Abstract
Cholesterol can be detrimental or vital, and must be present in the right place at the right time and in the right amount. This is well known in the heart and the vascular system. However, in the CNS cholesterol is still an enigma, although several of its fundamental functions in the brain have been identified. Brain cholesterol has attracted additional attention owing to its close connection to ApoE, a key polymorphic transporter of extracellular cholesterol in humans. Indeed, both cholesterol and ApoE are so critical to fundamental activities of the brain, that the brain regulates their synthesis autonomously. Yet, similar control mechanisms of ApoE and cholesterol homeostasis may exist on either sides of the blood–brain barrier. One indication is that the APOE ε4 allele is associated with hypercholesterolemia and a proatherogenic profile on the vascular side and with increased risk of Alzheimer’s disease on the CNS side. In this review, we draw attention to the association between cholesterol and ApoE in the aging and diseased brain, and to the behavior of the ApoE4 protein at the molecular level. The attempt to correlate in vivo and in vitro observations is challenging but crucial for developing future strategies to address ApoE-related aberrations in cholesterol metabolism selectively in the brain.
Keywords: aging, Alzheimer’s disease, apolipoprotein E, apolipoprotein E4, cholesterol, lipoprotein, neurodegeneration, neuronal function, synaptic plasticity
General background & significance of ApoE
Originally termed the ‘arginine-rich protein’, ApoE was identified approximately 35 years ago in human plasma and associated with cholesteryl ester-rich VLDLs [1]. Since then, tremendous progress has been made in our understanding of its role in regulating plasma cholesterol homeostasis. It was not until approximately two decades after the initial discovery of ApoE that it was found in the brain, where it is the major transporter of cholesterol. The importance of cholesterol in the brain and in the peripheral organs had been recognized much earlier. The findings that ApoE is linked to neurodegenerative disorders have spurred further interest in cholesterol metabolism in the brain. While ApoE has several different functions, its role in regulating lipoprotein metabolism is the most significant. This article focuses on the role of ApoE in cholesterol homeostasis in the aging and diseased brain.
ApoE is encoded by the polymorphic APOE gene located on chromosome 19 in humans [2,3]. Variation in the APOE gene sequence results in three common alleles, ε2, ε3 and ε4, represented with frequencies of 8%, 77% and 15%, respectively, in the population. The products of the three alleles are the isoforms, ApoE2, E3 and E4, which differ in the amino acids at positions 112 and 158 (Table 1). APOE ε4 is considered the ancestral gene among primates based on its similarities with primate APOE [4]. APOE ε3 arose from APOE ε4 during recent stages of human evolution [5]. A subsequent single base change from APOE ε3 gave rise to APOE ε2. The shift in diet from a largely plant-based (low-cholesterol) to a meat-based (lipid-rich, high-cholesterol) diet possibly led to the selection for ‘meat-adaptive genes’, such as APOE ε3, which is relatively less susceptible to diseases such as cardiovascular disease and Alzheimer’s disease (AD) [5]. ApoE3 is considered an anti-atherogenic protein. On the other hand, individuals homozygous for the APOE ε2 allele are prone to developing familial type III hyperlipoproteinemia and premature atherosclerosis. The inheritance of one or more of the APOE ε4 alleles predisposes the bearer to hypercholesterolemia, as well as AD and cerebral amyloid angiopathy, affecting both the age of onset and the severity of these diseases. Approximately 65% of individuals with late-onset familial and sporadic AD bear the APOE ε4 allele [6]. The precise mechanism by which ApoE4 is associated with AD is hotly debated and some aspects will be discussed in this article.
Table 1.
Characteristics of the human ApoE isoforms.
| Isoform | Amino acid at position 112 158 | Allelic frequency | LDLr binding | LRP binding | VLDLr binding | Associated risk |
|---|---|---|---|---|---|---|
| ApoE2 | Cys Cys | 0.070 | No | Yes | Yes | CVD, less prone to AD? |
| ApoE3 | Cys Arg | 0.783 | Yes | Yes | Yes | – |
| ApoE4 | Arg Arg | 0.147 | Yes | Yes | Yes | AD & CVD |
AD: Alzheimer’s disease; CVD: Cardiovascular disease; LDLr: LDL receptor; LRP: LDLr-related protein; VLDLr: VLDL receptor.
ApoE in the brain
While plasma ApoE originates predominantly from the liver and macrophages, the CNS ApoE is synthesized locally in the brain. The plasma pool of ApoE does not appear to exchange with the brain pool owing to the presence of the blood–brain barrier (BBB), as deduced by the failure to detect liver-derived ApoE in the cerebrospinal fluid (CSF) of liver transplant recipients [7]. Similarly, there is no mixing of the cholesterol pools between the brain and the periphery [8,9]. ApoE is the predominant and most widely studied apolipoprotein in the brain, although other apolipoproteins, such as ApoJ, ApoD, ApoAI and ApoAIV, are present within the CNS. In the brain, ApoE is mainly synthesized by the astrocytes [10–12]. Microglia [13] and neurons under select pathological or physiological conditions [14–17] are also able to synthesize some ApoE.
The direct study of ApoE in the brain parenchyma is difficult, but based on the observations that plasma ApoE is present in lipoproteins, it is assumed that the same is true for the brain [18]. Studies of astrocyte-derived lipoproteins and lipoproteins isolated from the CSF suggest that brain astrocytes secrete ApoE in discoidal HDL-like particles composed of phospholipids and unesterified cholesterol. It is not known if lipid-free ApoE is also secreted by astrocytes. ApoE isolated from CSF is present in both discoidal and spherical lipoprotein complexes [19–26]. Owing to its structural flexibility (discussed later), ApoE is able to associate with discoidal as well as spherical HDL-sized particles. Thus, it appears that before reaching the CSF some of these particles accumulate cholesterol and form spherical lipoproteins upon esterification of free cholesterol [18,27]. Large triglycerides-containing lipoprotein particles have not been detected in the CNS.
Cholesterol dynamics in the brain
In recent years, interest in brain cholesterol metabolism and ApoE has increased dramatically after the recognition that cholesterol plays a pivotal role in neurodegenerative disorders such as AD and Niemann–Pick type C disease, and that ApoE is the only risk factor consistently associated with nonfamilial forms of AD. Cholesterol is crucial in the development and normal functioning of the CNS and plays a vital role in the development and maintenance of neuronal plasticity [28–30], in synaptic vesicle transport along microtubules [31] and in neurotransmitter release [30].
The brain is the organ richest in cholesterol [8], with the CNS accounting for 20–25% of the total-body cholesterol; interestingly, the brain accounts for only 5% of the body mass [32]. In the brain, cholesterol is a major component of myelin, is present in membranes of neurons and glial cells and is also localized to lipoprotein particles. Peripheral and brain cholesterol pools are separated by the BBB and are independently regulated [9]. The half-life of brain cholesterol was reported to be 6 months to 1 year [33] or even 5 years in other studies [34], much longer than its half-life in plasma, which is in the order of hours. Cholesterol synthesis in the brain is sufficient to meet the demands of the CNS during development and in adult life; this local synthesis decreases with age [9,35,36]. Brain cells maintain cholesterol homeostasis by regulation of cholesterol synthesis and cholesterol uptake through ApoE-related receptors [9,37]. Glial cells produce two- to three-times more cholesterol than neurons. As discussed above, astrocytes secrete cholesterol in HDL-like particles with ApoE. With respect to neurons, although they can synthesize cholesterol, there is sufficient evidence indicating that the growth of neuronal processes and effective synapse formation depend on cholesterol supplied by the astrocytes (reviewed in [9,38]). In this regard, we have participated in the studies that demonstrated that when cholesterol synthesis is inhibited in neurons, axonal elongation is supported by lipoprotein-derived cholesterol [39,40]. As neurons mature they reduce endogenous cholesterol synthesis and become more dependent on exogenous cholesterol [37,41]. Pfrieger has hypothesized that after astrocyte differentiation is completed neurons rely constitutively on lipoprotein-derived cholesterol [42].
A small amount of cholesterol is transported from the brain to the CSF via an ApoE-dependent mechanism [12], however from a quantitative viewpoint, the most important mechanism of elimination of cholesterol from the brain is its conversion to 24S-hydroxycholesterol, which takes place in brain neurons [34,43]. Contrary to cholesterol, 24S-hydroxycholesterol is able to cross the BBB [34,43,44]. 24S-hydroxycholesterol might also play other important roles in brain cholesterol homeostasis (discussed later).
Functions of ApoE in lipid transport in the brain
The fact that ApoE is expressed in the brain and that neurons and glial cells have ApoE receptors suggests the following concepts:
There is a dynamic exchange of ApoE among brain cells
ApoE is a major transporter of extracellular lipids and cholesterol in the brain
ApoE-mediated cholesterol exchange occurs between neuronal and non-neuronal cells
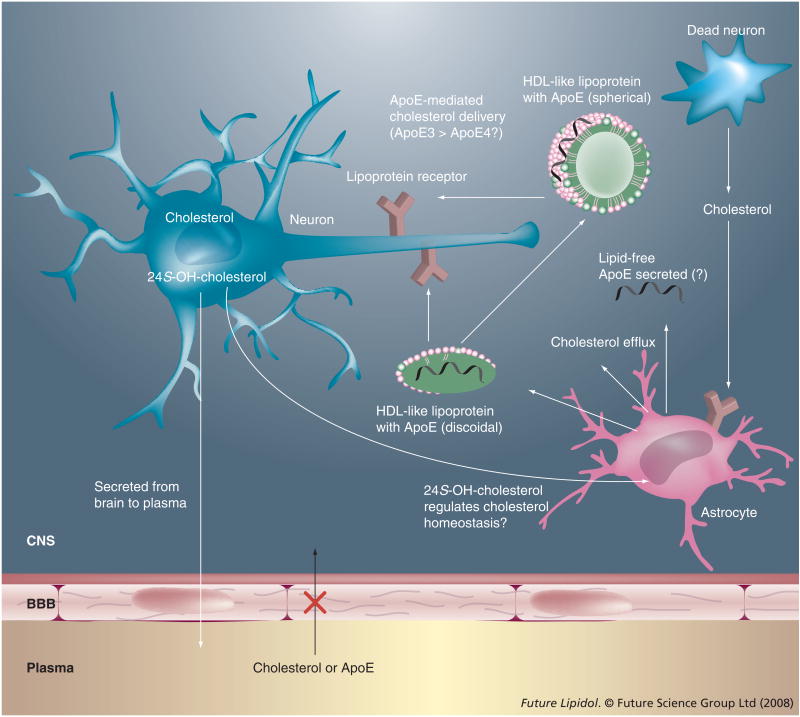
The synthesis and distribution of cholesterol among neurons and glial cells mediated by ApoE is schematically represented in Figure 1.
Figure 1. ApoE-mediated cholesterol shuttle in the brain.
ApoE shuttles cholesterol from the astrocytes to the neurons via lipoprotein complexes (green disc or sphere) by serving as a ligand for lipoprotein receptors (brown). The overall concept is that ApoE3 may mediate this function better than ApoE4. Conversion of cholesterol to 24S-OH-cholesterol is a major pathway for elimination of brain cholesterol. It also probably plays a role in regulating cholesterol synthesis in the astrocytes.
24S-OH-cholesterol: 24S-hyroxycholesterol; BBB: Blood–brain barrier.
ApoE may also play a crucial role in lipid clearance and recycling, particularly after injury [27,45]. The presence of ApoE-containing lipoproteins in the CSF indicates its role in brain lipid clearance. Moreover, following injury of the CNS there is a dramatic upregulation of ApoE that parallels cholesterol and lipid debris clearance from the site of injury [11,46–48]. Further supporting this cholesterol-’scavenging’ role of ApoE, degeneration products are not efficiently cleared from the hippocampus after injury in ApoE-null mice [49].
The importance of ApoE in cholesterol transport in the brain could explain the contribution of ApoE to the regulation of synapse formation, plasticity and repair [45,50]. The regulation of cholesterol supply to neurons by ApoE-containing lipoproteins is not completely understood and further studies are required, although some mechanisms are emerging. Two very important aspects that warrant further investigation are the neuronal location at which cholesterol transport takes place (synapses vs cell bodies) and the possibility that cholesterol delivery is regulated by electrical activity [42]. Future studies may link the contribution of ApoE in cell signaling directly or indirectly to the cholesterol status in the neuronal membrane and its microdomains.
Cholesterol in normal aging
Aging is the primary risk factor for AD and other neurodegenerative disorders. Therefore, in order to identify the roles of ApoE and cholesterol in the pathophysiology of neurodegeneration, it is essential to understand the changes that take place in cholesterol and ApoE during normal brain aging. Some other aspects of brain aging have been reviewed elsewhere [51,52].
Numerous studies have demonstrated that the human brain decreases in weight and size during aging. The brain volume reduction becomes more significant over the age of 70 years [53]. During normal aging, brain cholesterol levels change depending on the region considered, with some regions showing no change in cholesterol while others show a 40% decrease [54]. De novo synthesis of cholesterol in the brain is reduced, at least in the hippocampus [35], and cholesterol content in the cortex decreases linearly from early in life (~20 years old), and more rapidly after 80 years of age [55]. This decrease seems to represent the loss of axons, dendrites and astrocyte processes [55].
Macroscopic brain changes during normal aging are not due to dramatic neuronal loss, which is undetectable or relatively mild, but are perhaps due to a reduction in neuronal size and the number of synapses (reviewed in [53]). Synapse loss and reduction in the number and density of spines during aging might correlate with deteriorating cognitive performance. These changes may depend on ApoE and cholesterol availability.
