Abstract
Considerable effort has been directed toward the development of methods to selectively activate specific subtypes of neurons. Focus has been placed on the heterologous expression of proteins that are capable of exciting neurons in which they are expressed. Here we describe the heterologous expression of the invertebrate FMRFamide-gated sodium channel from Helix aspersa (HaFaNaC) in hippocampal slice cultures. HaFaNaC was co-expressed with a fluorescent protein (GFP, dsRed or tdTomato) in CA3 pyramidal neurons of rat hippocampal slice cultures using single cell electroporation. Pressure application of the agonist FMRFamide to HaFaNaC-expressing neuronal somata produced large prolonged depolarizations and bursts of action potentials (AP). FMRFamide responses were inhibited by amiloride (100 µM). In contrast, pressure application of FMRFamide to the axons of neurons expressing HaFaNaC produced no response. Fusion of GFP to the N-terminus of HaFaNaC showed that GFP-HaFaNaC was absent from axons. Bath application of FMRFamide produced persistent AP firing in HaFaNaC-expressing neurons. This FMRFamide-induced increase in the frequency of APs was dose-dependent. The concentrations of FMRFamide required to activate HaFaNaC-expressing neurons were below that required to activate the homologous acid sensing ion channel normally found in mammalian neurons. Furthermore, the mammalian neuropeptides neuropeptide FF and RFRP-1, which have amidated RF C-termini, did not affect HaFaNaC-expressing neurons. Antagonists of NPFF receptors (BIBP3226) also had no effect on HaFaNaC. Therefore, we suggest that heterologous-expression of HaFaNaC in mammalian neurons could be a useful method to selectively and persistently excite specific subtypes of neurons in intact nervous tissue.
Keywords: single-cell electroporation, activation, heterologous expression, invertebrate ion channel, mammalian neuron
Introduction
Determining the role that specific subtypes of neurons play in neuronal network function is essential for understanding how the nervous system operates and how dysfunction of specific subtypes of neurons contributes to psychiatric and neurological diseases. One method to study the role of specific neuronal subtypes in neural network function is by activating a specific set of neurons in a neural network with either direct electrical stimulation of nervous tissue or chemical stimulation through the application of an exogenous excitatory molecule (e.g. exogenous application of the ubiquitous excitatory neurotransmitter glutamate). However, electrical stimulation and excitatory chemical application activate all axons and neuronal processes in the exposed tissue and therefore neither stimulation technique can selectively activate a specific subtype of neuron in intact nervous tissue.
In order to selectively activate specific subtypes of neurons, attempts have been made to heterologously express proteins that, when activated, are capable of depolarizing and exciting only those neurons in which they are expressed (Zemelman et al., 2002; Zemelman et al., 2003; Banghart et al., 2004; Lima and Miesenbock, 2005; Boyden et al., 2005; Li et al., 2005; Nagel et al., 2005; Volgraf et al., 2006; Bi et al., 2006; Ishizuka et al., 2006; Schroll et al., 2006; Chambers et al., 2006; Arenkiel et al., 2007; Han and Boyden, 2007; Petreanu et al., 2007; Zhang and Oertner, 2007; Zhang et al., 2007; Adamantidis et al., 2007; Aravanis et al., 2007; Arenkiel et al., 2008). In order to be effective, the heterologously expressed activator protein cannot be endogenously expressed in the neural system under investigation. The mechanism of activation of the protein should not affect any endogenous molecules in the system under study, and molecules present in the system under study should not affect the activator protein. In the case of the mammalian nervous system, light- or ligand-gated ion channels from non mammalian species have provided a source for such proteins (Zemelman et al., 2002; Nagel et al., 2003; Banghart et al., 2004; Kramer et al., 2005; Boyden et al., 2005; Li et al., 2005; Herlitze and Landmesser, 2006; Parrish et al., 2006; Chambers et al., 2006). Therefore, in this study we have investigated the potential utility of heterologously expressing the invertebrate FMRFamide-gated sodium channel (FaNaC) (Lingueglia et al., 1995; Jeziorski et al., 2000; Perry et al., 2001; Furukawa et al., 2006) in mammalian neurons for their selective activation.
FaNaC was initially cloned from Helix aspersa (Lingueglia et al., 1995), but others have also been found in Helisoma trivolis, Lymnaea stagnalis, and Aplysia kurodai (Lingueglia et al., 1995; Jeziorski et al., 2000; Perry et al., 2001; Furukawa et al., 2006). FaNaCs are activated by the amidated peptide FMRFamide (Price and Greenberg, 1977) and are primarily permeated by sodium ions (Lingueglia et al., 1995). Importantly, when heterologously expressed in cell lines or oocytes, activation of FaNaC shows little desensitization (Lingueglia et al., 1995; Green and Cottrell, 1999; Jeziorski et al., 2000; Green and Cottrell, 2002). Of the different species, FaNaC from H. aspersa (HaFaNaC) has a higher affinity for FMRFamide, produces larger currents than FaNaC from other species (Lingueglia et al., 1995; Jeziorski et al., 2000; Perry et al., 2001; Furukawa et al., 2006). Thus, HaFaNaC may be the best candidate of the FaNaC proteins for gene targeted activation of mammalian neurons. However, there may be some limitations to the use of HaFaNaC as a gene targeted method for the activation of neurons in intact mammalian nervous tissue. Although FMRFamide is not found in mammals, other longer peptides with amidated RF C-termini (RFamide peptides) have been found in the mammalian central nervous system (CNS) (Yang et al., 1985; Perry et al., 1997; Vilim et al., 1999; Hinuma et al., 2000; for review see Fukusumi et al., 2006). Fortunately, RFamide peptides with an extended N-terminus do not appear to activate HaFaNaC (Lingueglia et al., 1995; Cottrell, 1997). In contrast, the mammalian NPFF receptors are activated by FMRFamide (Tang et al., 1984; Yang et al., 1985; Raffa, 1988; Brussaard et al., 1989; Raffa, 1989; Roumy and Zajac, 1998; Bonini et al., 2000; Hinuma et al., 2000; Liu et al., 2001). Furthermore, acid sensing ion channels (ASIC), which are homologous to HaFaNaC, are found in the mammalian CNS and are modulated by FMRFamide (Lingueglia et al., 1995; Askwith et al., 2000; Xie et al., 2003; Lingueglia et al., 2006). Therefore, although HaFaNaC may be capable of activating mammalian neurons in which it is heterologously expressed, activation of HaFaNaC by FMRFamide may have secondary effects on endogenous receptors and ion channels present in the mammalian CNS.
In this study, we show that heterologous expression of HaFaNaC in hippocampal CA3 pyramidal neurons permitted those neurons to be potently and persistently activated by application of FMRFamide. Furthermore, these neurons were activated at FMRFamide concentrations below that known to modulate ASICs. Lastly, an antagonist of NPFF receptors had no effect on HaFaNaC activation in CA3 pyramidal neurons. Therefore, the heterologous expression of HaFaNaC in mammalian neurons may provide a method for the selective activation of neurons of choice in the mammalian CNS.
Experimental Procedures
cDNA constructs
HaFaNaC constructs were contracted out to sequencing companies for subcloning into the mammalian expression vector pCMVTnT (Promega, Madison, WI). HaFaNaC cDNA (EMBL accession number: X92113) was donated by Drs. Eric Lingueglia and Michel Lazdunski (CNRS - Université de Nice-Sophia, Antipolis, France). To generate pCMVTnT/HaFaNaC, the entire coding sequence of HaFaNaC was PCR amplified and ligated between Xba I and Sal I sites (Epoch Biolabs, Sugar Land, Texas). GFPHaFaNaC fusion construct was created by Eton Bioscience Inc. (San Diego, CA) using overlap extension PCR. GFP was PCR amplified out of pmaxGFP (Amaxa, Germany) and fused to the N-terminus of the HaFaNaC full length coding sequence and ligated between EcoR I and Xba I sites. Kozak consensus sequences were added immediately upstream of the 5’ ATG start codon of each construct to enhance translation efficiency (Kozak, 1987).
The red fluorescent protein tdTomato (Shaner et al., 2004; Shaner et al., 2005) (donated by R. Tsien, University of California, San Diego) was ligated out of the bacterial vector pRSET-B and subcloned into BamH I and EcoR I sites in pcDNA3.1 (+) (Invitrogen, Carlsbad, CA). pCMV-DsRed Express vector (Clontech, Mountain View, CA) and pmaxGFP (Amaxa Inc., Germany) were also used for visualization. pEGFP/Synaptophysin (SynGFP) was kindly provided by Ed Ruthazer (McGill University, Montreal). Prior to electroporation into cells, plasmids were purified using Qiagen EndoFree kits (Hilden, Germany).
Hippocampal slice cultures
The use of animals adhered to a protocol approved by the Institutional Animal Care and Use Committee at Virginia Commonwealth University. Postnatal day 7 (P7) or P8 Sprague Dawley rat pups (Zivic Laboratories) were deeply anesthetized with isoflurane, decapitated, and their hippocampus dissected aseptically under an Olympus SZ61 dissection microscope. Organotypic hippocampal slice cultures were prepared using the method developed by Stoppini (Stoppini et al., 1991) with the following modifications. 300 µm transverse slices were cut using a Stoelting Tissue Chopper, and 2 to 3 slices were transferred to organotypic Millicell-CM inserts (Millipore, Bedford, MA) in 60 mm petri dishes containing 2 ml of media (50% Minimum Essential Medium, 25% Horse serum and 25% Hanks Balanced Salt Solution, 36 mM glucose, 25 mM HEPES, 1 % penn/strep, pH 7.2). After one day in culture, culture media was replaced with fresh media containing no antibiotic. Culture media was replaced thereafter biweekly. Cultures were allowed to grow for 5–7 days prior to single cell electroporation.
