate that elevated Aβ1–42 among first-degree relatives of sporadic AD patients may be indicative of increased Aβ production due to genetic and/or other risk factors.
Alteration in cholesterol levels and homeostasis have been associated with AD pathology (18,19), although possible reduction in AD risk with statin use remains controversial owing to the inconsistency of past findings (25). Furthermore, data from several recent studies, including data from another ADAPT ancillary study, suggest lowered risk of AD among statin users (26–28). However, plasma Aβ remains unaltered after statin treatment among AD or hypercholesterolemia patients (29). A significant increase in blood Aβ1–42 levels among statin users in the ADAPT subpopulation was observed and is similar to the findings among males from a non–family-based sample (30). Because individuals with hypercholesterolemia are likely to be statin users and also at increased risk for AD, it is possible that higher Aβ1–42 among statin users may be coincidental and just a reflection of this increased risk of AD. However, longitudinal studies are required to determine the consequence of statin therapy on Aβ1–42 levels as it relates to AD risk.
Several studies show that HDL in human serum binds to Aβ via interaction with apoA, apoJ, and apoC-III (31–34). We found a significant positive correlation of HDL levels with Aβ1–42 levels among statin nonusers. Similarly, a previous study evaluating plasma Aβ1–42 among 18 healthy individuals found a correlation between HDL and Aβ1–42 (35). However, Mayeux and colleagues reported an association of HDL with Aβ1–40 instead (2). These discrepancies may be due to differences in population characteristics or disease stages, but nevertheless suggest a probable association of HDL with peripheral Aβ. Thus, these findings suggest that a positive association of Aβ1–42 with HDL may be due to its clearance from the brain. Longitudinal follow-up of this ADAPT subpopulation is required to fully understand this association between HDL and Aβ1–42.
As with Blasko and colleagues, we observed no association of Aβ1–42 with the use of anti-hypertensives (30). Instead, elevated Aβ1–40 among the ADAPT subpopulation on antihypertensives and a negative interaction between use of antihypertensives and SBP on Aβ1–40 levels were detected. These findings may suggest that among individuals not on antihypertensives, association of SBP and Aβ1–40 levels may be indicative of increased risk for AD and may reflect increased Aβ1–40 production. However, among individuals who use antihypertensives, the observed association with Aβ1–40 may indicate increased clearance from the brain, but further studies are required to fully understand the mechanisms behind increased production versus increased clearance with respect to blood Aβ measurements in sporadic AD.
Consistent with other reported studies, our study showed a significant positive association between Aβ1–40 and serum creatinine (2,36). The cause of this relationship is still unclear, and its examination may be helpful in furthering our understanding of AD pathology.
Naproxen, a nonselective cyclooxygenase inhibitor, has been shown to preferentially lower Aβ1–42 in vitro (37). Because the ADAPT cohort received NSAIDs, we evaluated the potential effect of these interventions on both Aβ1–40 and Aβ1–42 in the ADAPT subpopulation and found no difference between the effects of placebo, celecoxib, and naproxen treatments on Aβ1–40 and Aβ1–42 levels. Although data from a pooled study of six different cohorts suggest that reduced incidence of AD is associated with NSAID use, there was no apparent advantage attributed to Aβ-lowering compounds (38). More recently, a disease-modifying clinical trial for AD using R-flurbiprofen, a selective Aβ1–42-lowering NSAID, failed to show any efficacy (39). However, recent findings from the main ADAPT cohort show reduced incidence of AD in the naproxen group compared with the groups receiving celecoxib or placebo (40). Therefore, further investigation is required to determine the full impact of NSAIDs on AD pathogenesis and whether Aβ-lowering NSAID compounds have any effect on peripheral Aβ levels.
There is a growing consensus that changes in blood Aβ levels parallel AD onset and progression, although these findings remain discrepant with respect to specific species, that is, Aβ1–42 or Aβ1–40 (2–6). Despite this discrepancy, evidence is now emerging in favor of the more pathologic form, Aβ1–42, being elevated at the stage of mild cognitive impairment or an even earlier prodromal stage of AD, possibly owing to increased production (2,5,7). These findings suggest that increased risk of AD associated with family history may be mediated in part through the enrichment of individuals with vascular risk factors and may be reflective of presymptomatic AD pathology. It remains to be determined, however, whether association of Aβ with medication used to treat vascular risk factors is indicative of prevention or amelioration of AD. Nonetheless, this exploratory study, which provides baseline data, and longitudinal evaluation of peripheral Aβ will provide a better understanding of the significance of this association in AD etiology.
ACKNOWLEDGMENTS
We thank Robert and Diane Roskamp for their generous support. The support for ADAPT was provided by National Institutes of Aging through National Institutes of Health (NIH) grant number NIH/7U01AG15477-02.
