Abstract
Synaptic accumulation of glutamate causes neuronal death in many neurodegenerative pathologies such as amyotrophic lateral sclerosis. Drugs capable of increasing glutamate uptake could therefore be therapeutically effective. We screened in a cell-based assay a library of 1040 FDA-approved drugs and nutrients for compounds that could enhance glutamate uptake. Nordihydroguaiaretic acid (NDGA), an anti-inflammatory drug that inhibits lipoxygensases, potently enhanced glutamate uptake in MN-1 cells. Given subcutaneously at 1 mg/day for 30 days in mice, NDGA increased glutamate uptake in spinal cord synaptosomes persistently throughout the treatment. However, when administered following the same regimen to the SOD1-G93A transgenic mouse model of ALS at disease onset, NDGA did not extend survival of these mice. We found that NDGA failed to sustain increased glutamate uptake in the SOD1-G93A mice despite an initial upregulation measured during the first 10 days of treatment. SOD1-G93A mice displayed a progressive increase in spinal cord expression levels of the efflux transporter P-glycoprotein beginning at disease onset. This increase was not a specific consequence of the NDGA treatment as we have measured it in untreated SOD1-G93A mice. Because P-glycoproteins control the extrusion of a broad range of toxins and xenobiotics and are responsible for drug resistance in many diseases including cancer and brain diseases such as epilepsy, we propose that the failure of NDGA in maintaining glutamate uptake upregulated in SOD1-G93A mice and its therapeutic inefficacy are due to acquired pharmacoresistance mediated by the increased expression of P-glycoprotein.
Keywords: Amyotrophic lateral sclerosis, glutamate transporter, excitotoxicity, pharmacoresistance, nordihydroguaiaretic acid, neurodegeneration, arachidonic acid, P-glycoprotein, neuroinflammation, SOD1
INTRODUCTION
Glutamate is the predominant excitatory neurotransmitter in the mammalian central nervous system (CNS). Abnormal accumulation of glutamate in the synaptic cleft and excessive activation of glutamate receptors contribute to neuronal death in acute insults to the CNS. The process, known as 'excitotoxicity', also contributes to neuronal loss in neurodegenerative diseases, including amyotrophic lateral sclerosis (ALS) (Pasinelli and Brown, 2006). Excitotoxicity is based on altered extracellular concentrations of glutamate, since it is this pool that can be toxic to neurons. The intracellular concentration of glutamate (1–10 mM) is 1,000 to 10,000-fold greater than the extracellular concentration (<1–10 µM) (Danbolt, 2001; Clements et al., 1992; Herman and Jahr, 2007). In the excitatory synapses of the nervous system termination of the excitatory neurotransmission occurs exclusively via a re-uptake mechanism mediated by high-affinity glutamate carriers. There are 5 high affinity, Na+-dependent glutamate transporters: EAAT1 (GLAST), EAAT2 (GLT-1), EAAT3 (EAAC1), EAAT4, and EAAT5. These transporters exhibit distinct properties in substrates affinity or inhibitors sensitivity. For instance, dihydrokainate (DHK) is a non-transportable EAAT2 (GLT-1) inhibitor ineffective, if not at millimolar concentration, on the other glutamate transporter subtypes.
Several studies indicate that genetic and pharmacological upregulation of expression levels and activity of the glutamate transporter EAAT2 (GLT-1) can offer neuroprotection in vitro and in vivo in animal models of neurodegeneration (Guo et al., 2003; Rothstein et al., 2005). On the contrary, loss of EAAT2 expression levels can adversely worsen the course of neurodegenerative diseases such as ALS, at least in animal models (Pardo et al., 2006). Therefore, a correct functioning of the glutamate transporters in general and of the EAAT2 subtype in particular is of fundamental importance. Rescuing or increasing glutamate transport in neurological disorders characterized by an excitotoxic component by modulating the activity of a specific glutamate transporter subtype or the overall cellular glutamate uptake could constitute an efficacious treatment for such pathological conditions.
Based on these premises, we established a cell-based assay to screen for compounds with glutamate transport enhancing activity. We screened a structurally diverse library of 1040 FDA-approved drugs and nutrients known to cross the blood brain barrier (MicroSource Discovery Systems, NINDS custom collection™) (Heemskerk et al., 2002) using an in vitro activity assay based on a clonal neural hybrid cell line, referred to here as MN-1 (Salazar-Grueso et al., 1991). The natural dicatechol nordihydroguaiaretic acid (NDGA), a generic lipoxygenase inhibitor with anti-inflammatory and anti-oxidant properties (Salari et al., 1984), was particularly potent in increasing the high affinity glutamate transport activity in MN-1 cells. NDGA was also active in vivo since we measured increased glutamate transport activity in synaptosomes prepared from mice treated up to 30 days with NDGA. However, when NDGA was chronically administered to the SOD1-G93A mouse model of ALS to determine if it could be therapeutically effective in this chronic neurodegenerative disease characterized by glutamate transport downregulation (Bendotti et al., 2001; Rothstein et al., 1993; Rothstein et al., 1992), we observed an initial increase in glutamate transport in spinal cord synaptosomes after 10 days of treatment that vanished at 20 and 30 days of treatment. Kaplan-Meier survival analysis showed that the SOD1-G93A mice did not benefit from the NDGA treatment, presumably in part because the NDGA treatment failed in keeping glutamate uptake upregulated. Interestingly, SOD1-G93A mice showed increased expression levels of P-glycoprotein (P-gp) in the spinal cord during the progression of the disease. This increase was likely caused by the disease mechanisms and not related to the NDGA administration as we have observed it in untreated SOD1-G93A mice. Moreover, in vitro assay showed that NDGA increased P-gp basal activity measured in vesicles strongly suggesting that it could be used as substrate by this transporter in vivo (Garrigues et al., 2002; Gebhardt et al., 2002). As these proteins are involved in multi-drug resistance via a transport-mediated mechanism of extrusion of xenobiotics out of the cells in which they are expressed, we suggest that their constitutive increased expression levels in the spinal cord of SOD1-G93A mice could be responsible for the poor efficacy of NDGA in maintaining elevated glutamate transport activity in vivo, compromising its potential therapeutic efficacy.
MATERIALS AND METHODS
MN-1 cell culture, screening assay and glutamate transport activity measurement
We used a clonal neural hybrid cell line (MN-1) that expresses motor neuron characteristics and showed consistent glutamate transport activity and expression of Na+-dependent, high affinity glutamate transporters EAAT1 (GLAST), EAAT2 (GLT-1) and EAAT3 (EAAC1). Cells were grown in 24-well plates in DMEM supplemented with 10% FBS and penicillin/streptomycin. Compounds from the NINDS library collection or pharmacological agents were added for 12 hours to the cells at 37 °C in Opti-MEM (Gibco) at 1 µM in 0.1% DMSO or at concentrations otherwise indicated. Control groups received DMSO 0.1%. The next day compounds are removed by three washes with PBS and glutamate transport activity was measured for 15 min at room temperature by adding the uptake buffer in the presence of 1 µM glutamate (1:30 isotopic dilution with 3H-glutamate, NEN). Uptake buffer contained (in mM): 5 HEPES/Na, 145 NaCl, 2.5 KCl, 1.2 CaCl2, 1.2 MgCl2, 1.2 K2HPO4, 10 glucose, pH 7.4. Glutamate uptake is terminated by rapid aspiration followed by washing the cells with ice-cold uptake stopping buffer containing (in mM): 5 Tris-HCl, 145 CholineCl, 2.5 KCl, 1.2 CaCl2, 1.2 MgCl2, 1.2 K2HPO4, 10 Glucose, pH 7.4. Cells are harvested with lysis buffer containing 0.5 N NaOH and 0.1% Triton X-100, and accumulated radioactivity measured by liquid scintillation counting. For saturation experiments the uptake solution contained L-glutamate in the concentration range 1–250 µM and 0.5 µCi of L-3H-glutamate as radiotracer.