Single cell electroporation
Organotypic hippocampal slices were placed on the fixed stage of an Olympus BX51WI microscope equipped with DIC optics. The image of CA3 pyramidal neurons was collected through a 60x (0.9 N.A.) water immersion objective lens, captured with a DAGE-MTI IR1000 CCD camera and displayed on a monochrome video monitor (Vitek VTM-14A, Audio Video Supply, San Diego, CA). Glass pipettes (borosilicate glass (8250, 1.65/1.0 mm)) were pulled using a Narishige PP830 pipette puller (East Meadow, NY). Tips were backfilled with 2 µl of solution containing 49.5 ng HaFaNaC cDNA and 16.5 ng fluorescent marker cDNA in sterile-filtered saline (in mM): 125 NaCl, 3.0 KCl, 1.2 CaCl2, 1.2 MgSO4, 1.25 NaH2PO4, 25 NaHCO3, and 25 glucose). Pipette tips were visually guided in close apposition to an individual neuron’s cell body. For electroporation, a train of square wave voltage pulses (−10 V, 200 Hz, 150 pulses, 1 ms duration) was delivered to the pipette from a model 2200 analog stimulation isolation unit (A-M systems) (Haas et al., 2001; Rae and Levis, 2002; Rathenberg et al., 2003). One or two CA3 pyramidal neurons were electroporated per slice. Following electroporation, cultures were washed with and incubated overnight in culture medium containing 1% penicillin/streptomycin.
Electrophysiological recordings
Four to nine days after electroporation, slices were submerged and continuously perfused in a glass-bottom recording chamber with warmed saline (33–35°C) consisting of (in mM): 125 NaCl, 3.0 KCl, 1.2 CaCl2, 1.2 MgSO4, 1.25 NaH2PO4, 25 NaHCO3, and 25 glucose saturated with 95% O2/5% CO2. The recording chamber was mounted on the same microscope used for electroporation. Whole cell patch pipettes (2–5 MΩ, borosilicate glass (8250, 1.65/1.0 mm)) were filled with (in mM): 130 K Gluconate, 8 NaCl, 2 MgATP, 0.1 NaGTP, 10 HEPES, pH 7.25. Whole cell patch clamp recordings were obtained from co-transfected CA3 pyramidal neurons (GFP, DsRed or tdTomato + HaFaNaC) identified by epifluorescence (100 W Hg lamp, Osram, Munich, Germany; GFP - Endow GFP Bandpass emission filter set (excitation filter 470/40 band pass, dichromatic beamsplitter 495 long pass, emission filter 525/50 band pass); dsRed and tdTomato – dsRed 2 filter set (excitation filter 540/40 band pass, dichromatic beamsplitter 570 long pass, emission filter 600/50 band pass) Chroma Technology, Rockingham, VT, USA). To examine the electrophysiological FMRFamide responses of HaFaNaC-expressing neurons, FMRFamide was applied either by bath perfusion or focally to the soma or axons by pressure application (PV 820 Pneumatic PicoPump, WPI USA). All other drugs were applied by bath perfusion. Electrophysiological responses were recorded with a Dagan BVC 700A amplifier (Dagan Corp. Minneapolis, MN) or a Model 2400 patch clamp amplifier (A-M Systems, Port Angeles, WA). For voltage clamp recordings, whole cell capacitance and series resistance were compensated 70 to 80% and membrane potentials were adjusted for a calculated 10 mV junction potential. Electrophysiological data was digitized by a PCI-6221 A/D board (National Instruments, Austin, TX) and stored and analyzed on a personal computer using WCP or WinEDR software (Dr. J Dempster, University of Strathclyde, Glasgow, Scotland). For quantification, the area of depolarizing responses to FMRFamide application were integrated from the initiation of the depolarization until the response returned to the resting membrane potential of the control responses.
Confocal Microscopy
To determine the localization of the GFPHaFaNaC in transfected neurons, slices containing GFPHaFaNaC and DsRed or tdTomato expressing neurons were examined by fluorescence confocal laser scanning microscopy. Slices were fixed with 4% paraformaldehyde in 0.1 M phosphate buffer, washed 6 × 10 minutes, mounted with Vectashield mounting medium (Vector Laboratories, Burlingame, CA) and examined with a Zeiss LSM 510 META confocal laser-scanning microscope (Jena, Germany) equipped with Argon (488 nm) and 561 DPSS (561nm) lasers. Cell body and axonal branches of transfected neurons were visualized using either a 40X oil immersion lens (1.3 N.A., pixel dimensions 0.1 µm2) or a 63X oil immersion lens (1.4 N.A., pixel dimensions 0.08 µm2).
Bandpass filters used included 500–530nm for the argon 488 laser and 575–615nm for 561 DPSS laser. Sequential scanning and 4 × line averaging was used to eliminate cross talk and minimize background noise.
Statistics
Statistics were performed using GraphPad Instat (GraphPad software, San Diego, CA). Statistical significances (P < 0.05) were determined by t-tests or repeated measures ANOVA followed by a Tukey-Kramer multiple comparisons post hoc test if statistical significances were found. When the distribution of the data did not pass the Kolmogorov-Smirnov test for normality, a non parametric Mann-Whitney test was used.
Reagents
All reagents were purchased from Fisher Scientific (Pittsburgh, PA, USA) or VWR (West Chester, PA, USA) unless otherwise indicated. Adenosine 5’triphosphate magnesium salt hydrate, amiloride hydrochloride hydrate and BIBP3226 were purchased from Sigma-Aldrich (St. Louis, MO). RFRP-1, H-Phenylalanine-Methionine-Arginine-Phenylalanine-NH2 (FMRFamide) and neuropeptide FF was purchased from Bachem (Torrance, CA). Minimum essential medium, horse serum, Hanks balanced salt solution and penicillin and streptomycin were purchased from Invitrogen (Carlsbad, CA). Tetrodotoxin was purchased from Tocris (Ellisville, MO).
Results
In this study we have examined the efficacy with which the invertebrate ligand-gated ion channel HaFaNaC can be expressed in mammalian neurons. In addition to its functional expression, we have investigated whether the expression of HaFaNaC or the application of FMRFamide would have any secondary confounding effects on mammalian neuronal function. Finally, we examined the subcellular localization of HaFaNaC in the somata, dendrites and axons of HaFaNaC-expressing mammalian neurons. We used single cell electroporation to express HaFaNaC with a fluorescent marker protein in CA3 pyramidal neurons of organotypic hippocampal slice cultures. To examine the efficacy of HaFaNaC expression, we measured transfected neuron electrophysiological responses to FMRFamide application with whole cell patch clamp methods.
Functional expression of HaFaNaC in mammalian neurons
To determine if HaFaNaC could be expressed in cultured hippocampal neurons, we used whole cell patch clamp methods to examine electrophysiological responses to focally applied FMRFamide on the somata of HaFaNaC-expressing neurons. HaFaNaC-expressing neurons had similar input resistances (HaFaNaC 176.0 ± 23.2 MΩ, N = 13; untransfected 149.0 ± 28.3 MΩ, N = 12, Mann-Whitney test P = 0.2471), resting membrane potentials (HaFaNaC −72.1 ± 1.2 mV, N = 13; untransfected −70.6 ± 1.3 mV, N=13, two tailed, unpaired t-test p=0.3891), AP threshold (HaFaNaC −47.2 ± 0.8 mV, N=13; untransfected −50.0 ± 1.7 mV, N=13, Mann-Whitney test, P = 0.3358), AP amplitude (HaFaNaC 76.0 ± 4.2, N=13; untransfected 82.7 ± 5.0 for cells N=13, Mann-Whitney test, p=0.1129), and AP half-widths (HaFaNaC 0.96 ± 0.11 msec, N = 14; untransfected neurons 0.89 ± 0.08 msec, N = 13 Mann-Whitney test, P = 0.3673) when compared to untransfected neurons from the same slice cultures. Thus, the heterologous expression of HaFaNaC in CA3 pyramidal neurons did not affect passive or active electrical properties of neurons.
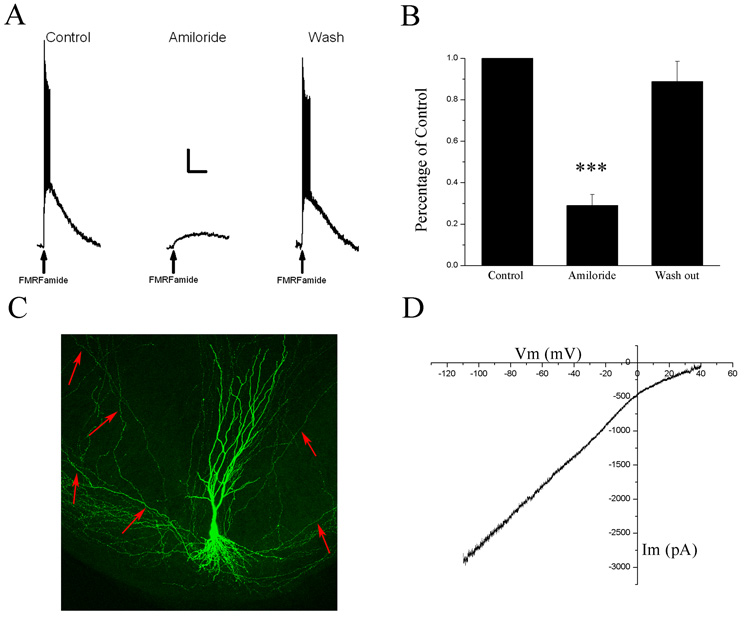
Focal application of FMRFamide (75–100 µM) to the somata of HaFaNaC-expressing neurons (Fig. 1C, example of a confocal reconstruction of GFP and HaFaNaC co-transfected CA3 pyramidal neuron) showed robust depolarizing responses that resulted in a burst of action potentials (Fig. 1A, N = 39). The depolarization elicited by FMRFamide was reversibly blocked by bath application of 100 µM amiloride (Fig. 1A, N = 11). The response of HaFaNaC-expressing neurons to FMRFamide application was quantified by measuring the area of the FMRFamide-induced depolarization (mV·msec). Amiloride significantly reduced the area of depolarization (Fig. 1B, repeated measures ANOVA P < 0.001, Tukey-Kramer post hoc test Control vs. Amiloride P < 0.001, N = 11) in a reversible manner (Tukey-Kramer post hoc test Amiloride vs. Wash P < 0.001, N = 11).