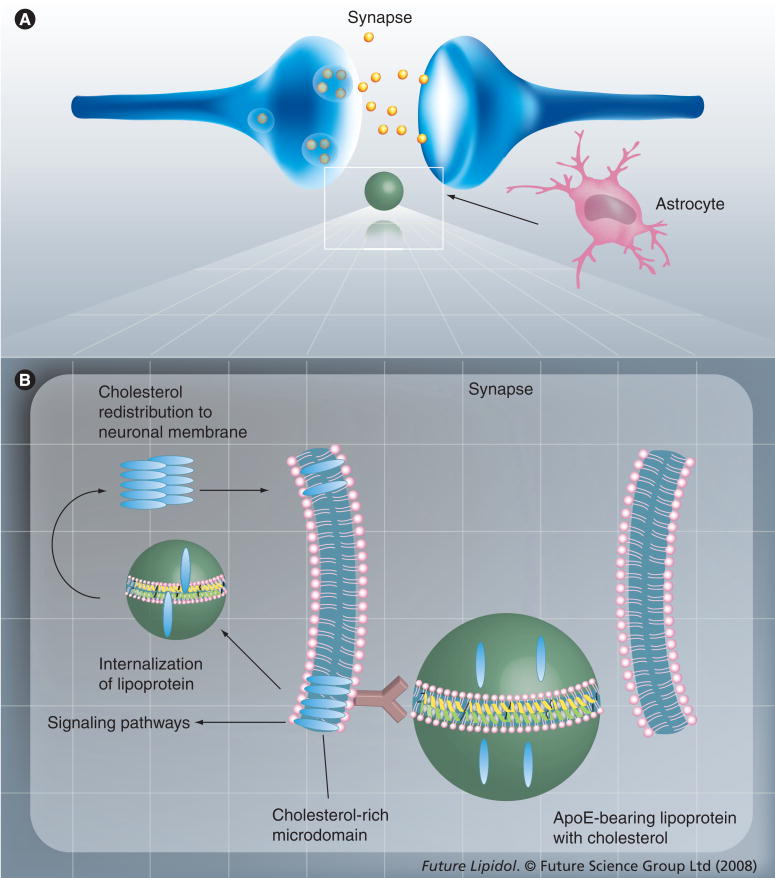
Cholesterol is a limiting factor in synapse development [30], dendrite differentiation [56], long-term potentiation [57] and axonal elongation [39,40]. Neurons have an intrinsic ability to form synapses. However, cholesterol delivered by ApoE-containing lipoproteins secreted by astrocytes dramatically enhances synapse formation [30] by increasing the number of synaptic vesicles and the formation of the machinery for releasing vesicles (Figure 2) [58]. The membranes of synaptic vesicles contain high amounts of cholesterol and cholesterol-interacting proteins [59,60]. Furthermore, cholesterol is required for synaptic vesicle biogenesis [60]. The lack of cholesterol supply to neurons causes failure of neurotransmission and decreased synaptic plasticity [61]. Cholesterol distributes primarily to cholesterol- and sphingolipid-rich microdomains (lipid rafts) in synaptic vesicle membranes, presynaptic active zones, postsynaptic membranes and at the edge of synapses, to promote cell adhesion [42]. The presence of these cholesterol-rich microdomains serves to compartmentalize membrane protein components in discrete microenvironments. Significantly, lipid rafts harbor several signaling proteins and allow organization and formation of complexes involved in the initiation of signaling cascades [62,63]. The molecular basis for the cholesterol dependence of synapses and synaptic vesicle formation has been explained by the requirement of cholesterol to support lipid rafts. There is evidence that postsynaptic proteins accumulate in lipid rafts, which are present in abundance in dendrites of cultured hippocampal neurons. Cholesterol depletion results in gradual loss of synapses and dendritic spines [64]. On the other hand, Thiele and collaborators made a case against the view that lipid rafts are crucial for synaptic vesicle formation by pointing out the low sphingolipid content of synaptic vesicles and the exclusion of the major cholesterol-binding protein synaptophysin from lipid rafts [60]. These authors propose an alternative role for cholesterol in synaptic vesicle formation, which is the association of cholesterol with oligomeric transmembrane cholesterol-binding proteins, such as synaptophysin. This association may induce the appropriate curvature of the nascent bud for vesicle formation.
Figure 2. Potential involvement of astrocyte-derived cholesterol in synapse formation.
(A) Cholesterol is delivered to neurons via ApoE-bearing HDL (green sphere) secreted by astrocytes. The box represents the synaptic site that is enlarged in (B). Following receptor-mediated lipoprotein uptake, there is intracellular redistribution of cholesterol (blue ovals) to the membrane, preferentially to the lipid rafts. Lipid rafts are discrete cholesterol-rich microdomains that harbor protein complexes involved in signaling pathways.
ApoE in normal aging
There is extensive evidence of the modulation of brain morphology by ApoE [65–68], which suggests that ApoE could have a pivotal role in aging. The role of ApoE in brain aging has been studied in animal models with conflicting results. Originally developed as an animal model to study the role of ApoE in atherogenesis, ApoE-null mice are valuable tools to study the function of ApoE in the human nervous system in general and during aging and disease in particular. In some studies ApoE-null mice displayed no signs of synaptic degeneration [69], showed normal brain histology and absence of neurodegenerative markers [70], had no signs of deterioration of the cholinergic system of the basal forebrain [49,71,72], and exhibited normal cholinergic activity and neuronal function [71,73–75].
Conversely, other studies demonstrated that ApoE-null mice develop severe spatial learning and memory deficits [76]. Poirier and colleagues suggested that the absence of ApoE makes these mice deficient in a procedural component of the Morris Water Maze test (commonly used to assess learning and spatial memory), rather than impaired in spatial memory per se [77]. Memory impairment in the absence of ApoE was associated with cholinergic deficits [78,79]. Infusion of recombinant ApoE via the intracerebroventricles improved cognitive deficits in ApoE-null mice [80], highlighting the importance of ApoE in this process. The impairment of spatial learning and memory of ApoE-null mice could be explained by synaptic changes associated with the lack of ApoE [81–87]. ApoE might also be important in age-associated neurodegeneration, since ApoE-null mice are more susceptible to neurodegeneration than the wild-type control animal [88,89]. The discrepancies in results from studies using ApoE-null mice have been explained by the genetic background strains [90], environmental variables and methodological approaches [91], and the behavioral test used in the studies [77].
In addition to the presence of ApoE, the levels of this protein might be important for maintaining brain homeostasis during aging. This concept was proposed based on a study that indicated that ApoE expression increases in the liver in an age-dependent manner [92]. In the brain, however, it is still unclear whether ApoE expression is altered during aging. Studies in mice showed that the expression level of ApoE decreases more than fivefold in the aged hypothalamus and cortex [93], but increases in the hippocampus [94]. In aged rats, glial expression of ApoE is elevated in a region-specific manner [95] and ApoE mRNA is modestly elevated in the striatum [96]. However, in a more recent study Gee and collaborators found no change in ApoE mRNA and protein in the cortex, striatum and hippocampus of aged rats [97]. The authors interpreted that the inability of the brain to increase ApoE levels upon aging may contribute to the reduced ability of the brain to respond to stress at an older age.
ApoE polymorphism & aging
Little information is available regarding the differential effects of ApoE isoforms in the normal human brain; however, each ApoE isoform is believed to have different effects on neuronal repair mechanisms [98]. The APOE ε4 allele has been associated with functional brain abnormalities, some of which are present even at a young age [99]. Reduction in cerebral glucose metabolism and reduced cognitive function are evident in healthy adults with ApoE4 [100,101]. Moreover, these individuals have relatively poor recovery after injuries to the brain [102–106].
The association of the ApoE genotype with aging has been extensively examined and conflicting results reported. While some studies indicated that APOE ε2 is over-represented in centenarians [107] and is associated with better learning activity in nondemented elderly subjects [108], others found that the ApoE polymorphism was not associated with longevity, at least in certain groups of octo- and nonagenarians [109]. The role of ApoE in cognition, a crucial process in successful aging, is also unresolved. A number of studies proposed that the expression of the APOE ε4 allele is linked to cognitive decline in the elderly [109–115] and may predispose people to dementia [116]. However, other research suggested that the APOE ε4 allele may not influence cognitive performance in adults without dementia [67,117]. Even in those studies in which ApoE genotype was demonstrated to play a role in aging, the mechanisms involved remain uncertain. One such mechanism could involve the transport of cholesterol from astrocytes to neurons for efficient synaptic plasticity.
ApoE isoforms & synaptic plasticity
With respect to the isoform-specific effect of ApoE in the maintenance of synaptic plasticity and cognitive function, a few studies demonstrate that both ApoE3 and ApoE4 successfully reversed presynaptic deficits and the cognitive impairment present in ApoE-null mice [80]. However, most evidence suggests that the ApoE4 isoform is less efficient than ApoE3 in facilitating brain function. Expression of ApoE3, but not of ApoE4, protects against neuronal damage and the age-dependent neurodegeneration seen in ApoE-null mice [91]. Compared with ApoE3 mice, ApoE4 mice display significantly lower excitatory synaptic transmission and dendritic arborization [118]. The number of synapses per neuron [69] and the number of dendritic spines [87] are also lower in mice expressing ApoE4 than those expressing ApoE3. While ApoE4 mice, but not ApoE3 mice, have synapto–dendritic alterations like ApoE-null mice, both ApoE4 and ApoE3 mice have better performance on cognitive tests [119] compared with ApoE-null mice. In addition, ApoE4-mice have a worse recovery from traumatic brain injury than ApoE3 mice [120] and are more susceptible to global cerebral ischemia [121] and lesions of the entrorhinal cortex [122].
Thus, an unresolved issue is whether ApoE4 performs certain brain functions less effectively than ApoE3 or whether it has detrimental effects, some of which could interfere with the neuroprotective actions of ApoE3. In some studies, the phenotype of mice expressing ApoE4 did not differ significantly from ApoE-null mice, suggesting a loss of function of ApoE4 in the maintenance of dendritic spines during normal aging [87]. In other studies, ApoE4 was not only less protective than ApoE3 but acted as a dominant-negative factor in ApoE function suggesting a gain of function [123]. Other indications of gain of an inhibitory function with ApoE4 come from studies in which ApoE-null mice and mice expressing ApoE3 or ApoE4 were exposed to environmental stimulation. Mice expressing ApoE3 and ApoE-null mice, but not ApoE4 mice, responded with an increase in synaptic markers in the hippocampus [124]. The observation that ApoE-null mice responded more than ApoE4 mice suggests that ApoE4 has gained an inhibitory function.
A few mechanisms have been proposed to explain the role of ApoE on synaptic function. There is evidence that ApoE may regulate intracellular calcium levels [125], significantly reducing excitatory synaptic transmission. ApoE may also have neurotrophic capabilities by binding to ApoE receptors and activating signaling proteins [126]. Here, we focus on the mechanisms that involve the transport, uptake and redistribution of cholesterol. It has been proposed that ApoE4 is unable to support the development of mature synapses and cannot provide the necessary lipids for extensive neuronal remodeling as efficiently as ApoE3 [118]. The variation may be explained by possible differences in the ability to bind to ApoE receptors (discussed later in this review) or in the composition of the brain lipoproteins formed with ApoE3 and ApoE4 [22,127–130].
ApoE & age-related oxidative stress
An additional aspect that may be relevant to the role of ApoE in aging is the influence of oxidative stress. Aging is associated with heightened oxidative stress manifest as increased oxidative insult to cellular and membrane components, including free radical-mediated damage to protein and lipids. Significantly, compared with wild-type mice, ApoE-null mice have higher levels of lipid peroxidation products and other oxidative stress markers in different parts of the brain [131], including increased levels of oxidized cholesterol [132,133] and increased susceptibility to oxidative modification [133,134]. Furthermore, oxidative stress and aging appear to be significant contributing factors towards impaired permeability and leakage of the BBB [135–137], which is exacerbated under conditions of ApoE deficiency. These observations warrant future studies examining the mechanistic effects of oxidative stress and ApoE deficiency in aging.
Cholesterol & ApoE in AD
The association between ApoE, cholesterol and AD has been reviewed by several researchers [32,37,45,138–142] and has been the subject of intense scrutiny by numerous groups. Among the many mechanisms that have been proposed to explain the link of AD with ApoE and cholesterol, in this review we emphasize the hypothesis that the isoform-specific effect of ApoE in AD is due to the differential ability to provide astrocyte-derived cholesterol to neurons, such that ApoE4 performs this activity less efficiently. We acknowledge that although the exchange of cholesterol between astrocytes and neurons mediated by ApoE is an attractive mechanism, we still need direct evidence that brain neurons require astrocyte-derived cholesterol in vivo [42] and that ApoE4 does provide less cholesterol than ApoE3. We highlight here the available information and point out the aspects that require further investigation.
Alzheimer’s disease is clinically characterized by progressive cognitive decline and dementia. The extracellular accumulation of amyloid β (Aβ) peptide, the intracellular presence of neurofibrillary tangles composed of hyperphosphorylated tau and the loss of cholinergic neurons in the brain are major neuropathological hallmarks of AD [143,144]. Accumulation of soluble and insoluble assemblies of Aβ (a peptide composed of 39–43 residues) in the brain parenchyma and in the cerebral vasculature is considered the primary event in AD pathogenesis by the amyloid hypothesis. The rest of the disease process results from the imbalance between Aβ production and clearance [145,146]. Aβ derives from the ubiquitously expressed transmembrane protein amyloid precursor protein (APP) by regulated intramembranous proteolysis [144]. Aβ is secreted under normal metabolic conditions and is present in normal CSF and plasma [147,148]. A gradual increase of Aβ levels in brain interstitial fluid and inside neurons [149,150] leads to Aβ oligomerization and eventually to fibrillization [144]. Soluble oligomeric Aβ assemblies are primary effectors in AD [151–153]. While in the familial form of AD Aβ accumulation correlates directly with increased production, in the nonfamilial forms it results from intricate interactions of factors that affect Aβ clearance and aggregation [154]. The familial and nonfamilial forms of the disease are almost phenotypically indistinguishable. The nonfamilial form occurs in approximately 95% of AD patients. The only risk factor consistently associated with the non-familial form of AD is the ε4 allele of the APOE gene [155–157].
In the brain, plasma, CSF and senile plaques Aβ is associated with ApoE [158–160]. Although the presence of amyloid plaques in the brain is required for a definitive identification of AD, there is no correlation between amyloid plaque abundance and the degree of dementia [161]. Conversely, the degree of degeneration of cholinergic neurons from the basal forebrain correlates closely with the degree of dementia in AD [162,163]. Hence, the salvage of cholinergic neurons in the brain by blocking Aβ neurotoxic effects is one of the major, but still elusive, therapeutic goals of current research in AD. The cholinergic deficit that results from basal forebrain neuron degeneration contributes significantly to the neuropsychiatric manifestations of the disease (reviewed in [143]). Moreover, the extent of cholinergic dysfunction is associated with inheritance of the APOE ε4 allele. In ApoE4 carriers with AD, the total number of cholinergic neurons is reduced to a greater extent [164–167], the compensatory mechanisms and repair (plastic neuronal remodeling) are impaired [166], and basal forebrain neurons have lower metabolic activity [168]. In addition, patients carrying the APOE ε4 allele responded less to cholinergic therapy than patients with the ε3 allele [164], although some studies demonstrated no difference in choline acetyltransferase activity between ε4 and ε3 patients [169–171].