Synaptosome preparation
Synaptosomes were prepared from spinal cords of mice according to our published protocol with small modifications (Volterra et al., 1992). Synaptosomes (50 µg/ml) were incubated at 37 °C for 15 min under shaking conditions in oxygenated Krebs/HCO−3 buffer containing (in mM): NaCl 124, KCl 4.6, CaCl2 1.2, MgCl2 × 6H2O 1.3, KH2PO4 0.42, NaHCO3 26.75, glucose 10, adjusted to pH 7.4 and 10 µM [3H]-glutamate (N.E.N. 41.9 Ci/mmol; isotopic dilution 1:4,000). The uptake was terminated by addition of ice-cold uptake stopping buffer with 100-fold excess cold glutamate and rapid filtration on 0.45 µm nitrocellulose filters. Filter blotted synaptosomes were counted by liquid scintillography for incorporated radioactivity. Accumulation of glutamate in synaptosomes in the presence of Na+ is usually between 100,000 and 140,000 d.p.m. at the isotopic dilution indicated above. Radioactivity retained after incubation in Choline+ uptake buffer was used to correct data to represent Na+-dependent uptake. Experimental samples were run at least in triplicate.
Data Analysis
Uptake kinetics was determined by non-linear regression analysis of saturation curves using Michaelis-Menten equation built in GraFit v5.0 (Eritacus Software). Log concentration-response curve were constructed for the determination of IC50 and data were fitted using a four-parameter logistic equation built in GraFit v5.0. IC50 values were converted to Ki (inhibition constant) values according to the equation Ki= IC50/1+[s]/Km where [s] equals the concentration of L-glutamate substrate (1 µM) and Km equals the estimated Km for glutamate calculated with the Michaelis-Menten transformation. All the data presented in this study represent the average ± s.e.m. of at least three independent determinations.
Western Blot analysis
Spinal cords were collected from SOD1-G93A mice at different stages of disease and from SOD1-wild type mice age-matched with end-stage SOD1-G93A mice and immediately homogenized on ice (glass-teflon homogenizer) in 30 volumes of PBS with 1% SDS and protease inhibitor mix (Complete ™). MN-1 cells were also collected and homogenized in the same buffer. The SDS-extracts were then incubated for 10 min. at room temperature, sonicated, centrifuged at 1,000 × g to remove unsolubilized material and immediately analyzed or stored at −80 °C. Immunoblots were probed with anti-P-glycoprotein (Mdr; polyclonal rabbit antibody H241; Santa Cruz Biotechnology; 1 µg/ml; this antibody recognizes both P-gp isoforms, Mdr1a and Mdr1b in rodents). Affinity-purified polyclonal antibodies against glutamate transporter subtypes were A522-541 (0.2 µg/ml; rabbit 8D0161, rat EAAT1 C-terminus), B12-26 (0.1 µg/ml; rabbit 26970, rat EAAT2 N-terminus), B493-508 (0.1 µg/ml, rabbit 84946, rat EAAT2 C-terminus), ABR556-573 (Affinity BioReagent; cat.#PA3-040, 1:1,000, mouse EAAT2 -terminus), and C161-177 (0.5 µg/ml, rat EAAT3 extracellular loop, Zymed lab). Band intensity was quantified using the Chemidoc system (BioRad). The anti-EAAT antibodies were raised against identical epitopes in mouse and rat isoforms.
Mice models of ALS and pharmacological treatment
Human SOD1-wild type (B6SJL-TgN(SOD1)2Gur; stock #002297; Jackson Lab) and SOD1-G93A mutant (B6SJL-TgN(G93A-SOD1)1Gur; stock #002726; Jackson Lab) transgenic mice were used for this study. Male SOD1-G93A mice were bred in house with B6SJL females. Offspring were assessed for the presence of the human SOD1 transgene and copy number by PCR. NDGA and placebo were delivered in a time-controlled release manner using subcutaneous implantation of matrix-driven delivery pellets (Innovative Research of America). The experimental groups receiving NDGA or placebo consisted of 17 mice, 6 males and 11 females. The pellet technology integrates the principles of drug diffusion, erosion and concentration gradient by generating a finished pellet with a biodegradable matrix that effectively and continuously releases the active product in the animal in a time-controlled manner. Placebo pellets containing all the components of the drug pellets except the active product itself were used in experiments as the proper control. The pellets were implanted subcutaneously on the back of the mouse neck using a 10 gauge precision trochar. We used pellets that were tailored for 10 days delivery and were administered at dosages that corresponded to about one tenth of the LD50 (dose that is lethal to 50% of the animals) as defined by FDA toxicology reports. For chronic regimen we implanted new pellets every 10 days. Signs of explicit toxicity such as inflammatory rectal lesions, hemorrhage and weight loss were monitored on a daily bases.
P-gp drug interaction assay
We used a P-gp drug interaction assay kit (Cayman Chemicals, cat. # 789201) to determine whether NDGA could affect the activity of P-gp. The system is based on the determination of basal or induced ATPase activity in membrane vesicles prepared from cells abundantly expressing P-gp proteins. P-gp mediated ATPase activity is measured by a spectrophotometric method (340 nm) based on continuous monitoring of ADP formation in the vesicle suspension medium (Garrigues et al., 2002).
Survival analysis of SOD1-G93A mice
Death, or end-stage of disease, was scored at the time-point at which the mice cannot right themselves within 30 s when laid on their sides. The time of euthanasia was recorded for survival analysis. Kaplan-Meier analysis was used for survival comparison.
RESULTS
Characterization of glutamate uptake in MN-1 neural cell line
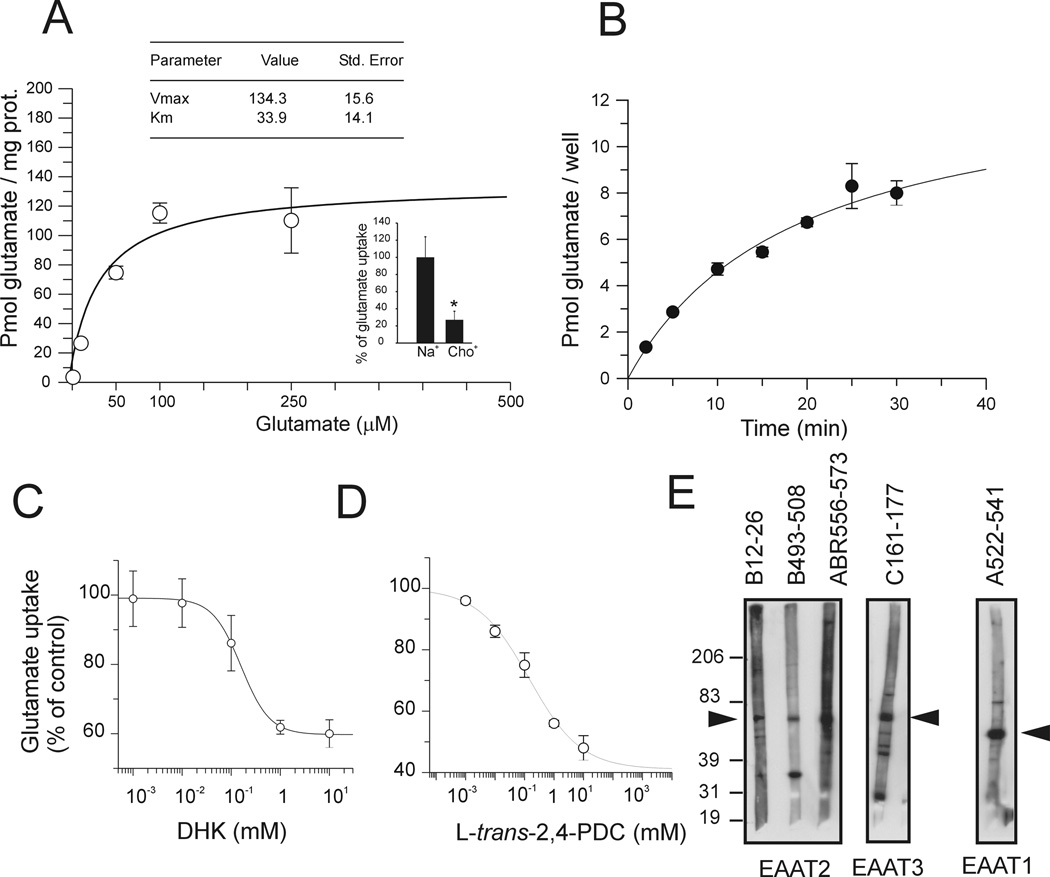
The neural cell line MN-1 displayed Na+-dependent, high-affinity glutamate transport activity. Na+ substitution from the incubation medium with equimolar concentration of Choline+ resulted in ~75% reduction in L-glutamate transport indicating that the accumulation of glutamate in these cells was predominantly Na+-dependent (Fig.1A inset). Moreover, saturation analysis showed that glutamate uptake followed a Michaelis-Menten kinetic with high affinity for glutamate (Km of 33.9 ± 14.1 µM) (Fig.1A). Uptake velocity measured at room temperature in the presence of 1 µM glutamate followed a saturation curve with Vmax of 13.3 ± 1.3 pmol glutamate/well and half saturation point reached at 18.8 ± 3.8 min. (Fig. 1B). We carried out basic pharmacological characterization of the Na+-dependent glutamate transport activity using the EAAT2-selective, non-transportable inhibitor dihydrokainic acid (DHK) and the competitive transportable EAAT1-4/non-transportable EAAT5 inhibitor L-trans-Pyrrolidine-2,4-dicarboxylic acid (trans-PDC). Maximum inhibition of glutamate uptake by DHK was ~40% (Ki = 155 ± 16.6 µM), whereas PDC inhibition was ~70% with Ki of 223 ± 11 µM (Fig.1C–D). Western blot analysis showed that MN-1 cells expressed at least the three major glutamate transporter subtypes, EAAT1 (GLAST), EAAT2 (GLT1) and EAAT3 (EAAC1). EAAT2 expression was detected by western blot using three different polyclonal antibodies directed against different epitopes of the transporter, whereas EAAT1 expression was detected with an antibody against the cytoplasmic C-terminal domain and EAAT3 with an antibody against the extracellular loop domain (Fig. 1E).