Figure 1. HaFaNaC can be functionally expressed in CA3 hippocampal neurons.
A. Pressure application of FMRFamide (arrow, 75 µM, 10 psi, 100 msec) onto the soma of a HaFaNaC-expressing neuron produced a depolarization and a burst of action potentials (Control). Bath application of 100 µM amiloride inhibited the FMRFamide response (Amiloride) in a reversible manner (Wash). Scale bars: horizontal 25 sec, vertical 10mV. B. Histogram of the area of FMRFamide-induced depolarizations (mV·msec) normalized to control values. Areas were significantly inhibited by amiloride (100 µM) in a reversible manner. C. A 3-D reconstruction of a through-focus series of images collected by confocal laser scanning microscopy of a CA3 pyramidal neuron transfected with HaFaNaC and pmaxGFP (at 3:1 ratio). CA3 pyramidal morphology appears unaffected by HaFaNaC. Red arrows point to axonal branches. D. Current – Voltage (I–V) relationship of a FMRFamide-induced response measured by a 30 second voltage ramp from −110 to +40 mV. I–V curve was constructed by subtracting the control voltage ramp from the voltage ramp in the presence of 100 µM FMRFamide.
To examine the ionic properties of HaFaNaC expressed in mammalian neurons, we performed slow voltage ramp experiments to determine the reversal potential of FMRFamide-induced ion currents of HaFaNaC expressing neurons. The HaFaNaC currents were produced by bath application of FMRFamide. Voltage-dependent sodium channels were blocked by extracellular tetrodotoxin (1 µM), voltage-dependent calcium channels were blocked by extracellular cadmium (200 µM), and voltage-dependent potassium channels were blocked by replacing intracellular potassium ions with cesium. The HaFaNaC current was isolated from remaining functional ion channels by subtracting control voltage ramp currents from voltage ramp currents in the presence of FMRFamide. The reversal potential for FMRFamide-activated HaFaNaC approached the equilibrium potential for sodium (Fig. 1D, mean +33 ± 4 mV, N = 5) suggesting a preference for sodium over other ions. This is consistent with the previous observations of the cloned HaFaNaC in oocytes (Lingueglia et al 1995) suggesting that HaFaNaC’s properties are not altered by expression in mammalian neurons. Therefore, HaFaNaC appears to be functionally expressed in CA3 pyramidal neurons of organotypical hippocampal slice cultures.
Efficacy of HaFaNaC-expression in mammalian neurons
Although HaFaNaC can be functionally expressed in CA3 pyramidal neurons (Fig. 1), we wanted to determine the concentrations of FMRFamide required to activate HaFaNaC-expressing mammalian neurons. To do this, we took advantage of the observation that cloned HaFaNaC produced little desensitization (Lingueglia et al., 1995). We recorded the frequency of action potentials produced by bath applying known concentrations of FMRFamide. We recorded membrane potentials of HaFaNaC-expressing neurons five minutes before, five minutes during and then five minutes following recovery from bath application of FMRFamide.
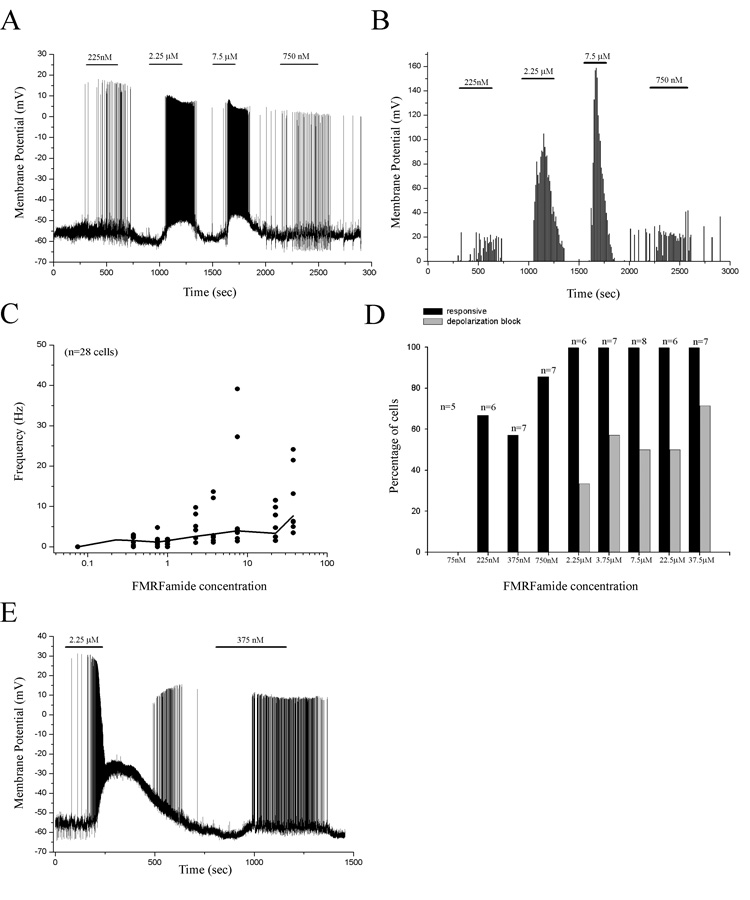
Repetitive applications of FMRFamide repeatedly depolarized a HaFaNaC-expressing neuron resulting in the continuous firing of APs (Fig. 2A). The frequency of APs in response to FMRFamide application was dose dependent, increasing with increasing concentrations (Fig. 2B, C). However, low concentrations of FMRFamide were not always sufficient to elicit AP firing in all neurons (Fig. 2D). Higher concentrations of FMRFamide (≥ 2.25 µM) were necessary to ensure the activation of 100% of HaFaNaC-expressing neurons (Fig. 2D, black bars). However, higher concentrations also produced depolarizations that were too large to support the continuous firing of APs (depolarization block, Fig. 2D, E, grey bars). Therefore, there is a concentration range (750 nM < optimal concentration < 2.25 µM) of FMRFamide in which nearly all HaFaNaC-expressing neurons in hippocampal slice cultures were sufficiently depolarized to permit persistent AP firing without resulting in depolarization block.
Figure 2. FMRFamide can persistently excite HaFaNaC-expressing neurons.
A. Bath application of low concentrations of FMRFamide repeatedly and persistently excited a HaFaNaC-expressing neuron in a dose-dependent manner. Bars indicate time and concentration of FMRFamide application. B. Plot of the number of action potentials that occurred in 10 sec bins throughout the experiment. Bath application of larger concentrations of FMRFamide produced larger numbers of action potentials with little adaptation. C. Plot of the frequency of action potentials for all neurons (dots) in response to bath application of FMRFamide. Line is the mean frequency of all neurons in response to different concentrations of FMRFamide. D. Plot of the percent of neurons that responded to specific concentrations of FMRFamide (black bars) and the percent of neurons that showed depolarization block at different concentrations of FMRFamide (gray bars). E. An example of a neuron that was persistently activated by bath application of low concentrations of FMRFamide (375 nM, right); however, larger concentrations of FMRFamide (2.25 µM) produced an appreciably larger depolarization that prevented the persistent firing of APs (left, depolarization block). Bars indicate time and concentration of FMRFamide application.
Subcellular localization of the HaFaNaC construct
Although our data suggest that HaFaNaC was functionally expressed in the somata of CA3 pyramidal neurons (Fig. 1), we wanted to determine which portions of the somatodendritic and axonal plasma membranes incorporated functional HaFaNaCs. To do this we applied FMRFamide focally to the different subcellular regions of transfected neurons. If HaFaNaC was expressed in axonal plasma membrane, discrete application of FMRFamide to the axon should result in a depolarization and back-propagation of an AP to the soma.