Footnotes
DISCLOSURE
We declare that the authors have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Tandon A, Rogaeva E, Mullan M, St George-Hyslop PH. Molecular genetics of Alzheimer’s disease: the role of beta-amyloid and the presenilins. Curr Opin Neurol. 2000;13:377–84. doi: 10.1097/00019052-200008000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Mayeux R, et al. Plasma Aβ40 and Aβ42 and Alzheimer’s disease: relation to age, mortality, and risk. Neurology. 2003;61:1185–9. doi: 10.1212/01.wnl.0000091890.32140.8f. [DOI] [PubMed] [Google Scholar]
- 3.Casoli T, et al. Release of beta-amyloid from high-density platelets: implications for Alzheimer’s disease pathology. Ann. N. Y. Acad. Sci. 2007;1096:170–8. doi: 10.1196/annals.1397.082. [DOI] [PubMed] [Google Scholar]
- 4.van Oijen M, Hofman A, Soares HD, Koudstaal PJ, Breteler MM. Plasma Aβ1–40 and Aβ1–42 and the risk of dementia: a prospective case-cohort study. Lancet Neurol. 2006;5:655–60. doi: 10.1016/S1474-4422(06)70501-4. [DOI] [PubMed] [Google Scholar]
- 5.Blasko I, et al. Conversion from cognitive health to mild cognitive impairment and Alzheimer’s disease: prediction by plasma amyloid beta 42, medial temporal lobe atrophy and homocysteine. Neurobiol Aging. 2008;29:1–11. doi: 10.1016/j.neurobiolaging.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Abdullah L, et al. The influence of diagnosis, intra- and inter-person variability on serum and plasma Aβ levels. Neurosci Lett. 2007;428:53–8. doi: 10.1016/j.neulet.2007.09.058. [DOI] [PubMed] [Google Scholar]
- 7.Luis CA, et al. Serum β-amyloid levels correlate with cognitive impairment. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2008;15:1–16. [Google Scholar]
- 8.Ertekin-Taner N, et al. Linkage of plasma Aβ42 to a quantitative locus on chromosome 10 in late-onset Alzheimer’s disease pedigrees. Science. 2000;290:2303–4. doi: 10.1126/science.290.5500.2303. [DOI] [PubMed] [Google Scholar]
- 9.Ertekin-Taner N, et al. Plasma amyloid β protein is elevated in late-onset Alzheimer disease families. Neurology. 2008;70:596–606. doi: 10.1212/01.WNL.0000278386.00035.21. [DOI] [PubMed] [Google Scholar]
- 10.De Jonghe C, et al. Evidence that Aβ42 plasma levels in presenilin-1 mutation carriers do not allow for prediction of their clinical phenotype. Neurobiol Dis. 1999;6:280–7. doi: 10.1006/nbdi.1999.0247. [DOI] [PubMed] [Google Scholar]
- 11.Scheuner D, et al. Secreted amyloid β-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat Med. 1996;2:864–70. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 12.Sleegers K, et al. Familial clustering and genetic risk for dementia in a genetically isolated Dutch population. Brain. 2004;127(Pt 7):1641–9. doi: 10.1093/brain/awh179. [DOI] [PubMed] [Google Scholar]
- 13.Houlden H, et al. Confirmation that familial clustering and age of onset in late onset Alzheimer’s disease are determined at the apolipoprotein E locus. Neurosci Lett. 1994;174:222–4. doi: 10.1016/0304-3940(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 14.Evans DA, et al. Apolipoprotein E epsilon4 and incidence of Alzheimer disease in a community population of older persons. JAMA. 1997;277:822–4. [PubMed] [Google Scholar]
- 15.Rosendorff C, Beeri MS, Silverman JM. Cardiovascular risk factors for Alzheimer’s disease. Am J Geriatr Cardiol. 2007;16:143–9. doi: 10.1111/j.1076-7460.2007.06696.x. [DOI] [PubMed] [Google Scholar]
- 16.Skoog I, et al. 15-year longitudinal study of blood pressure and dementia. Lancet. 1996;347:1141–5. doi: 10.1016/s0140-6736(96)90608-x. [DOI] [PubMed] [Google Scholar]
- 17.Launer LJ, et al. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol Aging. 2000;21:49–55. doi: 10.1016/s0197-4580(00)00096-8. [DOI] [PubMed] [Google Scholar]
- 18.Kivipelto M, et al. Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. BMJ. 2001;322:1447–51. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Notkola IL, et al. Serum total cholesterol, apolipoprotein E epsilon 4 allele, and Alzheimer’s disease. Neuroepidemiology. 1998;17:14–20. doi: 10.1159/000026149. [DOI] [PubMed] [Google Scholar]
- 20.Gentile MT, et al. β-amyloid deposition in brain is enhanced in mouse models of arterial hypertension. Neurobiol Aging. 2009;30:222–8. doi: 10.1016/j.neurobiolaging.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Deane R, Wu Z, Zlokovic BV. RAGE (yin) versus LRP (yang) balance regulates Alzheimer amyloid β-peptide clearance through transport across the blood-brain barrier. Stroke. 2004;35(11 Suppl 1):2628–31. doi: 10.1161/01.STR.0000143452.85382.d1. [DOI] [PubMed] [Google Scholar]