The mechanisms by which ApoE affects AD in an isoform-specific manner have been extensively studied. An important issue still under debate is whether the ApoE2/ApoE3 proteins protect the brain from AD or the ApoE4 protein initiates the pathology [155,172]. Supporters of the concept that ApoE has neuroprotective and neurotrophic functions in the normal, aging brain argue that ApoE2 and ApoE3 perform these functions more efficiently than ApoE4 [50]. On the other hand, some evidence suggests that it is the presence of ApoE4 and not the absence of ApoE3/ApoE2 that contributes to AD pathology [173,174]. Several hypotheses have been proposed to explain the association of ApoE4 with AD [175,176]. They include impairment of the antioxidative mechanisms [177–179], dysregulation of neuronal signaling pathways [180], altered phosphorylation of tau and neurofibrillary tangle formation [181–184], potentiation of Aβ-induced lysosomal leakage and apoptosis in neuronal cells [185], promotion of endosomal abnormalities linked to Aβ overproduction [186,187] and modulation of plaque formation, deposition and clearance of Aβ [188–194]. With respect to the clearance of Aβ from the extracellular milieu, we have recently demonstrated that although the peptide is internalized by neurons in a complex with ApoE, a mechanism of Aβ uptake independent of ApoE also exists [195].
The importance of cholesterol in the development and progression of AD has been established (reviewed in [37,139,154]). The best studied role of cholesterol in AD is in APP processing and Aβ generation. Increased cholesterol leads to increased cleavage of APP and increased Aβ production [196], while reduction of cellular cholesterol decreases the γ-secretase activity that is responsible for Aβ generation [197–201]. Conversely, at least one report demonstrated increased Aβ generation upon moderate reduction of cellular cholesterol levels [202]. The discrepancies in findings have been reconciled in a model that takes into consideration the impact of different levels of cholesterol on the structure of lipid rafts [203].
There is little consensus that total brain cholesterol is altered in patients with AD [204]. Some studies reported changes in cholesterol levels in specific brain areas in AD patients, in particular, in regions with extensive Aβ deposits and neurofibrillary tangles. In patients with ApoE4, these regions included the middle frontal gyrus [205] and frontal cortex [206], but not the unaffected cerebellum [205]. There is also indication that as the severity of the disease progresses, there is an increase in membrane-associated cholesterol [205], some of which may be part of the amyloid plaques [207]. In addition, cholesterol and cholesteryl ester accumulate in lipid droplets exclusively in Aβ-immunopositive neurons [208].
With respect to the role of peripheral cholesterol in AD, some studies indicate a direct correlation between plasma cholesterol levels and the incidence of AD [209–211] and epidemiological data suggest that treatment of the elderly with statins that inhibit cholesterol synthesis lowers the incidence of AD [212–214]. Yet, some of these correlations have not been confirmed [215], and the efficacy of statins to prevent AD has been questioned [37,216]. Besides, the mechanisms by which statins act in the brain are not well understood and not all statins are equally effective in lowering brain cholesterol levels [217]. In addition, the beneficial effect of statins could be a result of their anti-inflammatory rather than cholesterol-lowering action [218]. A previous review in this journal series addressed the issue of statins and their efficacy in preventing or treating AD [219]. The authors concluded that the epidemiological data provided little evidence to support a correlation between statin use and protection against AD.
As indicated earlier, we favor the hypothesis that neurodegeneration in AD may result from impaired delivery of cholesterol from astrocytes to neurons. This impaired delivery would be accentuated by the presence of Aβ and the expression of the ApoE4 isoform [42] (and references herein). Gong and collaborators concluded that the isoform-specific effect of ApoE in AD may be explained by the differential ability of ApoE3 and ApoE4 to supply cholesterol to neurons after injury [26]. The authors found that astrocytes expressing human ApoE3 generate lipoprotein particles that contain significantly less ApoE than astrocytes expressing ApoE4. However, the size of the lipoprotein particles did not differ significantly between those containing ApoE3 and ApoE4, as also noted by others [22]. This suggests that each lipoprotein particle could provide the same amount of lipids to neurons, if the two isoforms bear a similar receptor binding-competent conformation (see discussion on ApoE conformation below). Therefore, for this hypothesis to be correct, the levels of ApoE-containing lipopro-teins in the extracellular milieu of the brain would be the limiting factor.
Oxysterols & ApoE
As previously indicated, cholesterol is converted to 24S-hydroxycholesterol in brain neurons and crosses the BBB. It is also believed that the conversion to 24S-hydroxycholesterol is a mechanism to maintain cholesterol homeostasis in the brain. Almost all circulating 24S-hydroxycholesterol originates from the brain [220,221] and may reflect CNS cholesterol turnover [222]. The correlation between peripheral levels of 24S-hydroxycholesterol and cholesterol has been explained by the similar distribution of both sterols into lipoproteins [223]. Plasma concentrations of 24S-hydroxycholesterol are utilized as a biomarker and a diagnostic tool for neurological disorders. The circulating levels of 24S-hydroxycholesterol are age-dependent, decreasing dramatically in the first decades of life [220]. This decrease however, might not indicate a reduction of the flux of 24S-hydroxycholesterol from the brain to the periphery but might result from the difference in size increase of the brain (~ 30% in the first 2 years) and the liver (up to sixfold during this period), this last organ being responsible for 24S-hydroxycholesterol elimination [224]. Neuronal damage is accompanied by destruction of neuronal membranes, which provides higher levels of cholesterol to be converted into 24S-hydroxycholesterol. In agreement, significantly higher peripheral concentrations of 24S-hydroxycholesterol were found in AD and vascular demented patients [223]. Importantly, the latter study demonstrated that the ApoE genotype does not contribute significantly to the elevated plasma levels of 24S-hydroxycholesterol in AD patients. Plasma levels of 24S-hydroxycholesterol decreased as the severity of AD and vascular dementia increased, suggesting that this oxysterol derives from degenerating neurons at an early stage when the CNS atrophy is minimal [223,225]. By contrast, other studies report that plasma levels of 24S-hydroxycholesterol in AD patients were not significantly different than in control subjects [226]. The difference in findings could be explained by the degree of severity of the disease.
In contrast to plasma levels, the CSF levels of 24S-hydroxycholesterol seem to be more sensitive to changes in the brain and are not affected by hepatic clearance of this oxysterol. Therefore, CSF 24S-hydroxycholesterol levels may be better markers both for neurodegenerative diseases and for disturbances of the BBB [227,228]. In the early stages of AD there are significantly higher CSF concentrations of 24S-hydroxycholesterol, suggesting increased cholesterol turnover in the CNS during degeneration [226,229,230]. These levels decrease as the disease advances, possibly reflecting the loss of cells expressing cholesterol 24S-hydroxylase (CYP46A 1), the enzyme responsible for the conversion of brain cholesterol into 24S-hydroxycholesterol [226]. In AD and mild cognitive impairment, but not in normal individuals [231], the levels of 24S-hydroxycholesterol significantly correlate with CSF levels of ApoE. Furthermore, these levels correlated positively with the number of APOE ε4 alleles independent of dementia severity and of CSF cholesterol levels [226]. Two interpretations were provided for these findings: that the three APOE alleles differentially regulate transport of cholesterol, and/or that the activity of the 24S-hydroxylase is ApoE-dependent, with the APOE4 allele associated with higher 24S-hydroxylase activity. The elevation of 24S-hydroxycholesterol in CSF is consistent with a significant role for this oxysterol as a signaling molecule during neuronal degeneration. It has been shown that 24S-hydroxycholesterol synthesized by neurons from free cholesterol is able to induce expression of ApoE and ATP-binding cassette (ABC) transporters in astrocytes through activation of liver X receptor and to stimulate cellular cholesterol efflux [232]. 24S-hydroxycholesterol-mediated elevation of ABCA1 levels has been linked to increased levels of extracellular Aβ [233]. On the other hand, more recent studies in cultured cells showed that 24S-hydroxycholesterol affects APP processing by increasing α-secretase activity as well as the α-secretase:β-secretase activity ratio, a beneficial mechanism [234]. The roles of other oxysterols in neurodegeneration have been reviewed elsewhere [34,235].
Box 1. Specific features of ApoE4 compared with ApoE3 or ApoE2
Greater protease sensitivity & neurotoxicity of fragments[182,183,277,278,281,282]
Greater tendency to aggregate and cause aggregation of Aβ [189,243,283–285]
Increased plasma clearance [320]
Altered ability to promote cellular cholesterol efflux* [26,263,265–268]
*These functions may be dependent on the cell type studied. Aβ: Amyloid β peptide; LDLr: Low-density lipoprotein receptor.
Unique biochemical & biophysical features associated with ApoE4
In an effort to understand the unique physiological and pathological behavior associated with ApoE4, in this section we attempt to compare some biochemical, biophysical and structural features between the isoforms. It must be noted that some of the features described are relevant to the behavior of ApoE in peripheral tissues and the plasma as well. By the same token, there may be significant cell type-specific differences in the behavior of ApoE isoforms. The aberrant behavior of ApoE4 in CNS cholesterol homeostasis in normal aging and disease states may be attributed to one or a combination of the properties that are outlined in Box 1 and discussed below.
Structural features of lipid-free ApoE
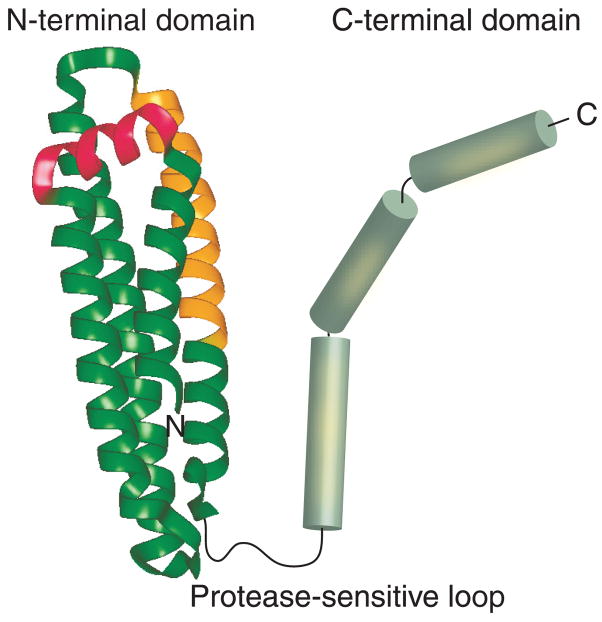
ApoE is comprised of two domains that are linked by a protease-sensitive loop (Figure 3): a 22 kDa N-terminal domain (residues 1–191) housing binding sites for the LDL receptor (LDLr) family of proteins and a 10 kDa C-terminal domain (residues 210–299) bearing high-affinity lipid-binding sites [236]. The N-terminal domain consists of a series of amphipathic α-helices that are folded into a four-helix bundle [237]. Receptor binding is believed to occur via a stretch of conserved basic residues predominantly located on helix 4 in the N-terminal domain [236]. In addition, ApoE bears a high-affinity heparan sulfate proteoglycan (HSPG)-binding site (residues 142–147) in the N-terminal domain and a low-affinity site (residues 243–272) in the C-terminal domain [238]. Interaction with cell surface-localized HSPG plays a major role in internalization of the lipoproteins, either directly or by optimal presentation of the lipoproteins to the lipoprotein receptors [239]. While the two domains may be independently folded in the case of ApoE3, it is believed that an interaction between the N- and C-terminal domains in ApoE4 plays a critical role in determining the functional behavior of this isoform [240–243].
Figure 3. Domain organization lipid-free ApoE.
ApoE2, ApoE3 and ApoE4 are comprised of an N-terminal and a C-terminal domain that are linked by a protease-sensitive loop. High resolution structural information is available only for the N-terminal domains of the three isoforms [237,240,323]. The N-terminal domain is composed of four long helices (green ribbon) that are folded into a helix-bundle architecture with a short linker helix (pink). Helix 4 bears the LDL receptor-binding site (gold) with overlapping heparan sulfate proteoglycan binding sites. The C-terminal domain is modeled as α-helices (green cylinders) and bears high affinity lipid binding and ApoE self-association sites (yellow patches).
A salt bridge between Arg61 in the N-terminal domain and Glu255 in the C-terminal domain of ApoE4, but not ApoE3 or ApoE2, constitutes the domain interaction. Although all three isoforms bear an Arg at position 61, high-resolution structural analysis of the N-terminal domain suggests that the side chain of this Arg is exposed for interaction with Glu255 only in the case of ApoE4. Recently, a weak interaction between the two domains was reported in a monomeric, albeit functional, full length ApoE3 variant [244]. Further studies are required to validate the physiological relevance of monomeric ApoE and to investigate whether the weak domain interaction reported in the ApoE3 variant is also present in the wild-type protein. Although it is not known if lipid-free ApoE exists in the CNS, it is essential to understand the structural organization of the intact wild-type ApoE isoforms at a high resolution in the absence of lipids in order to obtain valuable insights into the molecular basis of their physiological and pathological behavior in the brain.
Conformation of lipoprotein-associated ApoE
In the plasma, ApoE4 displays a binding preference for the larger VLDL-sized particles, while ApoE3 displays a preference for HDL-sized lipo-proteins [241]. The lipoprotein-binding preferences have been attributed to the domain interaction feature. However, in the CNS, ApoE3 and ApoE4 appear to be located primarily on HDL-like particles, as deduced from CSF analysis and in vitro studies examining ApoE isolated from astrocyte-conditioned medium [18]. It is not known whether the lipoprotein-associated forms of ApoE3 and ApoE4 on HDL-sized particles from the CNS display distinct conformational differences.