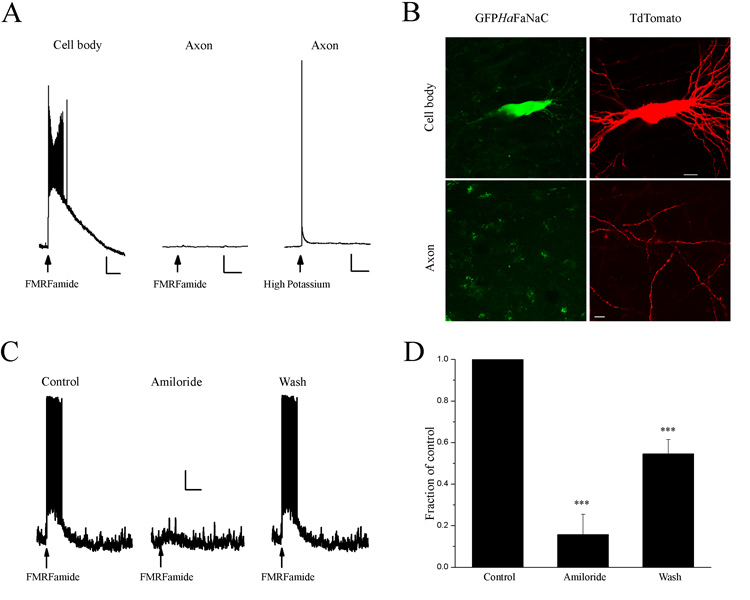
Pressure application of FMRFamide to the cell body of a HaFaNaC-expressing neuron caused a depolarization and a burst of APs (Fig. 3A, left panel). Axons were unequivocally identified by their beaded structure and projections in CA1 (Fig 1C). Pressure application of FMRFamide to the axon of the same cell did not cause a response (Fig. 3A, middle panel). To verify that the axonal segment of the same cell was capable of firing APs, a high potassium solution (130 mM) was applied to the same spot as the FMRFamide application and a back-propagated action potential was detected in the soma (Fig. 3A, right panel). Thus, these data suggest that HaFaNaC was not functionally incorporated at sufficient density in the axons of transfected neurons.
Figure 3. HaFaNaC is not functionally expressed in axons of CA3 pyramidal neurons.
A. Left. Pressure application of FMRFamide (arrow, 75 µM, 10 psi, 20 msec) onto the soma of a HaFaNaC-expressing CA3 hippocampal neuron produced a large depolarizing response. Middle. No response was observed when FMRFamide was applied to the axon of the same neuron. Right. A back-propagating action potential was observed in when high potassium (130 mM) was applied to the same axonal location as FMRFamide. Scale bars Vertical 10 mV, Horizontal Left 5 s, Middle and Right 200 ms. B. Confocal image of CA3 hippocampal neuron expressing GFPHaFaNaC (left panels) and TdTomato (right panels). TdTomato was present in the soma (top right) and axons (bottom right) whereas GFPHaFaNaC was present in soma (top left) but absent in the axon (bottom left). Scale bars: cell body images: 20 µm; axon: 10 µm. C. Left. Pressure application of FMRFamide (arrow, 75 µM, 10 psi, 20 msec) onto the soma of a GFPHaFaNaC-expressing CA3 hippocampal neuron produced a large depolarizing response. Middle. The response to FMRFamide was inhibited by amiloride (100 µM). Right. Recovery of FMRFamide response after removal of amiloride. Scale bars. Vertical 10 mV, Horizontal 2.5 s. D. Average area of FMRFamide-induced depolarization (mV·msec) for all neurons transfected with GFPHaFaNaC. FMRFamide responses were reversibly inhibited by amiloride (100 µM) in all neurons examined.
The observation that FMRFamide was incapable of producing a back-propagating AP in HaFaNaC-expressing neurons could be explained by a number of possibilities. First, HaFaNaC may not be trafficked to the axon of mammalian neurons. Second, HaFaNaC may not be incorporated into the axonal plasma membrane in sufficient number for FMRFamide to depolarize the axonal membrane to AP threshold. Third, HaFaNaC may be present but not functional in the plasma membrane of mammalian axons. In order to determine if HaFaNaC is present in the axon membrane, we tagged the intracellular N-terminus to HaFaNaC with GFP (GFPHaFaNaC) to visualize its subcellular location. When expressed in CA3 pyramidal neurons, GFPHaFaNaC was functional because pressure application of FMRFamide to neuronal somata resulted in a depolarization and burst of action potentials in 12 cells tested (Figure 3C). Similar to HaFaNaC (Fig. 1), FMRFamide-induced depolarizations of GFPHaFaNaC-expressing neurons was reversibly blocked by amiloride (Figure 3C and D, repeated measures ANOVA, p<0.001, Tukey-Kramer post hoc test control vs. amiloride P < 0.001, amiloride vs. wash P < 0.01, control vs. wash P < 0.001, N = 7). Thus, the fusion of GFP to the N-terminus of HaFaNaC did not appear to alter HaFaNaC function.
To determine the neuronal subcellular location of GFPHaFaNaC, we cotransfected CA3 pyramidal neurons with GFPHaFaNaC and a red fluorescent protein (dsRed or tdTomato) and examined their distributions by confocal laser scanning microscopy. The red fluorescent protein filled the entire neuron permitting the identification of its soma, dendrites and axons. GFPHaFaNaC was colocalized with red fluorescent protein in soma and proximal dendrites but not in distal axons located in CA1 (Fig 3B, N = 19). Although GFPHaFaNaC was not detected in the axons, under the same transfection conditions, the tagged vesicular protein SynGFP was observed in all axons of neurons in which it was transfected (N = 11, data not shown).
Potential Secondary Effects of HaFaNaC expression and FMRFamide
Although HaFaNaC and FMRFamide are not found in mammals, homologous ion channels (Lingueglia et al., 1995; Askwith et al., 2000; Xie et al., 2003; Lingueglia et al., 2006), homologous neuropeptides (RFamide peptides) (Yang et al., 1985; Perry et al., 1997; Vilim et al., 1999; Hinuma et al., 2000) and their receptors (Tang et al., 1984; Yang et al., 1985; Raffa, 1988; Brussaard et al., 1989; Raffa, 1989; Roumy and Zajac, 1998; Bonini et al., 2000; Hinuma et al., 2000; Liu et al., 2001) have been identified in the mammalian CNS. Activation or modulation of these ion channels or receptors by exogenously applied FMRFamide could limit the utility of the expression of HaFaNaC in mammalian neurons. Similarly, activation of HaFaNaC by mammalian RFamide peptides would also limit the usefulness of heterologously expressed HaFaNaC. Therefore, we examined if the application of FMRFamide could activate HaFaNaC in the absence of secondary effects in the mammalian CNS.
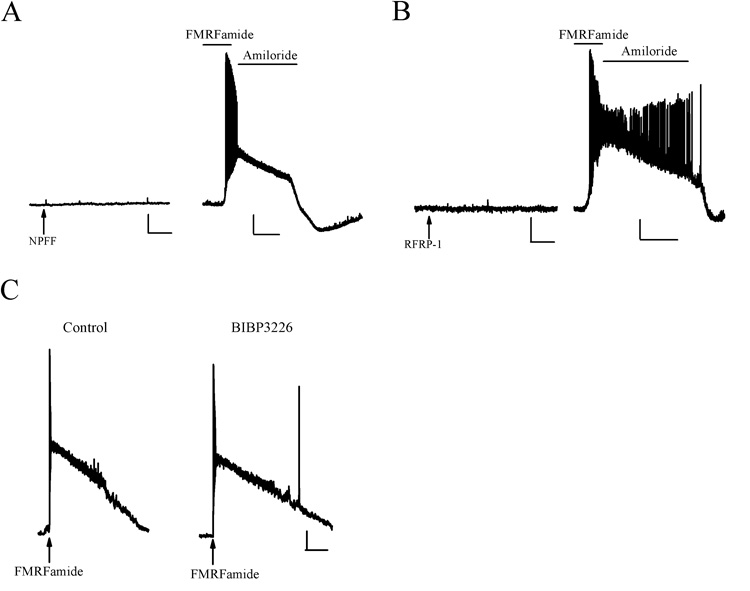
We first investigated the possibility that HaFaNaC might be activated by RFamide peptides located in the mammalian CNS. The RFamide peptides NPFF and RFRP-1 were applied to the somata of HaFaNaC-expressing CA3 pyramidal neurons. Neither NPFF (Fig. 4A) nor RFRP-1 (Fig. 4B) produced any response on the neurons. However, subsequent bath application of FMRFamide did depolarize these same neurons. Therefore, RFamide peptides of the mammalian CNS do not appear capable activating HaFaNaC in mammalian neurons.
Figure 4. Absence of endogenous effects on HaFaNaC and elimination of secondary effects of FMRFamide.
A. Pressure application of NPFF (arrow, 100 µM) did not affect a HaFaNaC-expressing CA3 pyramidal neuron (left). Bath application of FMRFamide (100 µM) to the same cell elicited a large depolarization that was suppressed by 100 µM amiloride (right). N=3. Scale bars: Vertical 10 mV (left and right), horizontal 2 s (left), 20s (right). B. Pressure application of RFRP-1 (arrow, 100 µM) did not affect a HaFaNaC-expressing CA3 pyramidal neuron (left). Bath application of FMRFamide to the same neuron produced a large depolarization that was suppressed by amiloride (100 µM, right). N = 3. Scale bars: Vertical 10 mV (left and right), horizontal 2s (left), 20s (right). C. Pressure application of FMRFamide (arrow, 100 µM) depolarized a HaFaNaC transfected neuron (left). The NPY-1 receptors antagonist BIBP3226, at concentrations known to block endogenous NPFF receptors (10 µM), did not block the FMRFamide-elicited depolarization (right). N = 3. Scale bars: Vertical 10 mV, horizontal 5 s.