- 22.Wenham PR, Newton CR, Price WH. Analysis of apolipoprotein E genotypes by the Amplification Refractory Mutation System. Clin Chem. 1991;37:241–4. [PubMed] [Google Scholar]
- 23.Caselli RJ, et al. Cognitive domain decline in healthy apolipoprotein E epsilon4 homozygotes before the diagnosis of mild cognitive impairment. Arch Neurol. 2007;64:1306–11. doi: 10.1001/archneur.64.9.1306. [DOI] [PubMed] [Google Scholar]
- 24.Cosentino S, et al. APOE epsilon 4 allele predicts faster cognitive decline in mild Alzheimer disease. Neurology. 2008;70(19 Pt 2):1842–9. doi: 10.1212/01.wnl.0000304038.37421.cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rockwood K. Epidemiological and clinical trials evidence about a preventive role for statins in Alzheimer’s disease. Acta Neurol Scand Suppl. 2006;185:71–7. doi: 10.1111/j.1600-0404.2006.00688.x. [DOI] [PubMed] [Google Scholar]
- 26.Haag MD, Hofman A, Koudstaal PJ, Stricker BH, Breteler MM. Statins are associated with a reduced risk of Alzheimer disease regardless of lipophilicity. The Rotterdam Study. J. Neurol. Neurosurg. Psychiatry. 2008 Oct 17; doi: 10.1136/jnnp.2008.150433. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Sparks DL, et al. Reduced risk of incident AD with elective statin use in a clinical trial cohort. Curr Alzheimer Res. 2008;5:416–21. doi: 10.2174/156720508785132316. [DOI] [PubMed] [Google Scholar]
- 28.Wolozin B, et al. Simvastatin is associated with a reduced incidence of dementia and Parkinson’s disease. BMC Med. 2007;5:20. doi: 10.1186/1741-7015-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Höglund K, Blennow K. Effect of HMG-CoA reductase inhibitors on beta-amyloid peptide levels: implications for Alzheimer’s disease. CNS Drugs. 2007;21:449–62. doi: 10.2165/00023210-200721060-00002. [DOI] [PubMed] [Google Scholar]
- 30.Blasko I, et al. Effects of medications on plasma amyloid β (Aβ) 42: longitudinal data from the VITA cohort. J Psychiatr Res. 2008;42:946–55. doi: 10.1016/j.jpsychires.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 31.Sousa M, Berglund ML, Saraiva MJ. Transthyretin in high density lipoproteins: association with apolipoprotein A-I. J. Lipid Res. 2000;41:58–65. [PubMed] [Google Scholar]
- 32.Matsubara E, Frangione B, Ghiso J. Characterization of apolipoprotein J-Alzheimer’s Aβ interaction. J Biol Chem. 1995;270:7563–7. doi: 10.1074/jbc.270.13.7563. [DOI] [PubMed] [Google Scholar]
- 33.Koudinov A, Matsubara E, Frangione B, Ghiso J. The soluble form of Alzheimer’s amyloid β protein is complexed to high density lipoprotein 3 and very high density lipoprotein in normal human plasma. Biochem Biophys Res Commun. 1994;205:1164–71. doi: 10.1006/bbrc.1994.2788. [DOI] [PubMed] [Google Scholar]
- 34.Wilson LM, et al. High density lipoproteins bind Aβ and apolipoprotein C-II amyloid fibrils. J Lipid Res. 2006;47:755–60. doi: 10.1194/jlr.C500022-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Balakrishnan K, et al. Plasma Aβ42 correlates positively with increased body fat in healthy individuals. J Alzheimers Dis. 2005;8:269–82. doi: 10.3233/jad-2005-8305. [DOI] [PubMed] [Google Scholar]
- 36.Arvanitakis Z, Lucas JA, Younkin LH, Younkin SG, Graff-Radford NR. Serum creatinine levels correlate with plasma amyloid β protein. Alzheimer Dis Assoc Disord. 2002;16:187–90. doi: 10.1097/00002093-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 37.Weggen S, et al. A subset of NSAIDs lower amyloidogenic Aβ42 independently of cyclooxygenase activity. Nature. 2001;414:212–6. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- 38.Szekely CA, et al. No advantage of Aβ 42-lowering NSAIDs for prevention of Alzheimer dementia in six pooled cohort studies. Neurology. 2008;70:2291–8. doi: 10.1212/01.wnl.0000313933.17796.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Green RC, Schneider LS, Hendrix SB, Zavitz KH, Swabb E. Safety and efficacy of tarenflurbil in subjects with mild Alzheimer’s disease: results from an 18-month multi-center phase 3 trial. Alzheimers Dement. 2008;4(4 Suppl):T165. [Google Scholar]
- 40.Breitner JCS. Onset of Alzheimer’s dementia occurs commonly without prior cognitive impairment: results from the Alzheimer’s disease anti-inflammatory prevention trial (ADAPT) Alzheimers Dement. 2008;4(4 Suppl):T130–1. doi: 10.1016/j.jalz.2007.10.007. [DOI] [PubMed] [Google Scholar]