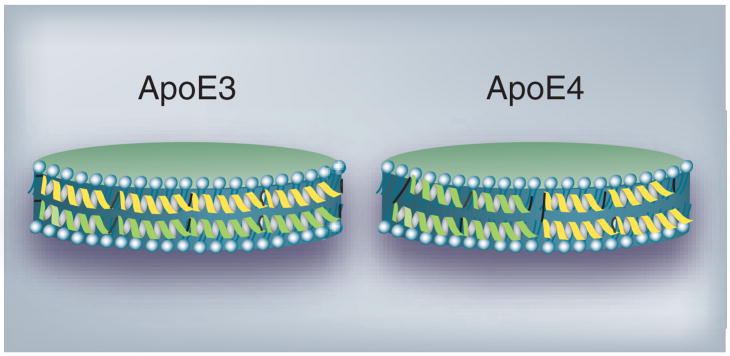
Our studies suggest a model of HDL-associated ApoE4, wherein positions 61 and 255 in the N-and C-terminal domains, respectively, may remain proximal following lipid interaction. In this model, ApoE4 adopts a conformation that resembles a ‘looped-back belt’ in reconstituted lipoproteins containing dimyristoylphosphatidyl-choline (DMPC) and recombinant protein (Figure 4) [245]. These lipoproteins have a similar geometry and size as nascent discoidal particles reported in the CNS and retain the functional conformation of ApoE in terms of LDLr binding and uptake ability; therefore, they serve as excellent models for structural studies. A similar conformation was reported for ApoE4 bound to dipalmitoylphosphatidylcholine (DPPC) [246]. Recently, a low-resolution (10 Å) x-ray analysis of DPPC particles containing two ApoE4 molecules in the absence of neutral core lipids was described [247]. The authors propose a spherical lipoprotein model with the ApoE molecules forming a helical hairpin bend around a core containing the fatty acyl chains of DPPC. On the other hand, an extended ‘belt’ model was suggested for ApoE3 bound to discoidal lipoproteins containing DMPC (Figure 4) [248]. It is not known if these conformational differences between lipid-associated ApoE3 and ApoE4 account for their differential physiological behavior. Future studies aimed at examining the structure and conformation of HDL-associated ApoE isoforms will aid in understanding possible isoform-specific differences in cholesterol metabolism in the CNS.
Figure 4. Discoidal HDL containing ApoE3 or ApoE4.
ApoE3 (left) and ApoE4 (right) are modeled as a series of α-helices (ribbons) circumscribing a bilayer of phospholipid molecules (blue-grey) yielding a discoidal lipoprotein particle. While ApoE3 is depicted in an extended organization like a ‘belt’, ApoE4 is modeled like a ‘looped-back belt’ with the molecule folded on itself. Only two ApoE molecules (one green and the other yellow) are shown per particle for simplicity.
Conformational flexibility of ApoE
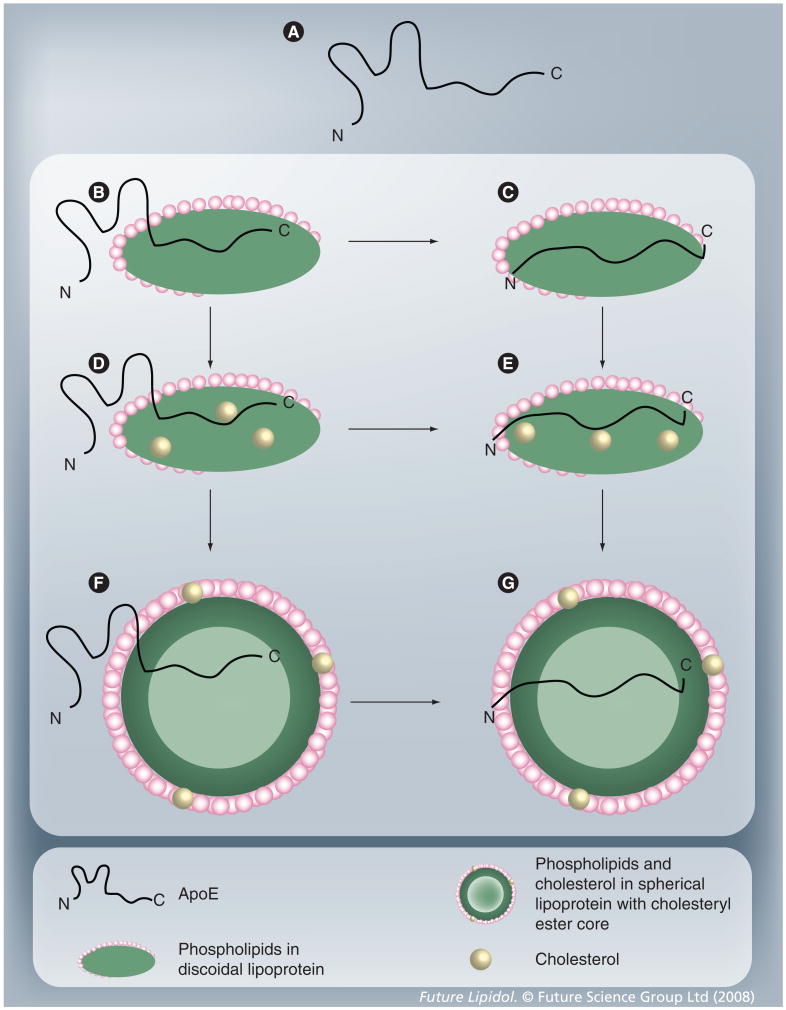
It can be envisaged that lipid-associated ApoE isoforms may exist in several different conformational states that are governed by the size, geometry and lipid composition and content of the lipoprotein particles, some of which are schematically depicted in Figure 5. Previously, it was proposed that lipid-associated ApoE isoforms may exist in at least two different conformations: one where the molecule is tethered to the lipoprotein particle only via the C-terminal domain with the N-terminal domain in a lipid-free helix-bundle state, and a second where the entire molecule is lipid-associated [249–251]. This model provides an attractive structural explanation for the regulation of cholesterol and lipoprotein metabolism by ApoE. Indeed, a differential ability of the two endogenously synthesized isoforms to associate with released cholesterol in astrocytes may be a consequence of their abilities to exist in multiple lipid-bound conformations [26]. However, the concept of a direct correlation between the structure of ApoE isoforms and their corresponding function will be difficult to demonstrate experimentally given the dynamic nature of the interaction between ApoE and lipids and the interconversion between lipid-associated states. Therefore, a monumental task lies ahead for the structural biologists: to understand and identify the molecular organization of ApoE isoforms in the discoidal and spherical HDL-like particles in the CNS and to determine whether cholesterol and other lipids alter the lipid-associated conformations. These structures will provide a basis for interpreting the isoform-specific interaction of Aβ with ApoE (discussed below) and possible Aβ-mediated alteration of ApoE/lipoprotein receptor interaction.
Figure 5. Conformational adaptability of ApoE.
(A) Lipid-free ApoE; (B and C) ApoE bound to discoidal phospholipid particles, with the N-terminal domain remaining unbound (B) or bound to lipids (C); (D and E) ApoE bound to discoidal phospholipid and cholesterol-containing particles, with the N-terminal domain remaining unbound (D) or bound to lipids (E); (F and G) ApoE bound to spherical phospholipid particles with cholesteryl ester core, with the N-terminal domain remaining unbound (F) or bound to lipids (G). Since these are not discrete states, numerous intermediate conformational states of ApoE with different extents of lipidation are possible between states (A) and (G).
ApoE isoform-specific differences in protein stability
Lipid interaction of ApoE involves extensive structural rearrangement of the two domains [249,251]. Since this interaction implies opening of the N-terminal domain helix bundle to expose the hydrophobic interior, several studies have attempted to understand the physiological basis of the ApoE–lipid interaction by comparing the relative abilities of the isoforms to unfold during chemical and heat denaturation. The three isoforms display a striking difference in stability of their N-terminal domains [252–254]. The N-terminal domain of ApoE4 is the least resistant to chemical and heat denaturation, while that of ApoE2 is the most resistant at neutral and low pH. The lipid binding affinity of ApoE4 is higher than those of ApoE2 and ApoE3, although the maximal binding capacity appears to be the same for the three isoforms [255,256]. This raises the question: does the lipid binding affinity correlate with the protein stability [252,257,258]? If so, the conformational flexibility of ApoE may be a key factor in regulating cellular cholesterol release and delivery to the neurons.
Ability of ApoE isoforms to promote cellular cholesterol efflux
In addition to its role in mediating binding and uptake of plasma lipoproteins, ApoE can promote cellular cholesterol efflux and HDL assembly via the ABCA1 transporter [259–262]. While some studies report no isoform-specific differences in the ability of ApoE to stimulate ABCA1-dependent cholesterol efflux [263–265], others note significant variations (ApoE3 > ApoE4 = ApoE2) [26,266,267]. The differences in observations may be attributed to the cell types used or to the level of expression of ABCA1. However, studies using neuronal cells and astrocytes suggest that ApoE4 is less efficient than ApoE3 in its ability to promote cholesterol efflux [266,268,269], suggesting a differential capacity to bind cellular cholesterol. Our studies demonstrate that the C-terminal lipid-binding domain of ApoE is necessary, sufficient and highly efficient at mediating cholesterol efflux, with activities comparable to that of intact ApoE or ApoAI [264]. Aβ interaction with the C-terminal domain of ApoE [270–275] can potentially modulate the ability of the latter to bind lipids [276], promote cholesterol efflux and generate HDL. More studies are required to clarify the role of ApoE isoforms in promoting cholesterol efflux from astrocytes and/or neurons.
Is the low protein stability of ApoE4 related to its neuropathological behavior?
As far as the pathological role in the brain is concerned, the lower stability of ApoE4 may account for:
Susceptibility of this isoform to protease degradation
Its ability to aggregate or promote aggregation of Aβ (more efficiently than ApoE3 or ApoE2)
Aberrations in the lysosomal recycling process and/or a tendency to disrupt the lysosomal membrane, alone or with Aβ.
Susceptibility of ApoE4 to protease degradation
It has been suggested that ApoE4 is relatively more susceptible to protease digestion than ApoE3 [182,277]. ApoE and its fragments have been identified in the amyloid plaques in brains affected by AD and in cultured neuronal cells [183,278]. The carboxyl terminal truncated ApoE4 resulting from proteolysis was found to induce neuropathology resembling that found in AD and behavioral deficits in ApoE4 transgenic mice. The C-terminal fragment may play a role in inducing and stabilizing the oligomeric form of Aβ [279]. In addition, N-terminal truncated fragments of varying lengths were also identified in neuroblastoma cells overexpressing ApoE [279] and in amyloid plaques of brains affected by AD [280]. The 22-kDa N-terminal fragment of ApoE4 has been shown to bear intense neurotoxic properties [277,281,282]. It is not known if the ApoE fragments were generated prior to or after interaction with Aβ. Furthermore, considering that lipid-free ApoE is more susceptible to protease activity than lipid-bound ApoE (the protease-sensitive loop linking the two domains is shielded in the lipid-bound state) [236,248], the presence of ApoE fragments suggests that they may have originated from lipid-free ApoE. Thus, the neurotoxicity associated with ApoE4 may be due to increased generation of toxic fragments. Whether the presence of these fragments in brains affected by AD is the cause or the consequence of amyloid aggregation and tangle formation needs further investigation.
The ability of ApoE4 to aggregate or promote aggregation of Aβ
ApoE4 has an inherent ability to aggregate into irregular protofilament-like structures that are neurotoxic to a line of cultured mouse neuronal cells [243]. The aggregation rate follows the order: ApoE4 > ApoE3 > ApoE2 and the aggregates are rich in α-helical structures. Additionally, ApoE4 has a greater tendency than ApoE3 or ApoE2 to promote Aβ aggregation in vitro and amyloid plaque deposition in vivo [189,283–285]. The tendency of ApoE4 to induce self-aggregation or aggregation of Aβ may be related to its low protein stability. More studies are needed to evaluate if cholesterol and other lipids play a role in determining the aggregation behavior of ApoE4 and if they contribute to the neuropathology associated with this isoform.
ApoE4 & aberration in the lysosomal recycling process
The decreased stability of ApoE4 at low pH may promote its interaction with the lysosomal membrane, where the protein is believed to adopt a molten globule conformation [286]. In addition, studies employing a line of mouse neuronal cells indicate that ApoE4 may potentiate Aβ-associated apoptosis and leakage of lysosomes [287,288]. Interestingly, compared with ApoE3, a pronounced accumulation of ApoE4 was noted in peripheral endosomes in human hepatoma cells [289,290]. This observation was attributed to the lower pH in these compartments, which possibly leads to protein aggregation and a decreased tendency of the protein to be recycled back to culture medium. It is not known if this behavior of ApoE4 affects intracellular cholesterol trafficking. Future studies are expected to shed light on the potential impact of isoform-specific differences on intracellular cholesterol redistribution in the neurons.
ApoE4 interaction with lipoprotein receptors: poor cholesterol delivery to neurons and/or Aβ clearance?
The lipid delivery function of ApoE is primarily mediated by its ability to serve as a ligand for the LDLr [291] and many of the members of the family of endocytic lipoprotein receptors. At least seven of these receptors have been identified in the brain: the LDLr, LDLr-related protein (LRP), VLDL receptor (VLDLr), ApoE receptor 2 (LRP8), glyco-protein 330/megalin and multiple epidermal growth factors domains (LRP4), SorLA or LR11 [292] and LRP1B [293]. These receptors are involved in diverse biological functions, including lipid delivery and signaling [291,294]. A requirement for ApoE interaction with the LDLr is its prior association with lipoproteins or lipids, although there is evidence that the lipid-free protein interacts with VLDLr [295] or LRP [296]. Lipoprotein association is accompanied by a dramatic conformational change in ApoE structure [238,249,250,297,298], which allows presentation of the multivalent ligand for optimal interaction with the ligand binding sites on the LDLr. Interestingly, while the LDLr displays poor interaction with ApoE2, VLDLr and LRP appear to bind all three isoforms [295].
In addition to being considered a risk factor for AD, ApoE4 is also associated with high plasma LDL-C and atherosclerosis. An increased clearance of plasma ApoE4 compared with ApoE3-containing VLDL was noted in transgenic mice [299]. One possible explanation for this observation is that the binding affinity to hepatic LDLr is slightly higher for ApoE4 than ApoE3 [300,301]. Increased internalization and catabolism of lipo-proteins (bearing ApoE4) is expected to downregulate the LDLr, which in turn leads to increased plasma cholesterol levels. This raises an important question: are there similar possibilities in the CNS? While the observed increased affinity may be due a net increase in the positive electrostatic potential in the microenvironment of the receptor binding region in ApoE4, it is not known if a similar difference in affinity is noted in the neuronal and non-neuronal cells. Furthermore, the isoform-specific associations between ApoE and VLDLr and LRP in cholesterol metabolism, aging and AD are less understood since these receptors, unlike the LDLr, are unable to discriminate between the different isoforms Table 1 [295]. In summary, several factors modulate the ApoE/lipoprotein receptor interaction, such as the type and location of the receptors, the cellular source of ApoE, the cell type studied, the extent and type of lipidation, and the receptor binding affinity of ApoE. Elucidating the significance of the various ApoE receptors in the brain and their relative contribution to cholesterol metabolism are currently active areas of research.