Although RFamide peptides do not appear to activate HaFaNaC, FMRFamide has been shown to activate NPFF receptors (Tang et al., 1984; Yang et al., 1985; Raffa, 1988; Brussaard et al., 1989; Raffa, 1989; Roumy and Zajac, 1998; Bonini et al., 2000; Hinuma et al., 2000; Liu et al., 2001). However, a neuropeptide Y1 receptor antagonist (BIBP3226) has been shown to block NPFF receptors (Mollereau et al., 2001; Mollereau et al., 2002). Therefore, we tested whether BIBP3226, at concentrations known to block NPFF receptors, could also block HaFaNaC when expressed in mammalian neurons. Bath application of BIBP3226 (10 µM) did not inhibit activation of HaFaNaC by pressure application of FMRFamide (Figure 4C, n= 3). This suggests that in the presence of BIBP3226, FMRFamide can activate HaFaNaC-expressing mammalian neurons without additionally activating endogenous NPFF receptors.
Discussion
We have shown that the invertebrate HaFaNaC can be functionally expressed in mammalian neurons in vitro. When HaFaNaC was expressed in CA3 pyramidal neurons of organotypic slice cultures, somatic application of FMRFamide was able to produce large depolarizations sufficient to elicit a burst of APs. The responses to FMRFamide were inhibited by amiloride and showed similar current-voltage relationships to those previously observed in cell lines and oocytes (Lingueglia et al., 1995). Importantly, FMRFamide activation of HaFaNaC showed little desensitization. This permitted bath application of FMRFamide to persistently activate CA3 pyramidal neurons in a dose-dependent manner. Furthermore, doses of FMRFamide required to activate CA3 pyramidal neurons were below those necessary for the modulation of ASICs (Askwith et al., 2000; Xie et al., 2003). In addition, endogenous RFamide peptides did not activate HaFaNaC in CA3 pyramidal neurons and an antagonist of NPFF receptors did not inhibit FMRFamide-activation of HaFaNaC. Therefore, coupled with pharmacological blockade in regions of the CNS containing NPFF-1 and NPFF-2 receptors, heterologous expression of HaFaNaC may provide a gene targeted method to selectively excite specific subtypes of neurons in intact mammalian CNS tissue.
When expressed in oocytes and cell lines, HaFaNaC activation by FMRFamide produced inward currents that displayed little desensitization (Lingueglia et al., 1995). These FMRFamide responses were inhibited by amiloride and had a reversal potential near the equilibrium potential for sodium. In this study, HaFaNaC was shown to have the same properties when expressed in organotypic CA3 pyramidal neurons. This suggests that the HaFaNaC can be functionally expressed in mammalian neurons and their properties are not significantly altered by expression in mammalian neurons.
As the heterologous expression of HaFaNaC was previously shown to display little desensitization (Lingueglia et al., 1995), we hypothesized that bath application of FMRFamide would persistently activate HaFaNaC-expressing CA3 pyramidal neurons in hippocampal slice cultures. Our study showed that FMRFamide could be repetitively applied by bath to excite CA3 pyramidal neurons and the response to FMRFamide was dose-dependent. Although there was a clear dose-dependence of FMRFamide application to individual HaFaNaC-expressing neurons, there was also significant variability of responses between different HaFaNaC-expressing neurons. This was likely due to the method of transfection. The number of HaFaNaC cDNA containing plasmids introduced into an individual neuron cannot be controlled with single cell electroporation and therefore the number of functionally expressed HaFaNaCs will vary from cell to cell. This variability could be reduced by maintaining the same copy number of HaFaNaC in all HaFaNaC-expressing neurons. This could be accomplished by using transgenic animal lines expressing HaFaNaC in specific neuronal cell types or the creation of HaFaNaC expressing animals through homologous recombination. Thus under more controlled conditions, the stable selective expression of HaFaNaC in specific neuronal subtypes could be used to activate neurons that display persistent and prolonged firing patterns in vivo (Grace, 1991; Kiyatkin, 1995; Richard et al., 1997; Detari et al., 1999; Apicella, 2002; Zhou et al., 2002; Dreher and Burnod, 2002; Wightman and Robinson, 2002; Dampney et al., 2003; Garcia-Rill et al., 2004; Heien and Wightman, 2006; Hikosaka, 2007; Fetter, 2007),. Furthermore, different degrees of activity in HaFaNaC expressing neuronal subtypes could be controlled by the concentration of FMRFamide applied to the preparation. This would provide insight into how changing the amount of activity of a neuronal subtype changes neural network function.
The utility of HaFaNaC expression in mammalian neurons in intact CNS tissue could be limited by the presence of homologous peptides, receptors and ion channels in the mammalian CNS (Tang et al., 1984; Yang et al., 1985; Raffa, 1988; Brussaard et al., 1989; Raffa, 1989; Perry et al., 1997; Roumy and Zajac, 1998; Vilim et al., 1999; Askwith et al., 2000; Bonini et al., 2000; Hinuma et al., 2000; Xie et al., 2003). However, activation of HaFaNaC is very sensitive to substitution of the amino acids that make up FMRFamide (Cottrell, 1997). First, N-terminal extensions to FMRFamide dramatically reduce efficacy at HaFaNaC. Thus RFamide-like peptides of the mammalian CNS will have reduced efficacy at HaFaNaC because they have N-terminal extensions of 4 to 50 amino acids in length (Fukusumi et al., 2006). Second, substitution of the amino acids at positions one and/or two of FMRFamide either dramatically reduce or completely eliminate the peptides efficacy at HaFaNaC (Cottrell, 1997). All mammalian RFamide peptides have amino acid substitutions at both position one and two which greatly reduces or eliminates their efficacy at HaFaNaC. This is consistent with our observations that even high (supra physiological) concentrations of the shortest length mammalian RFamide peptide NPFF had no effect on FMRFamide activation of HaFaNaC-expressing neurons, as previously observed by others (Lingueglia et al., 1995). We also showed that large concentrations of RFRP-1 had no effect on HaFaNaC-expressing neurons. Finally, because neuropeptides are released from nonsynaptic locations (Salio et al., 2006) and are normally found in the CNS at concentrations approximately 1000 to 10000 fold lower than required for the full agonist to activate HaFaNaC (Malin et al., 1990; Sundblom et al., 1997; Zangen et al., 1999; Kiyashchenko et al., 2002; Burlet-Schiltz et al., 2002; Guan et al., 2005; Hilke et al., 2005; Pallis et al., 2006), physiologically released RFamide peptides will have no effect on heterologously expressed HaFaNaC. Therefore, endogenous RFamides of the mammalian CNS will have no effect on heterologously expressed HaFaNaC.
HaFaNaC is a member of the ENaC/degenerens family of ion channels (Kellenberger and Schild, 2002) and therefore has sequence homology to ASICs, which are found in the mammalian CNS. Although FMRFamide has no direct effect on ASICS, FMRFamide has been shown to potentiate proton activation of ASICs (Askwith et al., 2000; Xie et al., 2003). However, bath concentrations used to activate HaFaNaC in CA3 pyramidal neurons were below those necessary to modulate the mammalian ASIC (Askwith et al., 2000; Xie et al., 2003). Therefore, it is possible to activate HaFaNaC without producing any confounding effect on ASIC function.
RFamide peptides activate several receptors in the mammalian CNS. However, some of these receptors are very selective for their agonist. For example, QRFP receptors does not depend on the RFamide C-terminus and instead requires amino acid sequences nearer their N-termini (Fukusumi et al., 2003). Thus other RFamides do not activate the QRFP receptors. Similarly, both the PrRP and the metastin receptors only bind their endogenous ligands (PrRPs and kisspeptins respectively) and are unaffected by other RFamides (Muir et al., 2001; Engstrom et al., 2003). In contrast, NPFF-1 and NPFF-2 receptors are relatively promiscuous and are activated by FMRFamide (Engstrom et al., 2003). However, both NPFF-1 and NPFF-2 can be blocked by the NPY-1 receptor antagonist BIBP3226 (Mollereau et al., 2001; Mollereau et al., 2002). Furthermore, at concentrations that inhibit mammalian NPFF-1 and NPFF-2, BIBP3226 had no affect on FMRFamide activation of HaFaNaC expressed in CA3 pyramidal neurons. Thus, if applied with BIBP3226, application of FMRFamide should have no affect on any endogenous RFamide receptors in the mammalian CNS. Therefore, the expression of HaFaNaC in specific subtypes of neurons in the mammalian CNS would permit the selective activation of those neurons through the application of FMRFamide.
In invertebrates, FaNaC appears to be present primarily in the soma and dendrites, although there is a possibility of small amounts also residing in axons of invertebrate neurons in vivo (Davey et al., 2001). However, FMRFamide was not capable of directly activating the axons of HaFaNaC-expressing CA3 pyramidal neurons and a functional N-terminally tagged GFP fusion HaFaNaC protein was not detected in the axons of CA3 pyramidal neurons. Thus, in order to excite HaFaNaC-expressing mammalian neurons, it is likely that FMRFamide must act on HaFaNaC receptors expressed on the somata of mammalian neurons. This may limit the utility of HaFaNaC expression in mammalian neurons because FMRFamide would not be able to evoke the release of transmitter directly from axon terminals. In brain slices, axon and soma of the HaFaNaC-expressing neurons would have to be intact for FMRFamide to elicit the release of transmitter, which limits the usefulness of HaFaNaC to local neurons because axons are often severed from their somata in brain slice preparations with long axonal projections. Axonal expression of HaFaNaC would also be beneficial for some in vivo experiments. For example, some neuronal subtypes project to multiple different regions of the brain. By activating the somata of these neuronal subtypes, neurotransmitter would be released in all its projection sites. However, if HaFaNaC was functionally expressed in the axon of mammalian neurons, FMRFamide could be applied discretely into one projection site of the neuronal subtype under investigation. This would allow the restricted release of transmitter into individual projection sites of the brain. Therefore, the functional expression of HaFaNaC in the axons would increase the utility of HaFaNaC as an activator of specific subtypes of neurons in the mammalian CNS. In the future, this might be accomplished by modifying the sequence of HaFaNaC to contain sequences of amino acids known to target the expression of voltage-dependent ion channels to axons of mammalian neurons (Garrido et al., 2001; Silverman et al., 2001; Garrido et al., 2003; Gu et al., 2003; Sampo et al., 2003; Rivera et al., 2005; Arnold, 2006; Chung et al., 2006; Gu et al., 2006; Pan et al., 2006).