Fig.1. Characterization of the glutamate uptake in MN-1 cells.
(A) Michaelis-Menten analysis of glutamate uptake in MN-1 cells was performed by subtracting Na+-independent glutamate uptake component (measured in the presence of Choline buffer) from the total glutamate uptake at any given glutamate concentration. (B) Time-course saturation analysis of glutamate uptake performed at 1 µM glutamate. Calculated half time to reach saturation was 18.8 ± 3.8 min. (C,D) Inhibitor IC50 values were estimated by non-linear regression analysis and were converted to Ki (inhibition constant) according to the equation Ki=IC50/1+[S]/Km where [S] equals 1 µM glutamate and Km is the estimated Km obtained by the Michaelis-Menten equation. (E) Western blot analysis of MN-1 homogenates (SDS-extract) performed with different antibodies raised against EAAT1-3 glutamate transporters.
In vitro characterization of NDGA effect on glutamate uptake
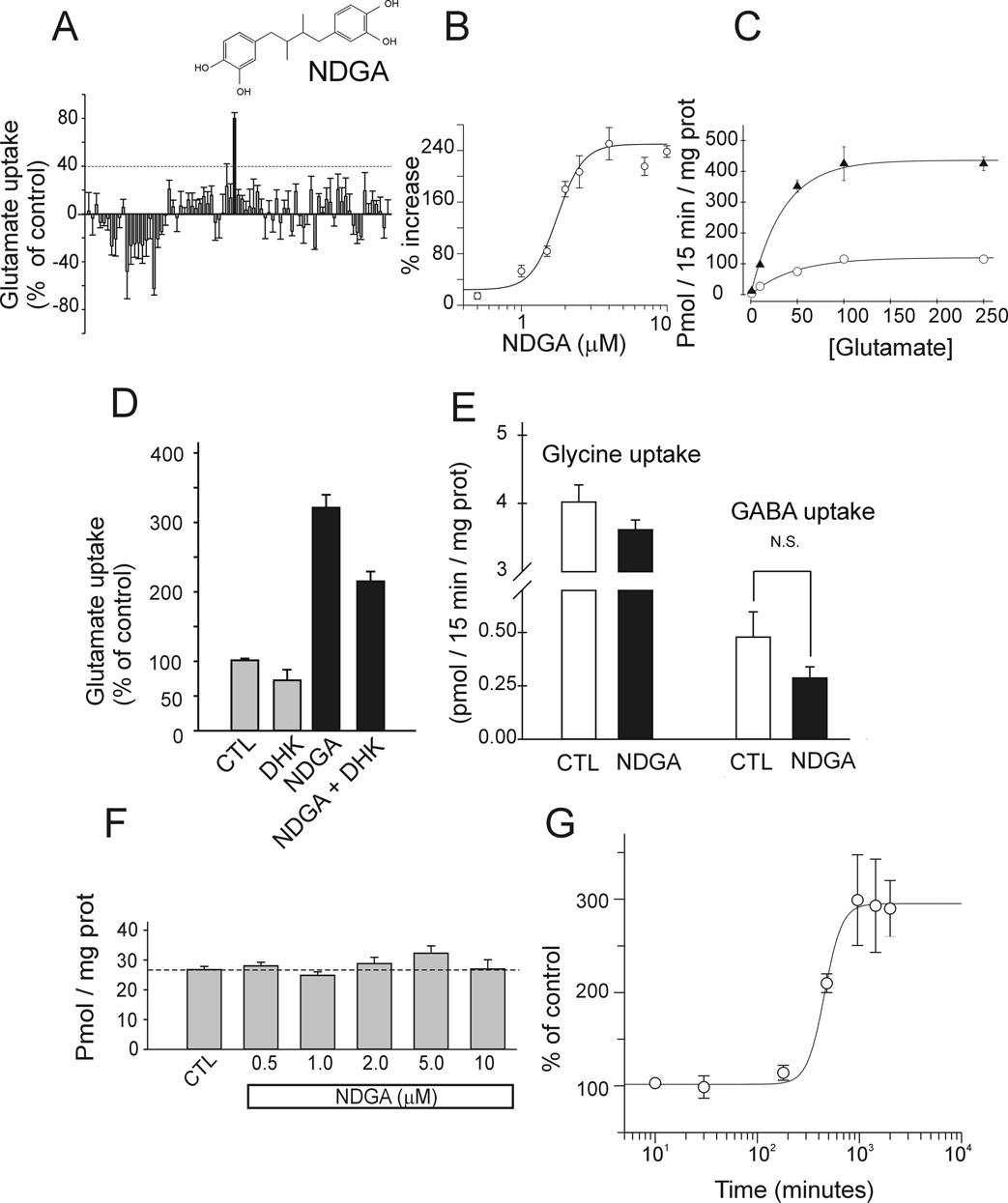
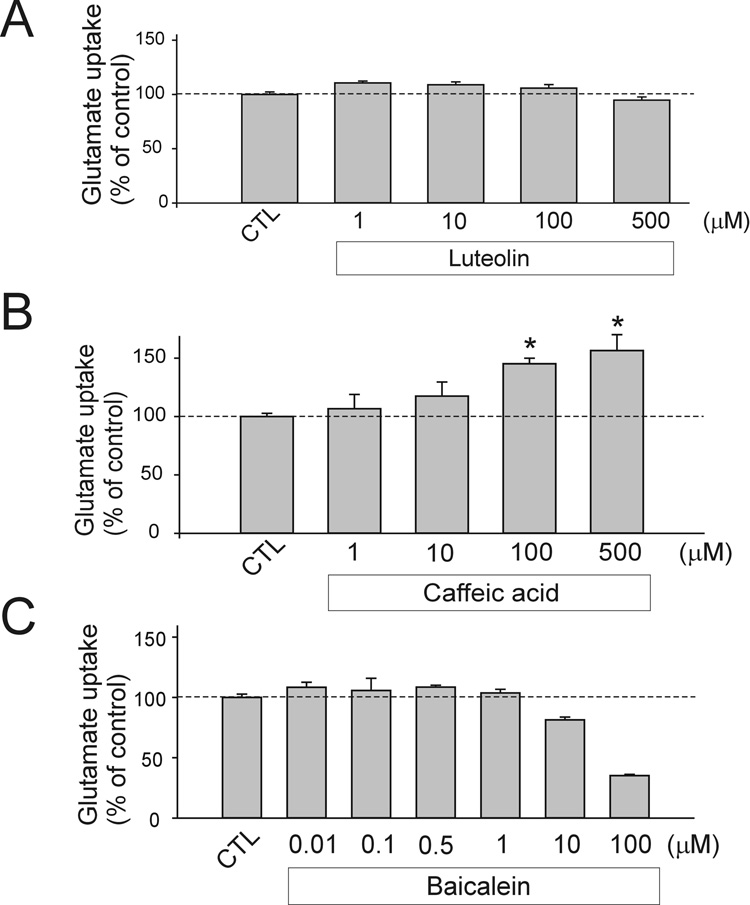
To identify compounds capable of increasing glutamate transport activity, we used the MN-1 neural cell line as screening platform (Salazar-Grueso et al., 1991). MN-1 cells were incubated with different compounds of the Microsource Discovery chemical library (Heemskerk et al., 2002) (NINDS custom collection) for 12 hours at 1 µM in 0.1% DMSO (Fig.2A). NDGA was identified as a potent glutamate transport enhancer and its effect characterized. Dose-response experiment showed that NDGA EC50 was 1.7 ± 0.2 µM (Fig. 2B). Increased Vmax of uptake accounted for the increase in glutamate transport whereas Km was not significantly affected (37.3 ± 12.8 µM for control vs. 27.4 ± 9.9 µM for NDGA) (Fig.2C). DHK-sensitive glutamate uptake accounted for a large portion of the Na+-dependent glutamate uptake (~40%) in MN-1. This DHK component likely reflects the activity of EAAT2 in these cells. EAAT2 appears therefore an important determinant of the MN-1 cells overall glutamate transport activity. Treatment with NDGA (4 µM) increased the DHK-sensitive component of glutamate uptake in MN-1 cells by ~3-fold, suggesting that EAAT2 activity was up-regulated (Fig.2D). NDGA-mediated increase in the Na+-dependent glutamate transport activity in cells could be achieved by alteration of the Na+ gradient across the plasma membrane, for example by modulation of the activity of the Na/K ATPase pump. To explore this possibility, we measured the effect of NDGA on other Na+-dependent uptake systems. We found that the same concentration of NDGA that increased glutamate uptake in MN-1 cells did not affect the Na+-dependent glycine and GABA uptake, indicating that the NDGA effect was selective toward the glutamate transport system and not due to alterations in the Na+ gradient across the cell membrane (Fig.2E). Suggesting a different mechanism of action other than alteration of the Na+ gradient was also the evidence that NDGA did not upregulate glutamate uptake if incubated only during the 15 minutes of the uptake assay (Fig.2F). Indeed, to achieve a meaningful glutamate uptake enhancing activity, NDGA must be incubated with MN-1 cells for at least 3 hours, with an EC50 of 456 ± 28 min (~ 8 hrs) for the time-course of the effect (Fig. 2G). NDGA is a compound that interferes with the arachidonic acid metabolic pathway; in particular NDGA blocks 5, 12, 15-Lipoxygenases (LOX). To determine if the LOX enzymatic pathway was involved in the NDGA-mediated glutamate uptake increase effect we incubated for 16 hours MN-1 cells with selective inhibitors of the different lipoxygenases and looked at the effect on glutamate transport. Caffeic acid, a potent inhibitor of 5- and 12-LOXs, increased glutamate uptake similarly to NDGA, despite with less efficacy. On the contrary, luteolin, a selective inhibitor of 15-LOX, and baicalein, a selective inhibitor of 12-LOX, failed to mimic NDGA effect, suggesting that the effect of NDGA was mediated through inhibition of 5-LOX (Fig.3A–C). Cycloxygenases were not involved in the NDGA effect as naproxene, a potent inhibitor of both COX-1 and 2 did not increase glutamate uptake in MN-1 cells (data not shown).
Fig.2. Characterization of the NDGA effect on glutamate uptake in MN-1 cells.
(A) Representative results obtained by screening plate #13 of the NINDS-FDA approved compound library collection. The positive hit corresponded to NDGA. An arbitrary cut-off of 40% increase (more than 2 times the average standard deviation) in glutamate uptake was chosen to select positive hits in the library. (B) Dose-response analysis for NDGA was estimated by non-linear regression analysis. EC50 for NDGA was 1.7 ± 0.2 µM. (C) Experiments to determine Michaelis-Menten kinetic parameters were performed in the presence (▲) and absence (○) of 4 µM NDGA. No change in Km for glutamate was measured. (D) DHK-sensitive component of glutamate uptake in MN-1 cells is increased ~3 times by 4 µM NDGA. Uptake was measured in the presence of 1 mM DHK. (E) 4 µM NDGA did not affect glycine and GABA uptake in MN-1 cells; N.S.: non significant (P=0.17). (F) Dose-response for acute treatment with NDGA; NDGA was incubated for 15 minutes during the uptake assay. (G) Time-dependent effect of 4 µM NDGA on glutamate uptake. Half maximum effect was 456 ± 28 min. NDGA was pre-incubated with the cells for the indicated time. Glutamate uptake was measured for 15 min. immediately after NDGA was removed.
Fig.3. 5-Lipoxygenase inhibition increases glutamate uptake in MN-1 cells.
Three different lipoxygenase inhibitors were tested on glutamate uptake in MN-1 cells. Inhibitors of the lipoxygenase pathway were incubated overnight at the indicated concentrations in 0.1% DMSO. Control groups were treated with 0.1% DMSO.
NDGA increased glutamate uptake in vivo in mice
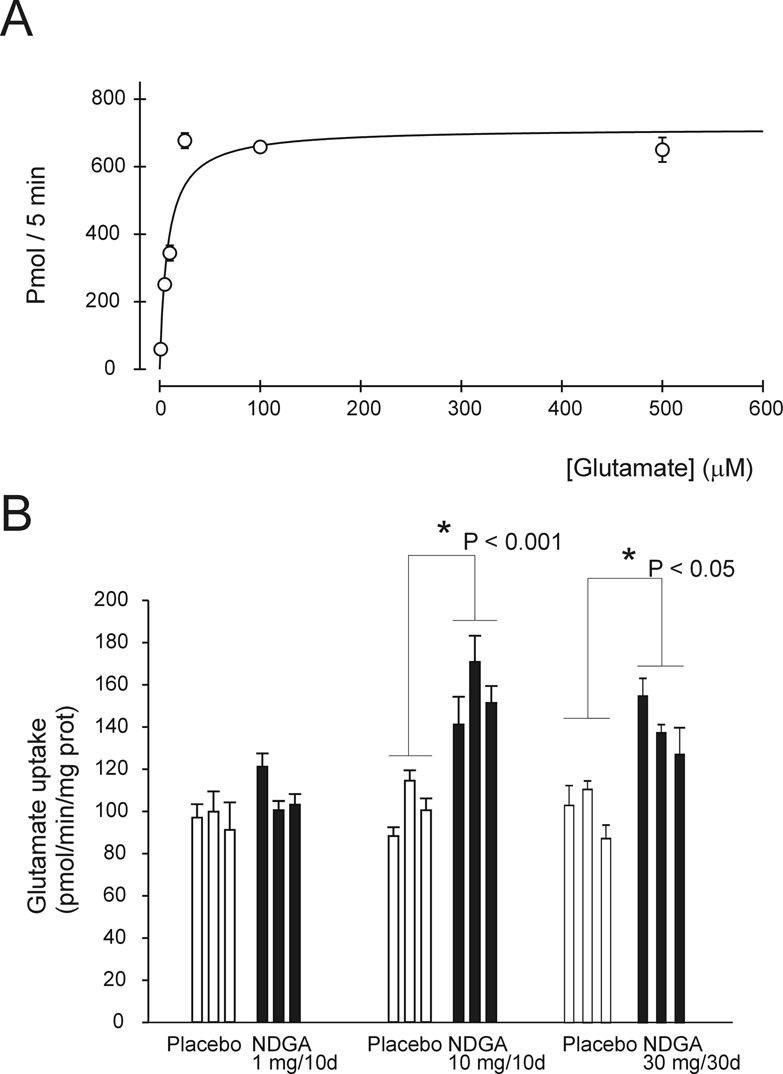
To determine whether NDGA was effective also in vivo, we measured glutamate uptake in synaptosomes isolated from spinal cord of mice treated with NDGA. Glutamate uptake in forebrain synaptosomes followed Michaelis-Menten kinetic, it was temperature dependent and showed high affinity and high capacity for glutamate (Km = 8 ± 3 µM; Vmax = 727 ± 64 pmol/5min/mg prot) (Fig. 4A). NDGA was administered to mice beginning at ~ 3 months of age at 5 and 50 mg/Kg body weight/day (average mouse weight is 20 g). Subcutaneous implantation of one NDGA-releasing pellet (tailored to release 10 mg of NDGA in10 days) significantly increased (~ +60%) glutamate uptake measured in spinal cord synaptosomes 10 days after it was implanted. Implantation of NDGA-pellets (10 mg/10 day) at 10-day intervals (3 pellets implanted for a total of 30 mg of NDGA delivered in 30 days) was able to keep upregulated glutamate uptake for 30 days (Fig.4B).