Additional consideration is warranted regarding the involvement of proteoglycans in effects elicited by ApoE in the neurons, at least via the LRP pathway. ApoE4, but not ApoE3, inhibits neurite outgrowth [302–305] or causes degeneration of neurites [282,306] via the LRP/HSPG pathway. In addition, the ApoE-mediated effects on lipid efflux from astrocytes and neurons [268] may involve this pathway, as noted for the macrophages [307]. Whereas this pathway has been studied in the liver regarding the clearance of remnant lipoproteins [308], less is known about its role in the CNS. Although both the N- and C-terminal domains of ApoE can interact with HSPG, albeit with different affinities, only the former is available for interaction in both lipid-free and lipoprotein-associated states. The C-terminal site appears to be masked owing to lipid association. The association of lipid-free ApoE with HSPG on the cell surface may have a direct bearing on CNS lipoprotein metabolism, because this lipid-free ApoE may serve as a reservoir that can be readily employed for cholesterol transport between neuronal and non-neuronal cells. A two-step binding mechanism has been proposed for the interaction of ApoE with heparin, a widely used model for HSPG that bears a higher sulfate content [309]. The initial step involves a rapid electrostatic interaction [310], while the subsequent step involves a slower hydrophobic interaction. No significant isoform-specific differences were noted in ApoE-heparin interactions [311]. Future studies may reveal whether the two-step mechanism is applicable to cell surface HSPG and whether the ApoE polymorphism affects this process. In addition to its relevance in cholesterol metabolism, the ApoE–HSPG interaction also has tremendous implications in AD [312,313] and aging, given the age-related changes that occur in the structure, composition and function of glycosaminoglycans [314].
APOE genotype & ApoE isoform levels in the brain
Lastly, an aspect that deserves mention is the concept that the poor neurological function of ApoE4 may simply be related to lower levels of this isoform being present in the brain. Some attempts have been made to use the CSF levels of ApoE as an indicator of its levels in the brain, in order to obtain a correlation between APOE genotype and brain ApoE levels ([201] and references therein). The overall results are inconclusive and more definitive studies are needed in this direction. While the catabolic rate of ApoE4 is the highest amongst all isoforms in the plasma [315], it appears that the rate may be lowered for this isoform in the CNS [316]. A recent study using LDLr-null mice [317] demonstrated that ApoE, but not cholesterol, accumulates in the brain. This suggests that the LDLr is a major factor affecting brain ApoE levels. In light of the observation that the plasma concentration of ApoE isoforms follows the order ApoE2 > ApoE3 > ApoE4 [318–322], while the risk associated with AD follows the order ApoE4 > ApoE3 > ApoE2, studies were carried out to correlate the brain tissue levels of ApoE with AD [45,139]. Although based on a small set of data, it was observed that ApoE levels in the CNS and AD were similarly related to genotype, with the highest ApoE levels associated with APOE ε2 and the lowest levels associated with APOE ε4. While attempts to increase ApoE levels in the CNS by administering agents that upregulate ApoE synthesis have yielded moderately promising results [45], more studies are needed in this direction to employ ApoE as a gene-based therapeutic target for treating AD.
Future perspective
While current evidence definitively indicates that elevated cholesterol is detrimental to vascular health, the same cannot be stated emphatically for the brain. However, the following three statements are well supported experimentally:
Cholesterol is vital for brain synaptic activities such as memory and learning
ApoE is critical for brain cholesterol homeostasis
ApoE4 is associated with neurodegenerative disorders
The next big challenge will be to obtain more direct information of the regulatory mechanisms of ApoE and cholesterol in vivo in the brain. In parallel, the rapid advancement of the spectroscopic tools available for protein structure analysis will allow intense examination of subtle differences in the molecular architecture of ApoE isoforms. Together, the new knowledge obtained will advance our understanding of the role of cholesterol and ApoE in the amyloid pathogenesis in AD and other neurological disorders of the CNS involving lipids. The knowledge of the regulation of cholesterol and ApoE in the peripheral tissues and plasma has served as a springboard for our understanding of corresponding events in the brain. However, it is not known whether there are differential requirements and regulation of cholesterol and ApoE on the CNS side of the BBB. Progress in these issues is crucial to developing potential preventive and therapeutic strategies targeting ApoE-related aberrations in cholesterol metabolism in the CNS.
Executive summary
Significance of ApoE
Cholesterol is a critical player in governing fundamental synaptic activities and neuronal plasticity in the brain.
ApoE aids in shuttling cholesterol between non-neuronal cells and neurons in the brain.
Cholesterol & ApoE in normal aging
Synaptic development and plasticity are governed by the availability of cholesterol and decline with aging, suggesting a pivotal role for ApoE in aging.
APOE ε4 appears to be correlated with poor cognitive performance, possibly related to altered cholesterol metabolism.
Cholesterol & ApoE in Alzheimer’s disease
ApoE4 is associated with a heightened risk for developing Alzheimer’s disease (AD), poor cognitive performance and accumulation of soluble and insoluble assemblies of amyloid β (Aβ).
The increased generation and poor clearance of Aβ in AD may be associated with altered ApoE4-mediated cholesterol metabolism.
Unique biochemical & biophysical features associated with ApoE4
Subtle conformational differences between ApoE isoforms may account for their differential behavior.
Both isoforms display conformational flexibility.
ApoE4 is the least stable of the three isoforms, which may be related to its neuropathological behavior, increased susceptibility to proteolysis, ability to promote Aβ aggregation and tendency to disrupt the lysosomal membrane.
ApoE4 is likely involved in poor cholesterol delivery to neurons and/or Aβ clearance.
Low expression levels of ApoE4 may account for its poor performance in cholesterol delivery.
Acknowledgments
We thank Dr Simonetta Sipione, Department of Pharmacology, University of Alberta, Edmonton, Canada, for critically reading the manuscript and for her insightful comments. We acknowledge Diane Kohlman for excellent assistance in preparing the manuscript. We apologize for any inadvertent omission of references.
Financial & competing interests disclosure
The authors were funded by the Canadian Institutes of Health Research, Alzheimer’s Society of Canada, Mr W Sim Trust (EPC), the American Heart Association, the Drake Family Trust (VN), the Alzheimer’s Association (EPC & VN) and the NIH. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Shore B, Shore V. An apolipoprotein preferentially enriched in cholesteryl ester-rich very low density lipoproteins. Biochem Biophys Res Commun. 1974;58:1–7. doi: 10.1016/0006-291x(74)90882-1. [DOI] [PubMed] [Google Scholar]
- 2.Das HK, McPherson J, Bruns GA, Karathanasis SK, Breslow JL. Isolation, characterization, and mapping to chromosome 19 of the human apolipoprotein E gene. J Biol Chem. 1985;260:6240–6247. [PubMed] [Google Scholar]
- 3.Lin-Lee YC, Kao FT, Cheung P, Chan L. Apolipoprotein E gene mapping and expression: localization of the structural gene to human chromosome 19 and expression of ApoE mRNA in lipoprotein- and non-lipoprotein-producing tissues. Biochemistry. 1985;24:3751–3756. doi: 10.1021/bi00335a050. [DOI] [PubMed] [Google Scholar]
- 4.Fullerton SM, Clark AG, Weiss KM, et al. Apolipoprotein E variation at the sequence haplotype level: implications for the origin and maintenance of a major human polymorphism. Am J Hum Genet. 2000;67:881–900. doi: 10.1086/303070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finch CE, Stanford CB. Meat-adaptive genes and the evolution of slower aging in humans. Q Rev Biology. 2004;79:3–50. doi: 10.1086/381662. [DOI] [PubMed] [Google Scholar]
- 6.Saunders AM, Strittmatter WJ, Schmechel D, et al. Association of apolipoprotein E allele ε4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 7.Linton MF, Gish R, Hubl ST, et al. Phenotypes of apolipoprotein B and apolipoprotein E after liver transplantation. J Clin Invest. 1991;88:270–281. doi: 10.1172/JCI115288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dietschy JM, Turley SD. Cholesterol metabolism in the brain. Curr Opin Lipidol. 2001;12:105–112. doi: 10.1097/00041433-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Dietschy JM, Turley SD. Thematic review series: brain lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res. 2004;45:1375–1397. doi: 10.1194/jlr.R400004-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Boyles JK, Pitas RE, Wilson E, Mahley RW, Taylor JM. Apolipoprotein E associated with astrocytic glia of the central nervous system and with nonmyelinating glia of the peripheral nervous system. J Clin Invest. 1985;76:1501–1513. doi: 10.1172/JCI112130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poirier J, Hess M, May PC, Finch CE. Astrocytic apolipoprotein E mRNA and GFAP mRNA in hippocampus after entorhinal cortex lesioning. Brain Res Mol Brain Res. 1991;11:97–106. doi: 10.1016/0169-328x(91)90111-a. [DOI] [PubMed] [Google Scholar]
- 12.Pitas RE, Boyles JK, Lee SH, Foss D, Mahley RW. Astrocytes synthesize apolipoprotein E and metabolize apolipoprotein E-containing lipoproteins. Biochim Biophys Acta. 1987;917:148–161. doi: 10.1016/0005-2760(87)90295-5. [DOI] [PubMed] [Google Scholar]
- 13.Xu PT, Schmechel D, Rothrock-Christian T, et al. Human apolipoprotein E2, E3, and E4 isoform-specific transgenic mice: human-like pattern of glial and neuronal immunoreactivity in central nervous system not observed in wild-type mice. Neurobiol Dis. 1996;3:229–245. doi: 10.1006/nbdi.1996.0023. [DOI] [PubMed] [Google Scholar]
- 14.Xu PT, Gilbert JR, Qiu HL, et al. Specific regional transcription of apolipoprotein E in human brain neurons. Am J Pathol. 1999;154:601–611. doi: 10.1016/S0002-9440(10)65305-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aoki K, Uchihara T, Sanjo N, et al. Increased expression of neuronal apolipoprotein E in human brain with cerebral infarction. Stroke. 2003;34:875–880. doi: 10.1161/01.STR.0000064320.73388.C6. [DOI] [PubMed] [Google Scholar]
- 16.Han SH, Einstein G, Weisgraber KH, et al. Apolipoprotein E is localized to the cytoplasm of human cortical neurons: a light and electron microscopic study. J Neuropathol Exp Neurol. 1994;53:535–544. doi: 10.1097/00005072-199409000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Harris FM, Tesseur I, Brecht WJ, et al. Astroglial regulation of apolipoprotein E expression in neuronal cells. Implications for Alzheimer’s disease. J Biol Chem. 2004;279:3862–3868. doi: 10.1074/jbc.M309475200. [DOI] [PubMed] [Google Scholar]
- 18.Fagan AM, Holtzman DM. Astrocyte lipoproteins, effects of apoE on neuronal function, and role of apoE in amyloid-β deposition in vivo. Microsc Res Tech. 2000;50:297–304. doi: 10.1002/1097-0029(20000815)50:4<297::AID-JEMT9>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 19.Pitas RE, Boyles JK, Lee SH, Hui D, Weisgraber KH. Lipoproteins and their receptors in the central nervous system. Characterization of the lipoproteins in cerebrospinal fluid and identification of apolipoprotein B, E(LDL) receptors in the brain. J Biol Chem. 1987;262:14352–14360. [PubMed] [Google Scholar]
- 20.Danik M, Champagne D, Petit-Turcotte C, Beffert U, Poirier J. Brain lipoprotein metabolism and its relation to neurodegenerative disease. Crit Rev Neurobiol. 1999;13:357–407. doi: 10.1615/critrevneurobiol.v13.i4.20. [DOI] [PubMed] [Google Scholar]
- 21.LaDu MJ, Gilligan SM, Lukens JR, et al. Nascent astrocyte particles differ from lipoproteins in CSF. J Neurochem. 1998;70:2070–2081. doi: 10.1046/j.1471-4159.1998.70052070.x. [DOI] [PubMed] [Google Scholar]
- 22.Fagan AM, Holtzman DM, Munson G, et al. Unique lipoproteins secreted by primary astrocytes from wild type, apoE−/−, and human apoE transgenic mice. J Biol Chem. 1999;274:30001–30007. doi: 10.1074/jbc.274.42.30001. [DOI] [PubMed] [Google Scholar]