Another limitation of HaFaNaC is the requirement for the application of an exogenous compound to activate HaFaNaC as opposed to light-gated ion channels (Banghart et al., 2004; Boyden et al., 2005; Li et al., 2005; Chambers et al., 2006). In the case of channelrhodopsin, light stimulation can rapidly elicit APs in neurons with millisecond precision (Boyden et al., 2005). It may be possible to improve the timing of HaFaNaC activation through the synthesis of a caged-FMRFamide molecule (Ellis-Davies, 2007). This may permit ultraviolet flashes of light to rapidly activate HaFaNaC through the local uncaging of a caged-FMRFamide. Alternatively, like the elegant strategy used to open and close a modified Drosophila Shaker channel (Banghart et al., 2004; Chambers et al., 2006), it may be possible to covalently attach FMRFamide to a photoisomerizable azobenzene molecule such that light would open and close HaFaNaC. The light-activated conformational changes in the azobenzene could permit the covalently attached FMRFamide to move in and out of its binding site on HaFaNaC (Cottrell, 2005). Therefore, improved strategies for the activation of HaFaNaC may improve HaFaNaC’s utility for activating specific subtypes of mammalian neurons.
In conclusion, we have described the utility of heterologously expressing HaFaNaC in mammalian neurons as a potential genetically targeted method to excite specific subsets of neurons in intact mammalian CNS tissue. FMRFamide activation of heterologous expressed HaFaNaC could be used to study the effect of the excitation of specific subsets of neurons that are tonically active in the mammalian CNS. Strategies to improve the kinetics of activation of HaFaNaC and modify its subcellular distribution may further improve the utility of HaFaNaC.
Acknowledgements
The authors would like to thank Drs. E. Lingueglia and M. Lazdunski for the donation of their FMRFamide-gated sodium channel, Dr. R.Y. Tsien for the tdTomato, Dr. E. Ruthazer for donating the GFP-tagged synaptophysin, and Dr. J Dempster for the gift of his electrophysiological software. Microscopy was performed at the VCU - Dept. of Neurobiology & Anatomy Microscopy Facility, supported, in part, with funding from NIH-NINDS Center core grant 5P30NS047463. A. Rory McQuiston is supported by a grant from the NIH-NIDA (5R01DA017110).
List of Abbreviations
- FaNaC
FMRFamide-gated sodium channel
- HaFaNaC
FMRFamide-gated sodium channel from Helix aspersa
- GFP
green fluorescent protein
- dsRed
red fluorescent protein from Discosoma sp.
- tdTomato
mutated form of dsRed
- AP
action potential
- PrRP
prolactin-releasing peptide
- RFRP
RFamide-related peptide
- QRFP
pyroglutamylated RFamide peptide
- ASIC
acid sensing ion channel
- PCR
polymerase chain reaction
- GFPHaFaNaC
FMRFamide-gated sodium channel from Helix aspersa with an N-terminal GFP fusion
- SynGFP
synaptophysin GFP fusion protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de LL. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella P. Tonically active neurons in the primate striatum and their role in the processing of information about motivationally relevant events. Eur J Neurosci. 2002;16:2017–2026. doi: 10.1046/j.1460-9568.2002.02262.x. [DOI] [PubMed] [Google Scholar]
- Aravanis AM, Wang LP, Zhang F, Meltzer LA, Mogri MZ, Schneider MB, Deisseroth K. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J Neural Eng. 2007;4:S143–S156. doi: 10.1088/1741-2560/4/3/S02. [DOI] [PubMed] [Google Scholar]
- Arenkiel BR, Klein ME, Davison IG, Katz LC, Ehlers MD. Genetic control of neuronal activity in mice conditionally expressing TRPV1. Nat Methods. 2008;5:299–302. doi: 10.1038/nmeth.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenkiel BR, Peca J, Davison IG, Feliciano C, Deisseroth K, Augustine GJ, Ehlers MD, Feng G. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron. 2007;54:205–218. doi: 10.1016/j.neuron.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold DB. Polarized targeting of ion channels in neurons. Pflugers Arch. 2006 doi: 10.1007/s00424-006-0155-5. [DOI] [PubMed] [Google Scholar]
- Askwith CC, Cheng C, Ikuma M, Benson C, Price MP, Welsh MJ. Neuropeptide FF and FMRFamide potentiate acid-evoked currents from sensory neurons and proton-gated DEG/ENaC channels. Neuron. 2000;26:133–141. doi: 10.1016/s0896-6273(00)81144-7. [DOI] [PubMed] [Google Scholar]
- Banghart M, Borges K, Isacoff E, Trauner D, Kramer RH. Light-activated ion channels for remote control of neuronal firing. Nat Neurosci. 2004;7:1381–1386. doi: 10.1038/nn1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi A, Cui J, Ma YP, Olshevskaya E, Pu M, Dizhoor AM, Pan ZH. Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration. Neuron. 2006;50:23–33. doi: 10.1016/j.neuron.2006.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini JA, Jones KA, Adham N, Forray C, Artymyshyn R, Durkin MM, Smith KE, Tamm JA, Boteju LW, Lakhlani PP, Raddatz R, Yao WJ, Ogozalek KL, Boyle N, Kouranova EV, Quan Y, Vaysse PJ, Wetzel JM, Branchek TA, Gerald C, Borowsky B. Identification and characterization of two G protein-coupled receptors for neuropeptide FF. J Biol Chem. 2000;275:39324–39331. doi: 10.1074/jbc.M004385200. [DOI] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Brussaard AB, Kits KS, ter MA, Mulder AH, Schoffelmeer AN. Peripheral injection of DNS-RFa, a FMRFa agonist, suppresses morphine-induced analgesia in rats. Peptides. 1989;10:735–739. doi: 10.1016/0196-9781(89)90105-8. [DOI] [PubMed] [Google Scholar]
- Burlet-Schiltz O, Mazarguil H, Sol JC, Chaynes P, Monsarrat B, Zajac JM, Roussin A. Identification of neuropeptide FF-related peptides in human cerebrospinal fluid by mass spectrometry. FEBS Lett. 2002;532:313–318. doi: 10.1016/s0014-5793(02)03686-4. [DOI] [PubMed] [Google Scholar]
- Chambers JJ, Banghart MR, Trauner D, Kramer RH. Light-induced depolarization of neurons using a modified Shaker K(+) channel and a molecular photoswitch. J Neurophysiol. 2006;96:2792–2796. doi: 10.1152/jn.00318.2006. [DOI] [PubMed] [Google Scholar]
- Chung HJ, Jan YN, Jan LY. Polarized axonal surface expression of neuronal KCNQ channels is mediated by multiple signals in the KCNQ2 and KCNQ3 C-terminal domains. Proc Natl Acad Sci U S A. 2006;103:8870–8875. doi: 10.1073/pnas.0603376103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell GA. The first peptide-gated ion channel. J Exp Biol. 1997;200:2377–2386. doi: 10.1242/jeb.200.18.2377. [DOI] [PubMed] [Google Scholar]
- Cottrell GA. Domain near TM1 influences agonist and antagonist responses of peptide-gated Na+ channels. Pflugers Arch. 2005;450:168–177. doi: 10.1007/s00424-005-1385-7. [DOI] [PubMed] [Google Scholar]
- Dampney RA, Horiuchi J, Tagawa T, Fontes MA, Potts PD, Polson JW. Medullary and supramedullary mechanisms regulating sympathetic vasomotor tone. Acta Physiol Scand. 2003;177:209–218. doi: 10.1046/j.1365-201X.2003.01070.x. [DOI] [PubMed] [Google Scholar]
- Davey F, Harris SJ, Cottrell GA. Histochemical localisation of FMRFamide-gated Na+ channels in Helisoma trivolvis and Helix aspersa neurones. J Neurocytol. 2001;30:877–884. doi: 10.1023/a:1020656915810. [DOI] [PubMed] [Google Scholar]
- Detari L, Rasmusson DD, Semba K. The role of basal forebrain neurons in tonic and phasic activation of the cerebral cortex. Prog Neurobiol. 1999;58:249–277. doi: 10.1016/s0301-0082(98)00084-7. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Burnod Y. An integrative theory of the phasic and tonic modes of dopamine modulation in the prefrontal cortex. Neural Netw. 2002;15:583–602. doi: 10.1016/s0893-6080(02)00051-5. [DOI] [PubMed] [Google Scholar]
- Ellis-Davies GC. Caged compounds: photorelease technology for control of cellular chemistry and physiology. Nat Methods. 2007;4:619–628. doi: 10.1038/nmeth1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engstrom M, Brandt A, Wurster S, Savola JM, Panula P. Prolactin releasing peptide has high affinity and efficacy at neuropeptide FF2 receptors. J Pharmacol Exp Ther. 2003;305:825–832. doi: 10.1124/jpet.102.047118. [DOI] [PubMed] [Google Scholar]
- Fetter M. Vestibulo-ocular reflex. Dev Ophthalmol. 2007;40:35–51. doi: 10.1159/000100348. [DOI] [PubMed] [Google Scholar]