Fig.4. In vivo effect of chronic administration of NDGA on glutamate uptake.
(A) Glutamate uptake was measured at 37 °C in synaptosomes prepared from spinal cord of non-transgenic mice. (B) Glutamate uptake was measured in spinal cord synaptosomes prepared from non-transgenic mice treated with placebo or NDGA containing pellets. Delivery of 30 mg of NDGA over 30 days was achieved by implanting 3 pellets, tailored to deliver 10mg over 10 days, at 10 day intervals (from day 0 – 20). Uptake was measured 10 and 30 days after the implantation of the first pellet.
NDGA treatment of SOD1-G93A mice model of ALS
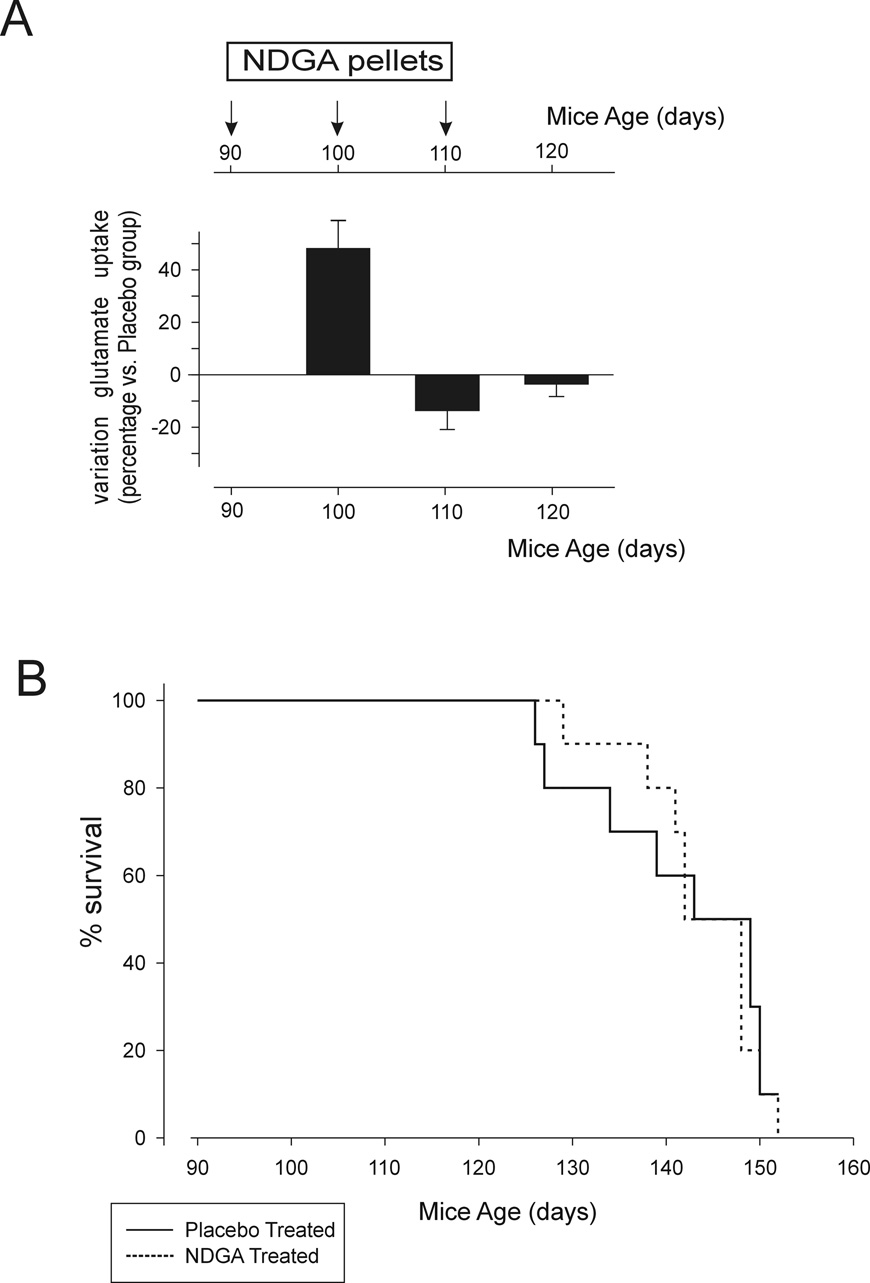
Impairment of glutamate uptake is well documented both in human ALS and in animal model of the disease (Maragakis and Rothstein, 2006). Genetically and pharmacologically increased expression levels and activity of the glutamate transporter EAAT2 offered some degree of neuroprotection in ALS (Guo et al., 2003; Rothstein et al., 2005; Pardo et al., 2006; Ganel et al., 2006). On this ground, we hypothesized that NDGA could also be therapeutically effective in prolonging the survival if administered to the transgenic SOD1-G93A ALS mice. NDGA was given to the SOD1-G93A mice in a time-controlled release manner using subcutaneous implantation of NDGA pellets (10 mg every 10 days). Drug or placebo pellets were implanted in mice beginning at approximately onset of disease (90 days of age) and then every 10 days. We chose to begin administering NDGA at disease onset as this regimen, if successful, would be more relevant as a potential therapeutic treatment of human ALS than administering the drug during the pre-symptomatic phase of the disease. In addition, impairment of glutamate uptake in ALS mice is only seen during disease progression, with significant loss of EAAT2 expression levels after the onset stage (Bendotti et al., 2001; Canton et al., 1998; Howland et al., 2002). In a parallel set of SOD1-G93A mice treated following the same NDGA or placebo regimen, we measured glutamate uptake in synaptosomes prepared from spinal cord of these mice at 100, 110 and 120 days. After 10 days from the first implantation of NDGA pellet, glutamate uptake activity was upregulated by approximately 50% compared to placebo treated mice. However, after 20 or 30 days of treatment, the increase in glutamate uptake dropped to the same levels as in placebo treated mice (Fig.5A). The failure of the NDGA treatment to sustain enhanced glutamate uptake activity in SOD1-G93A mice over time contrasted with the effect measured in non-transgenic control mice (Fig.4B). Kaplan-Meier survival plot in NDGA-treated versus placebo-treated SOD1-G93A mice showed that despite an initial, marginal positive shift in the survival curve, NDGA did not prolong the survival of the SOD1-93A mice (Fig.5B).
Fig.5. Effect of chronic administration of NDGA on SOD1-G93A mice model of ALS.
(A) Glutamate uptake measured in spinal cord synaptosomes prepared from SOD1-G93A mice treated with placebo or NDGA pellets (10mg/10day pellet). Data are expressed as percentage variation against placebo treated groups. Pellets were implanted in mice at 90 days of age and every 10 days until 110 days of age. We did not implant pellets in mice 120 days of age or older because these mice were already late in the disease and would not survive the anesthesia procedure for the implantation. Data represent average of measurements done in 3 mice per experimental group. (B) Kaplan-Meier survival plot of NDGA (dashed line) vs. placebo (full line) treated SOD1-G93A mice. NDGA or placebo was delivered as described in (A). Each group consisted of 17 mice (6 males + 11 females). The average survival of NDGA treated mice was not statistically different from control placebo treated mice, even when results were broken down by gender.