- 23.Ito J, Zhang LY, Asai M, Yokoyama S. Differential generation of high-density lipoprotein by endogenous and exogenous apolipoproteins in cultured fetal rat astrocytes. J Neurochem. 1999;72:2362–2369. doi: 10.1046/j.1471-4159.1999.0722362.x. [DOI] [PubMed] [Google Scholar]
- 24.Demeester N, Castro G, Desrumaux C, et al. Characterization and functional studies of lipoproteins, lipid transfer proteins, and lecithin:cholesterol acyltransferase in CSF of normal individuals and patients with Alzheimer’s disease. J Lipid Res. 2000;41:963–974. [PubMed] [Google Scholar]
- 25.Koch S, Donarski N, Goetze K, et al. Characterization of four lipoprotein classes in human cerebrospinal fluid. J Lipid Res. 2001;42:1143–1151. [PubMed] [Google Scholar]
- 26.Gong JS, Kobayashi M, Hayashi H, et al. Apolipoprotein E (ApoE) isoform-dependent lipid release from astrocytes prepared from human ApoE3 and ApoE4 knock-in mice. J Biol Chem. 2002;277:29919–29926. doi: 10.1074/jbc.M203934200. [DOI] [PubMed] [Google Scholar]
- 27.Ladu MJ, Reardon C, Van Eldik L, et al. Lipoproteins in the central nervous system. Ann NY Acad Sci. 2000;903:167–175. doi: 10.1111/j.1749-6632.2000.tb06365.x. [DOI] [PubMed] [Google Scholar]
- 28.Goritz C, Mauch DH, Nagler K, Pfrieger FW. Role of glia-derived cholesterol in synaptogenesis: new revelations in the synapse–glia affair. J Physiol Paris. 2002;96:257–263. doi: 10.1016/s0928-4257(02)00014-1. [DOI] [PubMed] [Google Scholar]
- 29.Koudinov AR, Berezov TT. Cholesterol, statins, and Alzheimer disease. PLoS Med. 2005;2:E81. doi: 10.1371/journal.pmed.0020081. author reply E85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30••.Mauch DH, Nagler K, Schumacher S, et al. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–1357. doi: 10.1126/science.294.5545.1354. This in vitro study suggests that neurons may outsource the cholesterol delivery function for synaptogenesis to the astrocytes. [DOI] [PubMed] [Google Scholar]
- 31.Klopfenstein DR, Tomishige M, Stuurman N, Vale RD. Role of phosphatidylinositol(4,5)bisphosphate organization in membrane transport by the Unc104 kinesin motor. Cell. 2002;109:347–358. doi: 10.1016/s0092-8674(02)00708-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michikawa M. Role of cholesterol in amyloid cascade: cholesterol-dependent modulation of tau phosphorylation and mitochondrial function. Acta Neurol Scand Suppl. 2006;185:21–26. doi: 10.1111/j.1600-0404.2006.00681.x. [DOI] [PubMed] [Google Scholar]
- 33.Andersson M, Elmberger P, Edlund C, Kristensson K, Dallner G. Rates of cholesterol, ubiquinone, dolichol and dolichyl-P biosynthesis in rat brain slices. FEBS Letters. 1990;269:5–18. doi: 10.1016/0014-5793(90)81107-y. [DOI] [PubMed] [Google Scholar]
- 34.Bjorkhem I, Heverin M, Leoni V, Meaney S, Diczfalusy U. Oxysterols and Alzheimer’s disease. Acta Neurol Scand Suppl. 2006;185:43–49. doi: 10.1111/j.1600-0404.2006.00684.x. [DOI] [PubMed] [Google Scholar]
- 35.Thelen KM, Falkai P, Bayer TA, Lutjohann D. Cholesterol synthesis rate in human hippocampus declines with aging. Neurosci Lett. 2006;403:15–19. doi: 10.1016/j.neulet.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 36.Yanagisawa K. Cholesterol and pathological processes in Alzheimer’s disease. J Neurosci Res. 2002;70:361–366. doi: 10.1002/jnr.10348. [DOI] [PubMed] [Google Scholar]
- 37.Shobab LA, Hsiung GY, Feldman HH. Cholesterol in Alzheimer’s disease. Lancet Neurol. 2005;4:841–852. doi: 10.1016/S1474-4422(05)70248-9. [DOI] [PubMed] [Google Scholar]
- 38.Lutjohann D. Cholesterol metabolism in the brain: importance of 24S-hydroxylation. Acta Neurol Scand Suppl. 2006;185:33–42. doi: 10.1111/j.1600-0404.2006.00683.x. [DOI] [PubMed] [Google Scholar]
- 39.de Chaves EI, Rusinol AE, Vance DE, Campenot RB, Vance JE. Role of lipoproteins in the delivery of lipids to axons during axonal regeneration. J Biol Chem. 1997;272:30766–30773. doi: 10.1074/jbc.272.49.30766. [DOI] [PubMed] [Google Scholar]
- 40.Posse De Chaves EI, Vance DE, Campenot RB, Kiss RS, Vance JE. Uptake of lipoproteins for axonal growth of sympathetic neurons. J Biol Chem. 2000;275:19883–19890. doi: 10.1074/jbc.275.26.19883. [DOI] [PubMed] [Google Scholar]
- 41••.Funfschilling U, Saher G, Xiao L, Mobius W, Nave KA. Survival of adult neurons lacking cholesterol synthesis in vivo. BMC Neurosci. 2007;8(1) doi: 10.1186/1471-2202-8-1. This in vivo study indicates that some adult neurons do not require endogenous cholesterol synthesis for survival and function and may acquire cholesterol from their surroundings, such as the glial cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pfrieger FW. Outsourcing in the brain: do neurons depend on cholesterol delivery by astrocytes? Bioessays. 2003;25:72–78. doi: 10.1002/bies.10195. [DOI] [PubMed] [Google Scholar]
- 43.Bjorkhem I. Crossing the barrier: oxysterols as cholesterol transporters and metabolic modulators in the brain. J Intern Med. 2006;260:493–508. doi: 10.1111/j.1365-2796.2006.01725.x. [DOI] [PubMed] [Google Scholar]
- 44.Wolozin B. Cyp46 (24S-cholesterol hydroxylase): a genetic risk factor for Alzheimer disease. Arch Neurol. 2003;60:16–18. doi: 10.1001/archneur.60.1.16. [DOI] [PubMed] [Google Scholar]
- 45.Poirier J. Apolipoprotein E represents a potent gene-based therapeutic target for the treatment of sporadic Alzheimer’s disease. Alzheimers Demen. 2008;4:S91–S97. doi: 10.1016/j.jalz.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 46.White F, Nicoll JA, Horsburgh K. Alterations in ApoE and ApoJ in relation to degeneration and regeneration in a mouse model of entorhinal cortex lesion. Exp Neurol. 2001;169:307–318. doi: 10.1006/exnr.2001.7655. [DOI] [PubMed] [Google Scholar]
- 47.Horsburgh K, Nicoll JA. Selective alterations in the cellular distribution of apolipoprotein E immunoreactivity following transient cerebral ischaemia in the rat. Neuropathol Appl Neurobiol. 1996;22:342–349. doi: 10.1111/j.1365-2990.1996.tb01113.x. [DOI] [PubMed] [Google Scholar]
- 48.Horsburgh K, Fitzpatrick M, Nilsen M, Nicoll JA. Marked alterations in the cellular localisation and levels of apolipoprotein E following acute subdural haematoma in rat. Brain Res. 1997;763:103–110. doi: 10.1016/s0006-8993(97)00411-3. [DOI] [PubMed] [Google Scholar]
- 49.Fagan AM, Murphy BA, Patel SN, et al. Evidence for normal aging of the septohippocampal cholinergic system in apoE−/− mice but impaired clearance of axonal degeneration products following injury. Exp Neurol. 1998;151:314–325. doi: 10.1006/exnr.1998.6818. [DOI] [PubMed] [Google Scholar]
- 50.Rebeck GW, Kindy M, LaDu MJ. Apolipoprotein E and Alzheimer’s disease: the protective effects of ApoE2 and E3. J Alzheimers Dis. 2002;4:145–154. doi: 10.3233/jad-2002-4304. [DOI] [PubMed] [Google Scholar]
- 51.Yankner BA, Lu T, Loerch P. The aging brain. Ann Rev Pathol Mech Dis. 2008;3:41–66. doi: 10.1146/annurev.pathmechdis.2.010506.092044. [DOI] [PubMed] [Google Scholar]
- 52.Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- 53.Esiri MM. Ageing and the brain. J Pathol. 2007;211:181–187. doi: 10.1002/path.2089. [DOI] [PubMed] [Google Scholar]
- 54.Soderberg M, Edlund C, Kristensson K, Dallner G. Lipid compositions of different regions of the human brain during aging. J Neurochem. 1990;54:415–423. doi: 10.1111/j.1471-4159.1990.tb01889.x. [DOI] [PubMed] [Google Scholar]
- 55.Svennerholm L, Bostrom K, Jungbjer B, Olsson L. Membrane lipids of adult human brain: lipid composition of frontal and temporal lobe in subjects of age 20 to 100 years. J Neurochem. 1994;63:1802–1811. doi: 10.1046/j.1471-4159.1994.63051802.x. [DOI] [PubMed] [Google Scholar]
- 56.Goritz C, Mauch DH, Pfrieger FW. Multiple mechanisms mediate cholesterol-induced synaptogenesis in a CNS neuron. Mol Cell Neurosci. 2005;29:190–201. doi: 10.1016/j.mcn.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 57.Koudinov AR, Koudinova NV. Cholesterol’s role in synapse formation. Science. 2002;295:2213. doi: 10.1126/science.295.5563.2213a. [DOI] [PubMed] [Google Scholar]
- 58.Ullian EM, Christopherson KS, Barres BA. Role for glia in synaptogenesis. Glia. 2004;47:209–216. doi: 10.1002/glia.20082. [DOI] [PubMed] [Google Scholar]
- 59.Breckenridge WC, Morgan IG, Zanetta JP, Vincendon G. Adult rat brain synaptic vesicles. II Lipid composition. Biochim Biophys Acta. 1973;320:681–686. doi: 10.1016/0304-4165(73)90148-7. [DOI] [PubMed] [Google Scholar]
- 60.Thiele C, Hannah MJ, Fahrenholz F, Huttner WB. Cholesterol binds to synaptophysin and is required for biogenesis of synaptic vesicles. Nat Cell Biol. 2000;2:42–49. doi: 10.1038/71366. [DOI] [PubMed] [Google Scholar]
- 61.Koudinov AR, Koudinova NV. Essential role for cholesterol in synaptic plasticity and neuronal degeneration. FASEB J. 2001;15:1858–1860. doi: 10.1096/fj.00-0815fje. [DOI] [PubMed] [Google Scholar]
- 62.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 63.Simons K, Ehehalt R. Cholesterol, lipid rafts, and disease. J Clin Invest. 2002;110:597–603. doi: 10.1172/JCI16390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hering H, Lin CC, Sheng M. Lipid rafts in the maintenance of synapses, dendritic spines, and surface AMPA receptor stability. J Neurosci. 2003;23:3262–3271. doi: 10.1523/JNEUROSCI.23-08-03262.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Love S, Siew LK, Dawbarn D, Wilcock GK, Ben-Shlomo Y, Allen SJ. Premorbid effects of APOE on synaptic proteins in human temporal neocortex. Neurobiol Aging. 2006;27:797–803. doi: 10.1016/j.neurobiolaging.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 66.Espeseth T, Westlye LT, Fjell AM, Walhovd KB, Rootwelt H, Reinvang I. Accelerated age-related cortical thinning in healthy carriers of apolipoprotein E ε4. Neurobiol Aging. 2008;29:329–340. doi: 10.1016/j.neurobiolaging.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 67.Cohen RM, Small C, Lalonde F, Friz J, Sunderland T. Effect of apolipoprotein E genotype on hippocampal volume loss in aging healthy women. Neurology. 2001;57:2223–2228. doi: 10.1212/wnl.57.12.2223. [DOI] [PubMed] [Google Scholar]
- 68.den Heijer T, Oudkerk M, Launer LJ, van Duijn CM, Hoffman A, Breteler MM. Hippocampal, amygdalar, and global brain atrophy in different apolipoprotein E genotypes. Neurology. 2002;59:746–748. doi: 10.1212/wnl.59.5.746. [DOI] [PubMed] [Google Scholar]
- 69.Cambon K, Davies HA, Stewart MG. Synaptic loss is accompanied by an increase in synaptic area in the dentate gyrus of aged human apolipoprotein E4 transgenic mice. Neuroscience. 2000;97:685–692. doi: 10.1016/s0306-4522(00)00065-8. [DOI] [PubMed] [Google Scholar]
- 70.Moghadasian MH, McManus BM, Nguyen LB, et al. Pathophysiology of apolipoprotein E deficiency in mice: relevance to apo E-related disorders in humans. FASEB J. 2001;15:2623–2630. doi: 10.1096/fj.01-0463com. [DOI] [PubMed] [Google Scholar]
- 71.Anderson R, Barnes JC, Bliss TV, et al. Behavioural, physiological and morphological analysis of a line of apolipoprotein E knockout mouse. Neuroscience. 1998;85:93–110. doi: 10.1016/s0306-4522(97)00598-8. [DOI] [PubMed] [Google Scholar]
- 72.Bronfman FC, Tesseur I, Hofker MH, Havekens LM, Van Leuven F. No evidence for cholinergic problems in apolipoprotein E knockout and apolipoprotein E4 transgenic mice. Neuroscience. 2000;97:411–418. doi: 10.1016/s0306-4522(00)00016-6. [DOI] [PubMed] [Google Scholar]
- 73.Anderson R, Higgins GA. Absence of central cholinergic deficits in ApoE knockout mice. Psychopharmacology (Berl) 1997;132:135–144. doi: 10.1007/s002130050329. [DOI] [PubMed] [Google Scholar]
- 74.Puolivali J, Miettinen R, Pradier L, Riekkinen P., Jr Apolipoprotein E-deficient mice are not more susceptible to the biochemical and memory deficits induced by nucleus basalis lesion. Neuroscience. 2000;96:291–297. doi: 10.1016/s0306-4522(99)00545-x. [DOI] [PubMed] [Google Scholar]
- 75.Krzywkowski P, Ghribi O, Gagne J, et al. Cholinergic systems and long-term potentiation in memory-impaired apolipoprotein E-deficient mice. Neuroscience. 1999;92:1273–1286. doi: 10.1016/s0306-4522(99)00061-5. [DOI] [PubMed] [Google Scholar]
- 76.Oitzl MS, Mulder M, Lucassen PJ, Havekes LM, Grootendorst J, de Kloet ER. Severe learning deficits in apolipoprotein E-knockout mice in a water maze task. Brain Res. 1997;752:189–196. doi: 10.1016/s0006-8993(96)01448-5. [DOI] [PubMed] [Google Scholar]
- 77.Champagne D, Dupuy JB, Rochford J, Poirier J. Apolipoprotein E knockout mice display procedural deficits in the Morris water maze: analysis of learning strategies in three versions of the task. Neuroscience. 2002;114:641–654. doi: 10.1016/s0306-4522(02)00313-5. [DOI] [PubMed] [Google Scholar]
- 78.Gordon I, Grauer E, Genis I, Sehayek E, Michaelson DM. Memory deficits and cholinergic impairments in apolipoprotein E-deficient mice. Neurosci Lett. 1995;199:1–4. doi: 10.1016/0304-3940(95)12006-p. [DOI] [PubMed] [Google Scholar]