- Fukusumi S, Fujii R, Hinuma S. Recent advances in mammalian RFamide peptides: the discovery and functional analyses of PrRP, RFRPs and QRFP. Peptides. 2006;27:1073–1086. doi: 10.1016/j.peptides.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Fukusumi S, Yoshida H, Fujii R, Maruyama M, Komatsu H, Habata Y, Shintani Y, Hinuma S, Fujino M. A new peptidic ligand and its receptor regulating adrenal function in rats. J Biol Chem. 2003;278:46387–46395. doi: 10.1074/jbc.M305270200. [DOI] [PubMed] [Google Scholar]
- Furukawa Y, Miyawaki Y, Abe G. Molecular cloning and functional characterization of the Aplysia FMRFamide-gated Na(+) channel. Pflugers Arch. 2006;451:646–656. doi: 10.1007/s00424-005-1498-z. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Homma Y, Skinner RD. Arousal mechanisms related to posture and locomotion: 1. Descending modulation. Prog Brain Res. 2004;143:283–290. [PubMed] [Google Scholar]
- Garrido JJ, Fernandes F, Giraud P, Mouret I, Pasqualini E, Fache MP, Jullien F, Dargent B. Identification of an axonal determinant in the C-terminus of the sodium channel Na(v)1.2. EMBO J. 2001;20:5950–5961. doi: 10.1093/emboj/20.21.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido JJ, Giraud P, Carlier E, Fernandes F, Moussif A, Fache MP, Debanne D, Dargent B. A targeting motif involved in sodium channel clustering at the axonal initial segment. Science. 2003;300:2091–2094. doi: 10.1126/science.1085167. [DOI] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- Green KA, Cottrell GA. Block of the helix FMRFamide-gated Na+ channel by FMRFamide and its analogues. J Physiol. 1999;519(Pt 1):47–56. doi: 10.1111/j.1469-7793.1999.0047o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KA, Cottrell GA. Activity modes and modulation of the peptide-gated Na(+) channel of Helix neurones. Pflugers Arch. 2002;443:813–821. doi: 10.1007/s00424-001-0750-4. [DOI] [PubMed] [Google Scholar]
- Gu C, Jan YN, Jan LY. A conserved domain in axonal targeting of Kv1 (Shaker) voltage-gated potassium channels. Science. 2003;301:646–649. doi: 10.1126/science.1086998. [DOI] [PubMed] [Google Scholar]
- Gu C, Zhou W, Puthenveedu MA, Xu M, Jan YN, Jan LY. The microtubule plus-end tracking protein EB1 is required for Kv1 voltage-gated K+ channel axonal targeting. Neuron. 2006;52:803–816. doi: 10.1016/j.neuron.2006.10.022. [DOI] [PubMed] [Google Scholar]
- Guan C, Zhang S, Sun Y, Zheng X. Assess the effect of ciliary neurotrophic factor on extracellular level of neuropeptide Y in paraventricular nucleus using microdialysis. Conf Proc IEEE Eng Med Biol Soc. 2005;4:3608–3611. doi: 10.1109/IEMBS.2005.1617262. [DOI] [PubMed] [Google Scholar]
- Haas K, Sin WC, Javaherian A, Li Z, Cline HT. Single-cell electroporation for gene transfer in vivo. Neuron. 2001;29:583–591. doi: 10.1016/s0896-6273(01)00235-5. [DOI] [PubMed] [Google Scholar]
- Han X, Boyden ES. Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution. PLoS ONE. 2007;2:e299. doi: 10.1371/journal.pone.0000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heien ML, Wightman RM. Phasic dopamine signaling during behavior, reward, and disease states. CNS Neurol Disord Drug Targets. 2006;5:99–108. doi: 10.2174/187152706784111605. [DOI] [PubMed] [Google Scholar]
- Herlitze S, Landmesser LT. New optical tools for controlling neuronal activity. Curr Opin Neurobiol. 2006 doi: 10.1016/j.conb.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Hikosaka O. GABAergic output of the basal ganglia. Prog Brain Res. 2007;160:209–226. doi: 10.1016/S0079-6123(06)60012-5. [DOI] [PubMed] [Google Scholar]
- Hilke S, Theodorsson A, Fetissov S, Aman K, Holm L, Hokfelt T, Theodorsson E. Estrogen induces a rapid increase in galanin levels in female rat hippocampal formation--possibly a nongenomic/indirect effect. Eur J Neurosci. 2005;21:2089–2099. doi: 10.1111/j.1460-9568.2005.04050.x. [DOI] [PubMed] [Google Scholar]
- Hinuma S, Shintani Y, Fukusumi S, Iijima N, Matsumoto Y, Hosoya M, Fujii R, Watanabe T, Kikuchi K, Terao Y, Yano T, Yamamoto T, Kawamata Y, Habata Y, Asada M, Kitada C, Kurokawa T, Onda H, Nishimura O, Tanaka M, Ibata Y, Fujino M. New neuropeptides containing carboxy-terminal RFamide and their receptor in mammals. Nat Cell Biol. 2000;2:703–708. doi: 10.1038/35036326. [DOI] [PubMed] [Google Scholar]
- Ishizuka T, Kakuda M, Araki R, Yawo H. Kinetic evaluation of photosensitivity in genetically engineered neurons expressing green algae light-gated channels. Neurosci Res. 2006;54:85–94. doi: 10.1016/j.neures.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Jeziorski MC, Green KA, Sommerville J, Cottrell GA. Cloning and expression of a FMRFamide-gated Na(+) channel from Helisoma trivolvis and comparison with the native neuronal channel. J Physiol. 2000;526(Pt 1):13–25. doi: 10.1111/j.1469-7793.2000.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev. 2002;82:735–767. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- Kiyashchenko LI, Mileykovskiy BY, Maidment N, Lam HA, Wu MF, John J, Peever J, Siegel JM. Release of hypocretin (orexin) during waking and sleep states. J Neurosci. 2002;22:5282–5286. doi: 10.1523/JNEUROSCI.22-13-05282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA. Functional significance of mesolimbic dopamine. Neurosci Biobehav Rev. 1995;19:573–598. doi: 10.1016/0149-7634(95)00029-1. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987;15:8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer RH, Chambers JJ, Trauner D. Photochemical tools for remote control of ion channels in excitable cells. Nat Chem Biol. 2005;1:360–365. doi: 10.1038/nchembio750. [DOI] [PubMed] [Google Scholar]
- Li X, Gutierrez DV, Hanson MG, Han J, Mark MD, Chiel H, Hegemann P, Landmesser LT, Herlitze S. Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proc Natl Acad Sci U S A. 2005;102:17816–17821. doi: 10.1073/pnas.0509030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima SQ, Miesenbock G. Remote control of behavior through genetically targeted photostimulation of neurons. Cell. 2005;121:141–152. doi: 10.1016/j.cell.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Lingueglia E, Champigny G, Lazdunski M, Barbry P. Cloning of the amiloride-sensitive FMRFamide peptide-gated sodium channel. Nature. 1995;378:730–733. doi: 10.1038/378730a0. [DOI] [PubMed] [Google Scholar]
- Lingueglia E, Deval E, Lazdunski M. FMRFamide-gated sodium channel and ASIC channels: a new class of ionotropic receptors for FMRFamide and related peptides. Peptides. 2006;27:1138–1152. doi: 10.1016/j.peptides.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Liu Q, Guan XM, Martin WJ, McDonald TP, Clements MK, Jiang Q, Zeng Z, Jacobson M, Williams DL, Jr., Yu H, Bomford D, Figueroa D, Mallee J, Wang R, Evans J, Gould R, Austin CP. Identification and characterization of novel mammalian neuropeptide FF-like peptides that attenuate morphine-induced antinociception. J Biol Chem. 2001;276:36961–36969. doi: 10.1074/jbc.M105308200. [DOI] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Hammond MV, Fowler DE, Rogillio RB, Brown SL, Sims JL, Leecraft BM, Yang HY. FMRF-NH2-like mammalian octapeptide: possible role in opiate dependence and abstinence. Peptides. 1990;11:969–972. doi: 10.1016/0196-9781(90)90018-z. [DOI] [PubMed] [Google Scholar]
- Miesenbock G. Genetic methods for illuminating the function of neural circuits. Curr Opin Neurobiol. 2004;14:395–402. doi: 10.1016/j.conb.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Miesenbock G, Kevrekidis IG. Optical imaging and control of genetically designated neurons in functioning circuits. Annu Rev Neurosci. 2005;28:533–563. doi: 10.1146/annurev.neuro.28.051804.101610. [DOI] [PubMed] [Google Scholar]
- Mollereau C, Gouarderes C, Dumont Y, Kotani M, Detheux M, Doods H, Parmentier M, Quirion R, Zajac JM. Agonist and antagonist activities on human NPFF(2) receptors of the NPY ligands GR231118 and BIBP3226. Br J Pharmacol. 2001;133:1–4. doi: 10.1038/sj.bjp.0704049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollereau C, Mazarguil H, Marcus D, Quelven I, Kotani M, Lannoy V, Dumont Y, Quirion R, Detheux M, Parmentier M, Zajac JM. Pharmacological characterization of human NPFF(1) and NPFF(2) receptors expressed in CHO cells by using NPY Y(1) receptor antagonists. Eur J Pharmacol. 2002;451:245–256. doi: 10.1016/s0014-2999(02)02224-0. [DOI] [PubMed] [Google Scholar]
- Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, Szekeres PG, Sarau HM, Chambers JK, Murdock P, Steplewski K, Shabon U, Miller JE, Middleton SE, Darker JG, Larminie CG, Wilson S, Bergsma DJ, Emson P, Faull R, Philpott KL, Harrison DC. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem. 2001;276:28969–28975. doi: 10.1074/jbc.M102743200. [DOI] [PubMed] [Google Scholar]
- Nagel G, Brauner M, Liewald JF, Adeishvili N, Bamberg E, Gottschalk A. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr Biol. 2005;15:2279–2284. doi: 10.1016/j.cub.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci U S A. 2003;100:13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallis EG, Spyraki C, Thermos K. Chronic antidepressant treatment modulates the release of somatostatin in the rat nucleus accumbens. Neurosci Lett. 2006;395:76–81. doi: 10.1016/j.neulet.2005.10.055. [DOI] [PubMed] [Google Scholar]
- Pan Z, Kao T, Horvath Z, Lemos J, Sul JY, Cranstoun SD, Bennett V, Scherer SS, Cooper EC. A common ankyrin-G-based mechanism retains KCNQ and NaV channels at electrically active domains of the axon. J Neurosci. 2006;26:2599–2613. doi: 10.1523/JNEUROSCI.4314-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish AR, Wang W, Wang L. Manipulating proteins for neuroscience. Curr Opin Neurobiol. 2006;16:585–592. doi: 10.1016/j.conb.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Perry SJ, Straub VA, Schofield MG, Burke JF, Benjamin PR. Neuronal expression of an FMRFamide-gated Na+ channel and its modulation by acid pH. J Neurosci. 2001;21:5559–5567. doi: 10.1523/JNEUROSCI.21-15-05559.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry SJ, Yi-Kung HE, Cronk D, Bagust J, Sharma R, Walker RJ, Wilson S, Burke JF. A human gene encoding morphine modulating peptides related to NPFF and FMRFamide. FEBS Lett. 1997;409:426–430. doi: 10.1016/s0014-5793(97)00557-7. [DOI] [PubMed] [Google Scholar]
- Petreanu L, Huber D, Sobczyk A, Svoboda K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat Neurosci. 2007;10:663–668. doi: 10.1038/nn1891. [DOI] [PubMed] [Google Scholar]
- Price DA, Greenberg MJ. Structure of a molluscan cardioexcitatory neuropeptide. Science. 1977;197:670–671. doi: 10.1126/science.877582. [DOI] [PubMed] [Google Scholar]
- Rae JL, Levis RA. Single-cell electroporation. Pflugers Arch. 2002;443:664–670. doi: 10.1007/s00424-001-0753-1. [DOI] [PubMed] [Google Scholar]
- Raffa RB. The action of FMRFamide (Phe-Met-Arg-Phe-NH2) and related peptides on mammals. Peptides. 1988;9:915–922. doi: 10.1016/0196-9781(88)90141-6. [DOI] [PubMed] [Google Scholar]
- Raffa RB. Supraspinal FMRFamide antagonizes morphine-induced horizontal, but not vertical, locomotor activity. Peptides. 1989;10:403–406. doi: 10.1016/0196-9781(89)90050-8. [DOI] [PubMed] [Google Scholar]
- Rathenberg J, Nevian T, Witzemann V. High-efficiency transfection of individual neurons using modified electrophysiology techniques. J Neurosci Methods. 2003;126:91–98. doi: 10.1016/s0165-0270(03)00069-4. [DOI] [PubMed] [Google Scholar]
- Richard P, Moos F, Dayanithi G, Gouzenes L, Sabatier N. Rhythmic activities of hypothalamic magnocellular neurons: autocontrol mechanisms. Biol Cell. 1997;89:555–560. [PubMed] [Google Scholar]
- Rivera JF, Chu PJ, Arnold DB. The T1 domain of Kv1.3 mediates intracellular targeting to axons. Eur J Neurosci. 2005;22:1853–1862. doi: 10.1111/j.1460-9568.2005.04384.x. [DOI] [PubMed] [Google Scholar]
- Roumy M, Zajac JM. Neuropeptide FF, pain and analgesia. Eur J Pharmacol. 1998;345:1–11. doi: 10.1016/s0014-2999(97)01604-x. [DOI] [PubMed] [Google Scholar]
- Salio C, Lossi L, Ferrini F, Merighi A. Neuropeptides as synaptic transmitters. Cell Tissue Res. 2006;326:583–598. doi: 10.1007/s00441-006-0268-3. [DOI] [PubMed] [Google Scholar]
- Sampo B, Kaech S, Kunz S, Banker G. Two distinct mechanisms target membrane proteins to the axonal surface. Neuron. 2003;37:611–624. doi: 10.1016/s0896-6273(03)00058-8. [DOI] [PubMed] [Google Scholar]
- Schroll C, Riemensperger T, Bucher D, Ehmer J, Voller T, Erbguth K, Gerber B, Hendel T, Nagel G, Buchner E, Fiala A. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr Biol. 2006;16:1741–1747. doi: 10.1016/j.cub.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol. 2004;22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- Shaner NC, Steinbach PA, Tsien RY. A guide to choosing fluorescent proteins. Nat Methods. 2005;2:905–909. doi: 10.1038/nmeth819. [DOI] [PubMed] [Google Scholar]
- Silverman MA, Kaech S, Jareb M, Burack MA, Vogt L, Sonderegger P, Banker G. Sorting and directed transport of membrane proteins during development of hippocampal neurons in culture. Proc Natl Acad Sci U S A. 2001;98:7051–7057. doi: 10.1073/pnas.111146198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Sundblom DM, Kalso E, Tigerstedt I, Wahlbeck K, Panula P, Fyhrquist F. Neuropeptide FF-like immunoreactivity in human cerebrospinal fluid of chronic pain patients and healthy controls. Peptides. 1997;18:923–927. doi: 10.1016/s0196-9781(97)00040-5. [DOI] [PubMed] [Google Scholar]
- Tang J, Yang HY, Costa E. Inhibition of spontaneous and opiate-modified nociception by an endogenous neuropeptide with Phe-Met-Arg-Phe-NH2-like immunoreactivity. Proc Natl Acad Sci U S A. 1984;81:5002–5005. doi: 10.1073/pnas.81.15.5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilim FS, Aarnisalo AA, Nieminen ML, Lintunen M, Karlstedt K, Kontinen VK, Kalso E, States B, Panula P, Ziff E. Gene for pain modulatory neuropeptide NPFF: induction in spinal cord by noxious stimuli. Mol Pharmacol. 1999;55:804–811. [PubMed] [Google Scholar]
- Volgraf M, Gorostiza P, Numano R, Kramer RH, Isacoff EY, Trauner D. Allosteric control of an ionotropic glutamate receptor with an optical switch. Nat Chem Biol. 2006;2:47–52. doi: 10.1038/nchembio756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman RM, Robinson DL. Transient changes in mesolimbic dopamine and their association with 'reward'. J Neurochem. 2002;82:721–735. doi: 10.1046/j.1471-4159.2002.01005.x. [DOI] [PubMed] [Google Scholar]
- Xie J, Price MP, Wemmie JA, Askwith CC, Welsh MJ. ASIC3 and ASIC1 mediate FMRFamide-related peptide enhancement of H+-gated currents in cultured dorsal root ganglion neurons. J Neurophysiol. 2003;89:2459–2465. doi: 10.1152/jn.00707.2002. [DOI] [PubMed] [Google Scholar]
- Yang HY, Fratta W, Majane EA, Costa E. Isolation, sequencing, synthesis, and pharmacological characterization of two brain neuropeptides that modulate the action of morphine. Proc Natl Acad Sci U S A. 1985;82:7757–7761. doi: 10.1073/pnas.82.22.7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangen A, Nakash R, Yadid G. Serotonin-mediated increases in the extracellular levels of beta-endorphin in the arcuate nucleus and nucleus accumbens: a microdialysis study. J Neurochem. 1999;73:2569–2574. doi: 10.1046/j.1471-4159.1999.0732569.x. [DOI] [PubMed] [Google Scholar]
- Zemelman BV, Lee GA, Ng M, Miesenbock G. Selective photostimulation of genetically chARGed neurons. Neuron. 2002;33:15–22. doi: 10.1016/s0896-6273(01)00574-8. [DOI] [PubMed] [Google Scholar]
- Zemelman BV, Nesnas N, Lee GA, Miesenbock G. Photochemical gating of heterologous ion channels: remote control over genetically designated populations of neurons. Proc Natl Acad Sci U S A. 2003;100:1352–1357. doi: 10.1073/pnas.242738899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wang LP, Boyden ES, Deisseroth K. Channelrhodopsin-2 and optical control of excitable cells. Nat Methods. 2006;3:785–792. doi: 10.1038/nmeth936. [DOI] [PubMed] [Google Scholar]
- Zhang W, Ge W, Wang Z. A toolbox for light control of Drosophila behaviors through Channelrhodopsin 2-mediated photoactivation of targeted neurons. Eur J Neurosci. 2007;26:2405–2416. doi: 10.1111/j.1460-9568.2007.05862.x. [DOI] [PubMed] [Google Scholar]
- Zhang YP, Oertner TG. Optical induction of synaptic plasticity using a light-sensitive channel. Nat Methods. 2007;4:139–141. doi: 10.1038/nmeth988. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Wilson CJ, Dani JA. Cholinergic interneuron characteristics and nicotinic properties in the striatum. J Neurobiol. 2002;53:590–605. doi: 10.1002/neu.10150. [DOI] [PubMed] [Google Scholar]