Increased expression levels of P-glycoprotein transporters in SOD1-G93A mice
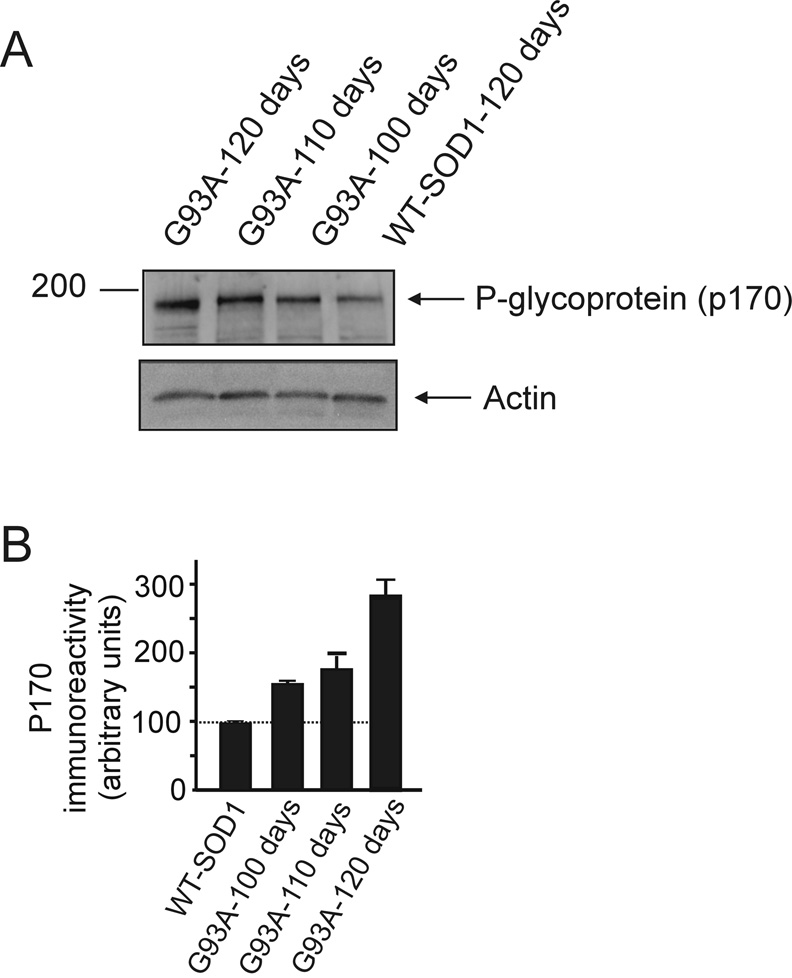
A possible cause for the lack of persistent effect of NDGA on glutamate uptake could be its decreased bio-availability during disease progression. This resistance to drug treatment is an important hurdle in the pharmacological therapy of many disorders, including brain cancer, epilepsy, depression and could be mediated, in addition or in alternative to drug-related mechanisms such as metabolic tolerance, by induction in certain cells of expression of drug efflux transporters that act by extruding both endogenous toxins and a broad range of xenobiotics (Loscher and Potschka, 2005). Expression levels of drug efflux transporters such as P-glycoprotein are increased in the brain following neuroinflammatory processes. Because ALS displays a neuroinflammatory component (Hensley et al., 2006), we looked at the expression levels of multi-drug resistance transporters such as P-glycoprotein (P-gp also known as ABCB1 transporter according to the HUGO nomenclature (Klein et al., 1999)) in spinal cord homogenates of SOD1-G93A mice during progression of the symptomatic phase of the disease. Western blot analysis performed in spinal cord homogenates of SOD1-G93A mice showed that immunoreactivity for P-gp remarkably increased during progression of the disease compared to age-matched SOD1-wild type mice (Fig.6 upper and lower panels) or even non-transgenic mice (not shown), suggesting that these drug efflux transporters are markedly up-regulated in the ALS mice. We also found that similar to Verapamil, a known substrate for P-gp (Langer et al., 2007; Hendrikse et al., 1998), NDGA modulates the activity of P-gp in isolated membrane vesicles (Table 1) (Seelig, 1998). Although this assay does not provide direct evidence that NDGA can be transported by P-gp, the modulation of P-gp activity strongly indicated that NDGA could serve as substrate for this transporter (Garrigues et al., 2002).
Fig.6. Expression profile of the drug transporter P-glycoprotein in the spinal cord of SOD1-G93A mice at different stages of disease.
(A) Western blot analysis of spinal cord homogenates (SDS-extracts) from SOD1-G93A mice at different stages of disease progression (100 days: early symptomatic; 110 days: symptomatic; 120 days: late symptomatic) and human SOD1-WT mouse spinal cord homogenate age-matched with symptomatic mice. The anti P-glycoprotein antibody was used at 1 µg/ml. (B) Immunoreactivity for P-glycoprotein was quantified by densitometry using Chemidoc system software (Biorad).
Table 1.
Effect of different concentrations of NDGA on basal P-gp activity measured in membrane vesicles
| NDGA (µM) | Relative activity |
|---|---|
| 0 | 1.00 |
| 0.05 | 1.18 ± 0.05 |
| 0.5 | 1.65 ± 0.38 |
| 50 | 2.05 ± 0.1 |
| Verapamil (µM) | |
| 30 | 1.7 ± 0.1 |
Basal activity was 150 ± 30 nmol/mg/min; Data are average ± s.e.m. of 4 experiments performed in duplicate
DISCUSSION
Excitotoxicity by overstimulation of glutamate receptors is one of the major causes of neuronal death (Choi, 1992). The therapeutic efficacy of glutamate receptor antagonists against in vitro model of excitotoxicity is well established, although the same approach pursued in vivo has led to disappointing results due to the vast deleterious side effects of these inhibitors. An alternative and probably much safer strategy to relieve neurons from the excitotoxic damage would therefore be to increase the clearance of synaptic glutamate by increasing the activity of glutamate transporters and glutamate uptake in general (Sheldon and Robinson, 2007). By screening a structurally diverse library of FDA-approved drugs and nutrients in a cell-based assay, we identified NDGA as a potent enhancer of glutamate transport activity. NDGA is effective both in vitro and in vivo. In vivo in control non-transgenic mice, the increase in glutamate uptake persisted upon chronic delivery of NDGA for at least 30 days, and peaked 10 days after the implantation of time-controlled NDGA delivery pellets. NDGA main pharmacological action is to inhibit the lipoxygenase (LOX) and cyclooxygenase (COX) metabolic pathways of arachidonic acid although the reported Ki values for these enzymes are dramatically different. Upregulation of glutamate uptake in MN-1 cells is achieved by inhibition of 5-Lipoxygenase as selective inhibitors of 12 and 15-Lipoxygenases did not affect glutamate uptake. The intimate mechanisms by which 5-LOX is involved in regulating the glutamate transport system in MN-1 cells and in vivo remain to be elucidated. Evidence gathered from this study suggests that glutamate transport upregulation, at least in MN-1 cells, may involve mechanisms at the gene expression and/or protein synthesis levels because a prolonged pre-incubation with NDGA (EC50 = ~ 8 hrs.) was required to promote the increase. It would remain to be established which glutamate transporter isoforms are affected by NDGA and whether the mechanisms involve a direct effect on the transporters themselves or through other regulatory proteins. Acute application of NDGA (present only during the 15 min. of uptake measurement) did not affect glutamate uptake in MN-1 cells ruling out an allosteric type of modulation by NDGA on the glutamate transporter molecules. Effect of NDGA on the regulation of homeostasis of Na+ gradient as a way to affect Na+-dependent transporters is also ruled out as other Na+-dependent transport mechanisms present in MN-1 cells, such as the GABA and Glycine uptakes are not affected.
As enhancer of glutamate transport activity in vitro and in vivo, NDGA could have the potential to be effective in a number of neurological conditions characterized by excitotoxic components. ALS is such a condition and we tested the effect on the disease phenotype of a chronic administration of NDGA in the mutant SOD1 mouse model of ALS. Similar therapeutic approaches have been already pursued in these mice by administering compounds like ceftriaxone or GPI-1046 that in vitro increased glutamate uptake mainly through increased expression of the glutamate transporter EAAT2 (Rothstein et al., 2005; Ganel et al., 2006).