- 79.Chapman S, Sabo T, Roses AD, Michaelson DM. Reversal of presynaptic deficits of apolipoprotein E-deficient mice in human apolipoprotein E transgenic mice. Neuroscience. 2000;97:419–424. doi: 10.1016/s0306-4522(00)00087-7. [DOI] [PubMed] [Google Scholar]
- 80.Masliah E, Samuel W, Veinbergs I, Mallory M, Mante M, Saitoh T. Neurodegeneration and cognitive impairment in apoE-deficient mice is ameliorated by infusion of recombinant apoE. Brain Res. 1997;751:307–314. doi: 10.1016/s0006-8993(96)01420-5. [DOI] [PubMed] [Google Scholar]
- 81.Masliah E, Mallory M, Ge N, Alford M, Veinbergs I, Roses AD. Neurodegeneration in the central nervous system of apoE-deficient mice. Exp Neurol. 1995;136:107–122. doi: 10.1006/exnr.1995.1088. [DOI] [PubMed] [Google Scholar]
- 82.Masliah E, Mallory M, Veinbergs I, Miller A, Samuel W. Alterations in apolipoprotein E expression during aging and neurodegeneration. Prog Neurobiol. 1996;50:493–503. doi: 10.1016/s0301-0082(96)00038-x. [DOI] [PubMed] [Google Scholar]
- 83.Nathan BP, Chang KC, Bellosta S, et al. The inhibitory effect of apolipoprotein E4 on neurite outgrowth is associated with microtubule depolymerization. J Biol Chem. 1995;270:19791–19799. doi: 10.1074/jbc.270.34.19791. [DOI] [PubMed] [Google Scholar]
- 84.Veinbergs I, Masliah E. Synaptic alterations in apolipoprotein E knockout mice. Neuroscience. 1999;91:401–403. doi: 10.1016/s0306-4522(98)00602-2. [DOI] [PubMed] [Google Scholar]
- 85.Veinbergs I, Jung MW, Young SJ, Van Uden E, Groves PM, Masliah E. Altered long-term potentiation in the hippocampus of apolipoprotein E-deficient mice. Neurosci Lett. 1998;249:71–74. doi: 10.1016/s0304-3940(98)00399-1. [DOI] [PubMed] [Google Scholar]
- 86.Krugers HJ, Mulder M, Korf J, Havekes L, de Kloet ER, Joels M. Altered synaptic plasticity in hippocampal CA1 area of apolipoprotein E deficient mice. Neuroreport. 1997;8:2505–2510. doi: 10.1097/00001756-199707280-00018. [DOI] [PubMed] [Google Scholar]
- 87.Ji Y, Gong Y, Gan W, Beach T, Holtzman DM, Wisniewski T. Apolipoprotein E isoform-specific regulation of dendritic spine morphology in apolipoprotein E transgenic mice and Alzheimer’s disease patients. Neuroscience. 2003;122:305–315. doi: 10.1016/j.neuroscience.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 88.Walker LC, Parker CA, Lipinski WJ, et al. Cerebral lipid deposition in aged apolipoprotein-E-deficient mice. Am J Pathol. 1997;151:1371–1377. [PMC free article] [PubMed] [Google Scholar]
- 89.Robertson TA, Dutton NS, Martins RN, Roses AD, Kakulas BA, Papadimitriou JM. Age-related congophilic inclusions in the brains of apolipoprotein E-deficient mice. Neuroscience. 1998;82:171–180. doi: 10.1016/s0306-4522(97)00284-4. [DOI] [PubMed] [Google Scholar]
- 90.Lominska C, Levin JA, Wang J, Sikes J, Kao C, Smith JD. Apolipoprotein E deficiency effects on learning in mice are dependent upon the background strain. Behav Brain Res. 2001;120:23–34. doi: 10.1016/s0166-4328(00)00365-x. [DOI] [PubMed] [Google Scholar]
- 91.Buttini M, Orth M, Bellosta S, et al. Expression of human apolipoprotein E3 or E4 in the brains of Apoe−/− mice: isoform-specific effects on neurodegeneration. J Neurosci. 1999;19:4867–4880. doi: 10.1523/JNEUROSCI.19-12-04867.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gee JR, Ding Q, Keller JN. Modulation of apolipoprotein E and interleukin-1β in the aging liver. Exp Gerontol. 2005;40:409–415. doi: 10.1016/j.exger.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 93.Jiang CH, Tsien JZ, Schultz PG, Hu Y. The effects of aging on gene expression in the hypothalamus and cortex of mice. Proc Natl Acad Sci USA. 2001;98:1930–1934. doi: 10.1073/pnas.98.4.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Terao A, Apte-Deshpande A, Dousman L, et al. Immune response gene expression increases in the aging murine hippocampus. J Neuroimmunol. 2002;132:99–112. doi: 10.1016/s0165-5728(02)00317-x. [DOI] [PubMed] [Google Scholar]
- 95.Morgan TE, Xie Z, Goldsmith S, et al. The mosaic of brain glial hyperactivity during normal ageing and its attenuation by food restriction. Neuroscience. 1999;89:687–699. doi: 10.1016/s0306-4522(98)00334-0. [DOI] [PubMed] [Google Scholar]
- 96.Pasinetti GM, Hassler M, Stone D, Finch CE. Glial gene expression during aging in rat striatum and in long-term responses to 6-OHDA lesions. Synapse. 1999;31:278–284. doi: 10.1002/(SICI)1098-2396(19990315)31:4<278::AID-SYN5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 97.Gee JR, Ding Q, Keller JN. Age-related alterations of Apolipoprotein E and interleukin-1β in the aging brain. Biogerontology. 2006;7:69–79. doi: 10.1007/s10522-005-6039-9. [DOI] [PubMed] [Google Scholar]
- 98.Poirier J, Minnich A, Davignon J. Apolipoprotein E, synaptic plasticity and Alzheimer’s disease. Ann Med. 1995;27:663–670. doi: 10.3109/07853899509019253. [DOI] [PubMed] [Google Scholar]
- 99.Reiman EM, Chen K, Alexander GE, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci USA. 2004;101:284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Reiman EM, Caselli RJ, Yun LS, et al. Preclinical evidence of Alzheimer’s disease in persons homozygous for the ε4 allele for apolipoprotein E. N Engl J Med. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 101.Flory JD, Manuck SB, Ferrell RE, Ryan CM, Muldoon MF. Memory performance and the apolipoprotein E polymorphism in a community sample of middle-aged adults. Am J Med Genet. 2000;96:707–711. doi: 10.1002/1096-8628(20001204)96:6<707::aid-ajmg1>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 102.Sundstrom A, Marklund P, Nilsson LG, et al. APOE influences on neuropsychological function after mild head injury: within-person comparisons. Neurology. 2004;62:1963–1966. doi: 10.1212/01.wnl.0000129268.83927.a8. [DOI] [PubMed] [Google Scholar]
- 103.Sundstrom A, Nilsson LG, Cruts M, Adolfsson R, Van Broeckhoven C, Nyberg L. Increased risk of dementia following mild head injury for carriers but not for non-carriers of the APOE ε4 allele. Int Psychogeriatr. 2007;19:159–165. doi: 10.1017/S1041610206003498. [DOI] [PubMed] [Google Scholar]
- 104.Teasdale GM, Nicoll JA, Murray G, Fiddes M. Association of apolipoprotein E polymorphism with outcome after head injury. Lancet. 1997;350:1069–1071. doi: 10.1016/S0140-6736(97)04318-3. [DOI] [PubMed] [Google Scholar]
- 105.Lichtman SW, Seliger G, Tycko B, Marder K. Apolipoprotein E and functional recovery from brain injury following postacute rehabilitation. Neurology. 2000;55:1536–1539. doi: 10.1212/wnl.55.10.1536. [DOI] [PubMed] [Google Scholar]
- 106.Laskowitz DT, Horsburgh K, Roses AD. Apolipoprotein E and the CNS response to injury. J Cereb Blood Flow Metab. 1998;18:465–471. doi: 10.1097/00004647-199805000-00001. [DOI] [PubMed] [Google Scholar]
- 107.Kervinen K, Savolainen MJ, Salokannel J, et al. Apolipoprotein E and B polymorphisms – longevity factors assessed in nonagenarians. Atherosclerosis. 1994;105:89–95. doi: 10.1016/0021-9150(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 108.Helkala EL, Koivisto K, Hanninen T, et al. The association of apolipoprotein E polymorphism with memory: a population based study. Neurosci Lett. 1995;191:141–144. doi: 10.1016/0304-3940(95)11575-h. [DOI] [PubMed] [Google Scholar]
- 109.Bader G, Zuliani G, Kostner GM, Fellin R. Apolipoprotein E polymorphism is not associated with longevity or disability in a sample of Italian octo- and nonagenarians. Gerontology. 1998;44:293–299. doi: 10.1159/000022030. [DOI] [PubMed] [Google Scholar]
- 110.Helkala EL, Koivisto K, Hanninen T, et al. Memory functions in human subjects with different apolipoprotein E phenotypes during a 3-year population-based follow-up study. Neurosci Lett. 1996;204:177–180. doi: 10.1016/0304-3940(96)12348-x. [DOI] [PubMed] [Google Scholar]
- 111.Bretsky P, Guralnik JM, Launer L, Albert M, Seeman TE. The role of APOE-ε4 in longitudinal cognitive decline: MacArthur Studies of Successful Aging. Neurology. 2003;60:1077–1081. doi: 10.1212/01.wnl.0000055875.26908.24. [DOI] [PubMed] [Google Scholar]
- 112.Reed T, Carmelli D, Swan GE, et al. Lower cognitive performance in normal older adult male twins carrying the apolipoprotein E ε4 allele. Arch Neurol. 1994;51:1189–1192. doi: 10.1001/archneur.1994.00540240033012. [DOI] [PubMed] [Google Scholar]
- 113.Bondi MW, Salmon DP, Galasko D, Thomas RG, Thal LJ. Neuropsychological function and apolipoprotein E genotype in the preclinical detection of Alzheimer’s disease. Psychol Aging. 1999;14:295–303. doi: 10.1037//0882-7974.14.2.295. [DOI] [PubMed] [Google Scholar]
- 114.Jonker C, Schmand B, Lindeboom J, Havekes LM, Launer LJ. Association between apolipoprotein E ε4 and the rate of cognitive decline in community-dwelling elderly individuals with and without dementia. Arch Neurol. 1998;55:1065–1069. doi: 10.1001/archneur.55.8.1065. [DOI] [PubMed] [Google Scholar]
- 115.Yaffe K, Cauley J, Sands L, Browner W. Apolipoprotein E phenotype and cognitive decline in a prospective study of elderly community women. Arch Neurol. 1997;54:1110–1114. doi: 10.1001/archneur.1997.00550210044011. [DOI] [PubMed] [Google Scholar]
- 116.Packard CJ, Westendorp DJ, Stott RG, et al. Association between apolipoprotein E4 and cognitive decline in elderly adults. J Am Geriatr Soc. 2007;55:1777–1785. doi: 10.1111/j.1532-5415.2007.01415.x. [DOI] [PubMed] [Google Scholar]
- 117.Small BJ, Basun H, Backman L. Three-year changes in cognitive performance as a function of apolipoprotein E genotype: evidence from very old adults without dementia. Psychol Aging. 1998;13:80–87. doi: 10.1037//0882-7974.13.1.80. [DOI] [PubMed] [Google Scholar]
- 118.Wang C, Wilson WA, Moore SD, et al. Human apoE4-targeted replacement mice display synaptic deficits in the absence of neuropathology. Neurobiol Dis. 2005;18:390–398. doi: 10.1016/j.nbd.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 119.Veinbergs I, Mallory M, Mante M, Rockenstein E, Gilbert JR, Masliah E. Differential neurotrophic effects of apolipoprotein E in aged transgenic mice. Neurosci Lett. 1999;265:218–222. doi: 10.1016/s0304-3940(99)00243-8. [DOI] [PubMed] [Google Scholar]
- 120.Sabo T, Lomnitski L, Nyska A, et al. Susceptibility of transgenic mice expressing human apolipoprotein E to closed head injury: the allele E3 is neuroprotective whereas E4 increases fatalities. Neuroscience. 2000;101:879–884. doi: 10.1016/s0306-4522(00)00438-3. [DOI] [PubMed] [Google Scholar]
- 121.Horsburgh K, McCulloch J, Nilsen M, Roses AD, Nicoll JA. Increased neuronal damage and apoE immunoreactivity in human apolipoprotein E, E4 isoform-specific, transgenic mice after global cerebral ischaemia. Eur J Neurosci. 2000;12:4309–4317. [PubMed] [Google Scholar]
- 122.White F, Nicoll JA, Roses AD, Horsburgh K. Impaired neuronal plasticity in transgenic mice expressing human apolipoprotein E4 compared to E3 in a model of entorhinal cortex lesion. Neurobiol Dis. 2001;8:611–625. doi: 10.1006/nbdi.2001.0401. [DOI] [PubMed] [Google Scholar]
- 123.Buttini M, Akeefe H, Lin C, et al. Dominant negative effects of apolipoprotein E4 revealed in transgenic models of neurodegenerative disease. Neuroscience. 2000;97:207–210. doi: 10.1016/s0306-4522(00)00069-5. [DOI] [PubMed] [Google Scholar]
- 124•.Levi O, Jongen-Relo AL, Feldon J, Roses AD, Michaelson DM. ApoE4 impairs hippocampal plasticity isoform-specifically and blocks the environmental stimulation of synaptogenesis and memory. Neurobiol Dis. 2003;13:273–282. doi: 10.1016/s0969-9961(03)00045-7. Suggests a gain of negative function of apoE4, which may specifically block environmental stimulation of synaptogenesis and memory. [DOI] [PubMed] [Google Scholar]
- 125.Veinbergs I, Everson A, Sagara Y, Masliah E. Neurotoxic effects of apolipoprotein E4 are mediated via dysregulation of calcium homeostasis. J Neurosci Res. 2002;67:379–387. doi: 10.1002/jnr.10138. [DOI] [PubMed] [Google Scholar]
- 126.Weeber EJ, Beffert U, Jones C, et al. Reelin and ApoE receptors cooperate to enhance hippocampal synaptic plasticity and learning. J Biol Chem. 2002;277:39944–39952. doi: 10.1074/jbc.M205147200. [DOI] [PubMed] [Google Scholar]
- 127.Sun Y, Wu S, Bu G, et al. Glial fibrillary acidic protein-apolipoprotein E (apoE) transgenic mice: astrocyte-specific expression and differing biological effects of astrocyte-secreted apoE3 and apoE4 lipoproteins. J Neurosci. 1998;18:3261–3272. doi: 10.1523/JNEUROSCI.18-09-03261.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.DeMattos RB, Brendza RP, Heuser JE, et al. Purification and characterization of astrocyte-secreted apolipoprotein E and J-containing lipoproteins from wildtype and human apoE transgenic mice. Neurochem Int. 2001;39:415–425. doi: 10.1016/s0197-0186(01)00049-3. [DOI] [PubMed] [Google Scholar]