However, the outcome of these animal trials was quite unsatisfactory, with scarce efficacy on the overall mice survival and duration of the disease. We found that despite the initial efficacy of NDGA in increasing glutamate uptake in spinal cord of the ALS mice, the effect progressively vanished as the disease progressed. This loss of efficacy is reminiscent of the multi-drug resistance observed in cancer cells during chemotherapy. All cells express efflux transporters that protect them from endogenous or exogenous potentially toxic substances (Loscher and Potschka, 2005). Most of these transporters belong to the superfamily of ATP-binding cassette proteins that drive the cellular extrusion of many drugs with different structures and uses. This phenotype that is referred to as multi-drug resistance (MDR) was first described for chemotherapy-resistant cancer cells that overexpressed one of these efflux transporters, P-glycoprotein. In the mice model of ALS the loss of NDGA effect on glutamate uptake coincided with increased expression levels in the spinal cord of the P-glycoprotein transporter, P-gp (MDR1). Several reports suggested that expression of efflux transporters such as P-gp are upregulated in neuroinflammatory conditions in endothelial cells, perivascular and parenchymal astrocytes that constitute the blood-brain barrier as well as in neurons (Volk et al., 2005). Remarkably, P-glycoprotein drug efflux transporters are upregulated in neurological conditions characterized by inflammatory processes like in epileptogenic brain tissues of patients with intractable epilepsy and in the rodent model of temporal lobe epilepsy and are thought to be responsible for the pharmacoresistance observed in 30% of epilepsy patients. Intriguingly, this upregulation of P-gp transporters appears to be regulated by excessive glutamate release which occurs in epileptic seizures (Bankstahl et al.; Zhu and Liu, 2004). Similarly, P-gp expression levels are upregulated in the mutant SOD1 mouse model of ALS, a neurodegenerative disease characterized by both neuroinflammation and glutamate excitotoxicity (Consilvio et al., 2004; Nguyen et al., 2002; Rothstein, 1995; McGeer and McGeer, 2002). The role of the efflux pump P-gp is of particular interested in the ALS context. Many pharmacological approaches have been attempted in ALS mice with little or no reproducible efficacy by testing a variety of structurally diverse compounds against molecular pathways believed to be altered by the disease mechanisms (Traynor et al., 2006). By extruding a broad range of xenobiotics from cells, P-gp confers the multidrug resistance phenotype on many cells, which invariably leads to poor clinical outcomes. It is therefore likely that the lack or loss of the NDGA efficacy in these mice could be consequential to its increased extrusion from the CNS, and the spinal cord in particular, due to the increased expression levels of P-gp proteins. The membrane assay we employed showed that NDGA interacts with P-gp as strongly as another known transported substrate structurally similar, verapamil (Langer et al., 2007; Hendrikse et al., 1998), suggesting that NDGA could be a substrate for these drug efflux transporters. This assay cannot accurately answer the question whether the tested compound that interacts with P-gp could also be a substrate for the drug transporter. Classification of drugs as substrates requires the use of radiolabeled compounds applied to cell cultures in monolayer. However, a comparison of cell systems and membrane assays have been recently described and as far as these studies are concerned it results that all compounds positive in the ATPase membrane assay are also potential substrates (Adachi et al., 2001; Polli et al., 2001). It has been proposed that the expression pattern of P-gp has a key role in the pathogenesis of brain diseases such as Alzheimer’s disease (Vogelgesang et al., 2002), Parkinson’s disease (Kortekaas et al., 2005) and brain HIV infection (Langford et al., 2004). ALS would seem to be no exception, although a more detailed analysis on the expression of P-gp and other drug efflux transporters in ALS mice and in human specimens would need to be undertaken. The product of the MDR1ab gene, the P-gp protein, has been shown to have an important role in the permeability of the blood-brain barrier (BBB) to a number of different compounds (Schinkel et al., 1996). Kirkinezos and colleagues created a transgenic SOD1-G93A ALS mouse with a permissive BBB in which the P-gp protein was knocked out (SOD1-G93A::MDR1−/−) and found that therapeutic treatment with Cyclosporin A, a compound that normally is not accumulated in the brain, improved survival of the ALS mice, although marginally compared to lack of effect observed in the transgenic ALS mouse with normal expression of P-gp (Kirkinezos et al., 2004). This observation underscored the importance of drug efflux proteins in modulating the efficacy of pharmacological treatments in vivo (Polli et al., 2001). The effect of these multi-drug resistance proteins on the pharmacokinetic and bioavailability of many potential therapeutic compounds is even more dramatic in diseases such as ALS in which pathological mechanisms lead to their upregulation. A logic outcome of our studies would be to repeat the NDGA trial in the ALS mice by co-administering together with NDGA a selective inhibitor of p-gp in order to increase NDGA bio-availability, in the spinal cord in particular. Similar approaches are followed in cancer therapeutics, where the anti-cancer drug is co-administered with potential blockers of multi-drug transporter proteins. Unfortunately, these inhibitors have quite toxic effects to cells because they basically block the function of crucial transporter proteins that act by expelling not only xenobiotics from cells, but also toxic endogenous catabolites (Loscher and Potschka, 2005). The specific mechanisms by which the expression and function of multi-drug transporters are regulated in neurological disorders like ALS should be investigated in detail, because this could open up new lines of investigation to prevent and reverse drug resistance, and if necessary reconsider many clinical trials that have been attempted in the mutant SOD1 animal model in which the function of these proteins could have compromised a positive outcome.
ACKNOWLEDGMENTS
This work was supported by the National Institute of Health grants RO1-NS44292, R21-NS058475 and the ALS Association (to D.T.). The Weinberg Unit for ALS research is also supported by the Farber Family Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Pasinelli P, Brown RH. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat Rev Neurosci. 2006;7:710–723. doi: 10.1038/nrn1971. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Clements JD, Lester RA, Tong G, Jahr CE, Westbrook GL. The time course of glutamate in the synaptic cleft. Science. 1992;258:1498–1501. doi: 10.1126/science.1359647. [DOI] [PubMed] [Google Scholar]
- Herman MA, Jahr CE. Extracellular glutamate concentration in hippocampal slice. J Neurosci. 2007;27:9736–9741. doi: 10.1523/JNEUROSCI.3009-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Lai L, Butchbach ME, Stockinger MP, Shan X, Bishop GA, Lin CL. Increased expression of the glial glutamate transporter EAAT2 modulates excitotoxicity and delays the onset but not the outcome of ALS in mice. Hum Mol Genet. 2003;12:2519–2532. doi: 10.1093/hmg/ddg267. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Pardo AC, Wong V, Benson LM, Dykes M, Tanaka K, Rothstein JD, Maragakis NJ. Loss of the astrocyte glutamate transporter GLT1 modifies disease in SOD1(G93A) mice. Exp Neurol. 2006;201:120–130. doi: 10.1016/j.expneurol.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Heemskerk J, Tobin AJ, Bain LJ. Teaching old drugs new tricks. TRENDS in Neurosciences. 2002;25:494–496. doi: 10.1016/s0166-2236(02)02236-1. [DOI] [PubMed] [Google Scholar]
- Salazar-Grueso EF, Kim S, Kim H. Embryonic mouse spinal cord motor neuron hybrid cells. Neuroreport. 1991;2:505–508. doi: 10.1097/00001756-199109000-00002. [DOI] [PubMed] [Google Scholar]
- Salari H, Braquet P, Borgeat P. Comparative effects of indomethacin, acetylenic acids, 15-HETE, nordihydroguaiaretic acid and BW755C on the metabolism of arachidonic acid in human leukocytes and platelets. Prostaglandins Leukot Med. 1984;13:53–60. doi: 10.1016/0262-1746(84)90102-1. [DOI] [PubMed] [Google Scholar]
- Bendotti C, Tortarolo M, Suchak SK, Calvaresi N, Carvelli L, Bastone A, Rizzi M, Rattray M, Mennini T. Transgenic SOD1 G93A mice develop reduced GLT-1 in spinal cord without alterations in cerebrospinal fluid glutamate levels. J Neurochem. 2001;79:737–746. doi: 10.1046/j.1471-4159.2001.00572.x. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Jin L, Dykes-Hoberg M, Kuncl RW. Chronic inhibition of glutamate uptake produces a model of slow neurotoxicity. Proc Natl Acad Sci U S A. 1993;90:6591–6595. doi: 10.1073/pnas.90.14.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Martin LJ, Kuncl RW. Decreased glutamate transport by the brain and spinal cord in amyotrophic lateral sclerosis. N Engl J Med. 1992;326:1464–1468. doi: 10.1056/NEJM199205283262204. [see comments] [DOI] [PubMed] [Google Scholar]
- Garrigues A, Nugier J, Orlowski S, Ezan E. A high-throughput screening microplate test for the interaction of drugs with P-glycoprotein. Anal Biochem. 2002;305:106–114. doi: 10.1006/abio.2002.5650. [DOI] [PubMed] [Google Scholar]
- Gebhardt C, Korner R, Heinemann U. Delayed anoxic depolarizations in hippocampal neurons of mice lacking the excitatory amino Acid carrier 1. J Cereb Blood Flow Metab. 2002;22:569–575. doi: 10.1097/00004647-200205000-00008. [DOI] [PubMed] [Google Scholar]
- Volterra A, Trotti D, Cassutti P, Tromba C, Salvaggio A, Melcangi RC, Racagni G. High sensitivity of glutamate uptake to extracellular free arachidonic acid levels in rat cortical synaptosomes and astrocytes. J Neurochem. 1992;59:600–606. doi: 10.1111/j.1471-4159.1992.tb09411.x. [DOI] [PubMed] [Google Scholar]
- Maragakis NJ, Rothstein JD. Mechanisms of Disease: astrocytes in neurodegenerative disease. Nat Clin Pract Neurol. 2006;2:679–689. doi: 10.1038/ncpneuro0355. [DOI] [PubMed] [Google Scholar]
- Ganel R, Ho T, Maragakis NJ, Jackson M, Steiner JP, Rothstein JD. Selective up-regulation of the glial Na+-dependent glutamate transporter GLT1 by a neuroimmunophilin ligand results in neuroprotection. Neurobiology of Disease. 2006;21:556. doi: 10.1016/j.nbd.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Canton T, Pratt J, Stutzmann JM, Imperato A, Boireau A. Glutamate uptake is decreased tardively in the spinal cord of FALS mice. Neuroreport. 1998;9:775–778. doi: 10.1097/00001756-199803300-00001. [DOI] [PubMed] [Google Scholar]
- Howland DS, Liu J, She Y, Goad B, Maragakis NJ, Kim B, Erickson J, Kulik J, DeVito L, Psaltis G, DeGennaro LJ, Cleveland DW, Rothstein JD. Focal loss of the glutamate transporter EAAT2 in a transgenic rat model of SOD1 mutant-mediated amyotrophic lateral sclerosis (ALS) Proc Natl Acad Sci U S A. 2002;29:29. doi: 10.1073/pnas.032539299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscher W, Potschka H. Drug resistance in brain diseases and the role of drug efflux transporters. Nat Rev Neurosci. 2005;6:591–602. doi: 10.1038/nrn1728. [DOI] [PubMed] [Google Scholar]
- Hensley K, Mhatre M, Mou S, Pye QN, Stewart C, West M, Williamson KS. On the relation of oxidative stress to neuroinflammation: lessons learned from the G93A-SOD1 mouse model of amyotrophic lateral sclerosis. Antioxid Redox Signal. 2006;8:2075–2087. doi: 10.1089/ars.2006.8.2075. [DOI] [PubMed] [Google Scholar]
- Klein I, Sarkadi B, Varadi A. An inventory of the human ABC proteins. Biochim Biophys Acta. 1999;1461:237–262. doi: 10.1016/s0005-2736(99)00161-3. [DOI] [PubMed] [Google Scholar]
- Langer O, Bauer M, Hammers A, Karch R, Pataraia E, Koepp MJ, Abrahim A, Luurtsema G, Brunner M, Sunder-Plassmann R, Zimprich F, Joukhadar C, Gentzsch S, Dudczak R, Kletter K, Muller M, Baumgartner C. Pharmacoresistance in Epilepsy: A Pilot PET Study with the P-Glycoprotein Substrate R-[11C]verapamil. Epilepsia. 2007;48:1774–1784. doi: 10.1111/j.1528-1167.2007.01116.x. [DOI] [PubMed] [Google Scholar]
- Hendrikse NH, Schinkel AH, de Vries EG, Fluks E, Van der Graaf WT, Willemsen AT, Vaalburg W, Franssen EJ. Complete in vivo reversal of P-glycoprotein pump function in the blood-brain barrier visualized with positron emission tomography. Br J Pharmacol. 1998;124:1413–1418. doi: 10.1038/sj.bjp.0701979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelig A. A general pattern for substrate recognition by P-glycoprotein. Eur J Biochem. 1998;251:252–261. doi: 10.1046/j.1432-1327.1998.2510252.x. [DOI] [PubMed] [Google Scholar]
- Choi DW. Excitotoxic cell death. J Neurobiol. 1992;23:1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- Sheldon AL, Robinson MB. The role of glutamate transporters in neurodegenerative diseases and potential opportunities for intervention. Neurochemistry International. 2007;51:333. doi: 10.1016/j.neuint.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk H, Potschka H, Loscher W. Immunohistochemical Localization of P-glycoprotein in Rat Brain and Detection of Its Increased Expression by Seizures Are Sensitive to Fixation and Staining Variables. J. Histochem. Cytochem. 2005;53:517–531. doi: 10.1369/jhc.4A6451.2005. [DOI] [PubMed] [Google Scholar]
- Bankstahl JP, Hoffmann K, Bethmann K, Löscher W. Glutamate is critically involved in seizure-induced overexpression of P-glycoprotein in the brain. Neuropharmacology. doi: 10.1016/j.neuropharm.2008.02.008. In Press, Accepted Manuscript. [DOI] [PubMed] [Google Scholar]
- Zhu H-J, Liu G-Q. Glutamate up-regulates P-glycoprotein expression in rat brain microvessel endothelial cells by an NMDA receptor-mediated mechanism. Life Sciences. 2004;75:1313. doi: 10.1016/j.lfs.2004.02.027. [DOI] [PubMed] [Google Scholar]
- Consilvio C, Vincent AM, Feldman EL. Neuroinflammation, COX-2, and ALS--a dual role? Exp Neurol. 2004;187:1–10. doi: 10.1016/j.expneurol.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Nguyen MD, Julien JP, Rivest S. Innate immunity: the missing link in neuroprotection and neurodegeneration? Nat Rev Neurosci. 2002;3:216–227. doi: 10.1038/nrn752. [DOI] [PubMed] [Google Scholar]
- Rothstein JD. Excitotoxic mechanisms in the pathogenesis of amyotrophic lateral sclerosis. Adv Neurol. 1995;68:7–20. [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Inflammatory processes in amyotrophic lateral sclerosis. Muscle Nerve. 2002;26:459–470. doi: 10.1002/mus.10191. [DOI] [PubMed] [Google Scholar]
- Traynor BJ, Bruijn L, Conwit R, Beal F, O'Neill G, Fagan SC, Cudkowicz ME. Neuroprotective agents for clinical trials in ALS: a systematic assessment. Neurology. 2006;67:20–27. doi: 10.1212/01.wnl.0000223353.34006.54. [DOI] [PubMed] [Google Scholar]
- Adachi Y, Suzuki H, Sugiyama Y. Comparative studies on in vitro methods for evaluating in vivo function of MDR1 P-glycoprotein. Pharm Res. 2001;18:1660–1668. doi: 10.1023/a:1013358126640. [DOI] [PubMed] [Google Scholar]
- Polli JW, Wring SA, Humphreys JE, Huang L, Morgan JB, Webster LO, Serabjit-Singh CS. Rational use of in vitro P-glycoprotein assays in drug discovery. J Pharmacol Exp Ther. 2001;299:620–628. [PubMed] [Google Scholar]
- Vogelgesang S, Cascorbi I, Schroeder E, Pahnke J, Kroemer HK, Siegmund W, Kunert-Keil C, Walker LC, Warzok RW. Deposition of Alzheimer's beta-amyloid is inversely correlated with P-glycoprotein expression in the brains of elderly non-demented humans. Pharmacogenetics. 2002;12:535–541. doi: 10.1097/00008571-200210000-00005. [DOI] [PubMed] [Google Scholar]
- Kortekaas R, Leenders KL, van Oostrom JC, Vaalburg W, Bart J, Willemsen AT, Hendrikse NH. Blood-brain barrier dysfunction in parkinsonian midbrain in vivo. Ann Neurol. 2005;57:176–179. doi: 10.1002/ana.20369. [DOI] [PubMed] [Google Scholar]
- Langford D, Grigorian A, Hurford R, Adame A, Ellis RJ, Hansen L, Masliah E. Altered P-glycoprotein expression in AIDS patients with HIV encephalitis. J Neuropathol Exp Neurol. 2004;63:1038–1047. doi: 10.1093/jnen/63.10.1038. [DOI] [PubMed] [Google Scholar]
- Schinkel AH, Wagenaar E, Mol CA, van Deemter L. P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J Clin Invest. 1996;97:2517–2524. doi: 10.1172/JCI118699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkinezos IG, Hernandez D, Bradley WG, Moraes CT. An ALS mouse model with a permeable blood-brain barrier benefits from systemic cyclosporine A treatment. J Neurochem. 2004;88:821–826. doi: 10.1046/j.1471-4159.2003.02181.x. [DOI] [PubMed] [Google Scholar]