- 129.Morikawa M, Fryer JD, Sullivan PM, et al. Production and characterization of astrocyte-derived human apolipoprotein E isoforms from immortalized astrocytes and their interactions with amyloid-β. Neurobiol Dis. 2005;19:66–76. doi: 10.1016/j.nbd.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 130.Wahrle SE, Jiang H, Parsadanian M, et al. ABCA1 is required for normal central nervous system ApoE levels and for lipidation of astrocyte-secreted apoE. J Biol Chem. 2004;279:40987–40993. doi: 10.1074/jbc.M407963200. [DOI] [PubMed] [Google Scholar]
- 131.Matthews RT, Beal MF. Increased 3-nitrotyrosine in brains of ApoE-deficient mice. Brain Res. 1996;718:181–184. doi: 10.1016/0006-8993(95)01576-0. [DOI] [PubMed] [Google Scholar]
- 132.Maor I, Kaplan M, Hayek T, Vaya J, Hoffman A, Aviram M. Oxidized monocyte-derived macrophages in aortic atherosclerotic lesion from apolipoprotein E-deficient mice and from human carotid artery contain lipid peroxides and oxysterols. Biochem Biophys Res Commun. 2000;269:775–780. doi: 10.1006/bbrc.2000.2359. [DOI] [PubMed] [Google Scholar]
- 133.Keller JN, Lauderback CM, Butterfield DA, Kindy MS, Yu J, Markesbery WR. Amyloid β-peptide effects on synaptosomes from apolipoprotein E-deficient mice. J Neurochem. 2000;74:1579–1586. doi: 10.1046/j.1471-4159.2000.0741579.x. [DOI] [PubMed] [Google Scholar]
- 134.Lauderback CM, Hackett JM, Keller JN, et al. Vulnerability of synaptosomes from apoE knock-out mice to structural and oxidative modifications induced by Aβ1–40: implications for Alzheimer’s disease. Biochemistry. 2001;40:2548–2554. doi: 10.1021/bi002312k. [DOI] [PubMed] [Google Scholar]
- 135.Fullerton SM, Shirman GA, Strittmatter WJ, Matthew WD. Impairment of the blood–nerve and blood–brain barriers in apolipoprotein E knockout mice. Exp Neurol. 2001;169:13–22. doi: 10.1006/exnr.2001.7631. [DOI] [PubMed] [Google Scholar]
- 136.Methia N, Andre P, Hafezi-Moghadam A, Economopoulos M, Thomas KL, Wagner DD. ApoE deficiency compromises the blood–brain barrier especially after injury. Mol Med. 2001;7:810–815. [PMC free article] [PubMed] [Google Scholar]
- 137.Hafezi-Moghadam A, Thomas KL, Wagner DD. ApoE deficiency leads to a progressive age-dependent blood–brain barrier leakage. Am J Physiol Cell Physiol. 2007;292:C1256–C1262. doi: 10.1152/ajpcell.00563.2005. [DOI] [PubMed] [Google Scholar]
- 138.Hirsch-Reinshagen V, Wellington CL. Cholesterol metabolism, apolipoprotein E, adenosine triphosphate-binding cassette transporters, and Alzheimer’s disease. Curr Opin Lipidol. 2007;18:325–332. doi: 10.1097/MOL.0b013e32813aeabf. [DOI] [PubMed] [Google Scholar]
- 139.Poirier J. Apolipoprotein E, cholesterol transport and synthesis in sporadic Alzheimer’s disease. Neurobiol Aging. 2005;26:355–361. doi: 10.1016/j.neurobiolaging.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 140.Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer’s disease. Proc Natl Acad Sci USA. 2006;103:5644–5651. doi: 10.1073/pnas.0600549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mahley RW, Huang Y, Weisgraber KH. Detrimental effects of apolipoprotein E4: potential therapeutic targets in Alzheimer’s disease. Curr Alzheimer Res. 2007;4:537–540. doi: 10.2174/156720507783018334. [DOI] [PubMed] [Google Scholar]
- 142.Fenili D, McLaurin J. Cholesterol and ApoE: a target for Alzheimer’s disease therapeutics. Curr Drug Targets CNS Neurol Disord. 2005;4:553–567. doi: 10.2174/156800705774322085. [DOI] [PubMed] [Google Scholar]
- 143.Auld DS, Kornecook TJ, Bastianetto S, Quirion R. Alzheimer’s disease and the basal forebrain cholinergic system: relations to β-amyloid peptides, cognition, and treatment strategies. Prog Neurobiol. 2002;68:209–245. doi: 10.1016/s0301-0082(02)00079-5. [DOI] [PubMed] [Google Scholar]
- 144.Selkoe DJ. Alzheimer disease: mechanistic understanding predicts novel therapies. Ann Intern Med. 2004;140:627–638. doi: 10.7326/0003-4819-140-8-200404200-00047. [DOI] [PubMed] [Google Scholar]
- 145.Golde TE. Alzheimer disease therapy: can the amyloid cascade be halted? J Clin Invest. 2003;111:11–18. doi: 10.1172/JCI17527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hardy J. Testing times for the ‘amyloid cascade hypothesis’. Neurobiol Aging. 2002;23:1073–1074. doi: 10.1016/s0197-4580(02)00042-8. [DOI] [PubMed] [Google Scholar]
- 147.Haass C, Schlossmacher MG, Hung AY, et al. Amyloid β-peptide is produced by cultured cells during normal metabolism. Nature. 1992;359:322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- 148.Seubert P, Vigo-Pelfrey C, Esch F, et al. Isolation and quantification of soluble Alzheimer’s β-peptide from biological fluids. Nature. 1992;359:325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- 149.Skovronsky DM, Doms RW, Lee VM. Detection of a novel intraneuronal pool of insoluble amyloid β protein that accumulates with time in culture. J Cell Biol. 1998;141:1031–1039. doi: 10.1083/jcb.141.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Walsh DM, Tseng BP, Rydel RE, Podlisny MB, Selkoe DJ. The oligomerization of amyloid β-protein begins intracellularly in cells derived from human brain. Biochemistry. 2000;39:10831–10839. doi: 10.1021/bi001048s. [DOI] [PubMed] [Google Scholar]
- 151.Lambert MP, Barlow AK, Chromy BA, et al. Diffusible, nonfibrillar ligands derived from Aβ1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Klein WL, Krafft GA, Finch CE. Targeting small Aβ oligomers: the solution to an Alzheimer’s disease conundrum? Trends Neurosci. 2001;24:219–224. doi: 10.1016/s0166-2236(00)01749-5. [DOI] [PubMed] [Google Scholar]
- 153.Kirkitadze MD, Bitan G, Teplow DB. Paradigm shifts in Alzheimer’s disease and other neurodegenerative disorders: the emerging role of oligomeric assemblies. J Neurosci Res. 2002;69:567–577. doi: 10.1002/jnr.10328. [DOI] [PubMed] [Google Scholar]
- 154.Puglielli L, Tanzi RE, Kovacs DM. Alzheimer’s disease: the cholesterol connection. Nat Neurosci. 2003;6:345–351. doi: 10.1038/nn0403-345. [DOI] [PubMed] [Google Scholar]
- 155.Ashford JW, Mortimer JA. Non-familial Alzheimer’s disease is mainly due to genetic factors. J Alzheimers Dis. 2002;4:169–177. doi: 10.3233/jad-2002-4307. [DOI] [PubMed] [Google Scholar]
- 156.Poirier J. Apolipoprotein E and cholesterol metabolism in the pathogenesis and treatment of Alzheimer’s disease. Trends Mol Med. 2003;9:94–101. doi: 10.1016/s1471-4914(03)00007-8. [DOI] [PubMed] [Google Scholar]
- 157.Tanzi RE, Bertram L. New frontiers in Alzheimer’s disease genetics. Neuron. 2001;32:181–184. doi: 10.1016/s0896-6273(01)00476-7. [DOI] [PubMed] [Google Scholar]
- 158.Namba Y, Ikeda K. Apolipoprotein B immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in senile dementia of Alzheimer type. Rinsho Shinkeigaku. 1991;31:826–830. [PubMed] [Google Scholar]
- 159.Permanne B, Perez C, Soto C, Frangione B, Wisniewski T. Detection of apolipoprotein E/dimeric soluble amyloid β complexes in Alzheimer’s disease brain supernatants. Biochem Biophys Res Commun. 1997;240:715–720. doi: 10.1006/bbrc.1997.7727. [DOI] [PubMed] [Google Scholar]
- 160.Wisniewski T, Golabek A, Matsubara E, Ghiso J, Frangione B. Apolipoprotein E: binding to soluble Alzheimer’s β-amyloid. Biochem Biophys Res Commun. 1993;192:359–365. doi: 10.1006/bbrc.1993.1423. [DOI] [PubMed] [Google Scholar]
- 161.Parihar MS, Hemnani T. Alzheimer’s disease pathogenesis and therapeutic interventions. J Clin Neurosci. 2004;11:456–467. doi: 10.1016/j.jocn.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 162.Perry EK, Tomlinson BE, Blessed G, Bergmann K, Gibson PH, Perry RH. Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia. Br Med J. 1978;2:1457–1459. doi: 10.1136/bmj.2.6150.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, Delon MR. Alzheimer’s disease and senile dementia: loss of neurons in the basal forebrain. Science. 1982;215:1237–1239. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]
- 164•.Poirier J, Delisle MC, Quirion R, et al. Apolipoprotein E4 allele as a predictor of cholinergic deficits and treatment outcome in Alzheimer disease. Proc Natl Acad Sci USA. 1995;92:12260–12264. doi: 10.1073/pnas.92.26.12260. Study provides strong support for the concept that apoE4 may play a significant role in the cholinergic dysfunction seen in Alzeimer’s disease (AD), suggesting that it may be a useful indicator for predicting possible poor response to acetylcholinesterase inhibition therapy in AD subjects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Soininen H, Kosunen O, Helisalmi S, et al. A severe loss of choline acetyltransferase in the frontal cortex of Alzheimer patients carrying apolipoprotein ε4 allele. Neurosci Lett. 1995;187:79–82. doi: 10.1016/0304-3940(95)11343-6. [DOI] [PubMed] [Google Scholar]
- 166.Arendt T, Schindler C, Bruckner MK, et al. Plastic neuronal remodeling is impaired in patients with Alzheimer’s disease carrying apolipoprotein ε4 allele. J Neurosci. 1997;17:516–529. doi: 10.1523/JNEUROSCI.17-02-00516.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Allen SJ, MacGowan SH, Tyler S, et al. Reduced cholinergic function in normal and Alzheimer’s disease brain is associated with apolipoprotein E4 genotype. Neurosci Lett. 1997;239:33–36. doi: 10.1016/s0304-3940(97)00872-0. [DOI] [PubMed] [Google Scholar]
- 168.Dubelaar EJ, Verwer RW, Hofman MA, Van Heerikhuize JJ, Ravid R, Swaab DE. ApoE ε4 genotype is accompanied by lower metabolic activity in nucleus basalis of Meynert neurons in Alzheimer patients and controls as indicated by the size of the Golgi apparatus. J Neuropathol Exp Neurol. 2004;63:159–169. doi: 10.1093/jnen/63.2.159. [DOI] [PubMed] [Google Scholar]
- 169.Corey-Bloom J, Tiraboschi P, Hansen LA, et al. E4 allele dosage does not predict cholinergic activity or synapse loss in Alzheimer’s disease. Neurology. 2000;54:403–406. doi: 10.1212/wnl.54.2.403. [DOI] [PubMed] [Google Scholar]
- 170.Svensson AL, Warpman U, Hellstrom-Lindahl E, Bogdanovic N, Lannfelt L, Nordberg A. Nicotinic receptors, muscarinic receptors and choline acetyltransferase activity in the temporal cortex of Alzheimer patients with differing apolipoprotein E genotypes. Neurosci Lett. 1997;232:37–40. doi: 10.1016/s0304-3940(97)00573-9. [DOI] [PubMed] [Google Scholar]
- 171.Tiraboschi P, Hansen LA, Masliah E, Alford M, Thal LJ, Corey-Bloom J. Impact of APOE genotype on neuropathologic and neurochemical markers of Alzheimer disease. Neurology. 2004;62:1977–1983. doi: 10.1212/01.wnl.0000128091.92139.0f. [DOI] [PubMed] [Google Scholar]
- 172.Strittmatter WJ. Apolipoprotein E and Alzheimer’s disease. Ann NY Acad Sci. 2000;924:91–92. doi: 10.1111/j.1749-6632.2000.tb05565.x. [DOI] [PubMed] [Google Scholar]
- 173.Teter B, Ashford JW. Neuroplasticity in Alzheimer’s disease. J Neurosci Res. 2002;70:402–437. doi: 10.1002/jnr.10441. [DOI] [PubMed] [Google Scholar]
- 174.Teter B, Xu PT, Gilbert JR, Roses AD, Galasko D, Cole GM. Defective neuronal sprouting by human apolipoprotein E4 is a gain-of-negative function. J Neurosci Res. 2002;68:331–336. doi: 10.1002/jnr.10221. [DOI] [PubMed] [Google Scholar]
- 175.Huang Y, Weisgraber KH, Mucke L, Mahley RW. Apolipoprotein E: diversity of cellular origins, structural and biophysical properties, and effects in Alzheimer’s disease. J Mol Neurosci. 2004;23:189–204. doi: 10.1385/JMN:23:3:189. [DOI] [PubMed] [Google Scholar]
- 176.Cedazo-Minguez A, Cowburn RF. Apolipoprotein E: a major piece in the Alzheimer’s disease puzzle. J Cell Mol Med. 2001;5:254–266. doi: 10.1111/j.1582-4934.2001.tb00159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Miyata M, Smith JD. Apolipoprotein E allele-specific antioxidant activity and effects on cytotoxicity by oxidative insults and β-amyloid peptides. Nat Genet. 1996;14:55–61. doi: 10.1038/ng0996-55. [DOI] [PubMed] [Google Scholar]
- 178.Ramassamy C, Averill D, Beffert U, et al. Oxidative damage and protection by antioxidants in the frontal cortex of Alzheimer’s disease is related to the apolipoprotein E genotype. Free Radic Biol Med. 1999;27:544–553. doi: 10.1016/s0891-5849(99)00102-1. [DOI] [PubMed] [Google Scholar]
- 179.Montine KS, Olson SJ, Amarnath V, Whetsell WO, Jr, Graham DG, Montine TJ. Immunohistochemical detection of 4-hydroxy-2-nonenal adducts in Alzheimer’s disease is associated with inheritance of APOE4. Am J Pathol. 1997;150:437–443. [PMC free article] [PubMed] [Google Scholar]
- 180.Herz J, Beffert U. Apolipoprotein E receptors: linking brain development and Alzheimer’s disease. Nat Rev Neurosci. 2000;1:51–58. doi: 10.1038/35036221. [DOI] [PubMed] [Google Scholar]
- 181.Strittmatter WJ, Saunders AM, Goedert M, et al. Isoform-specific interactions of apolipoprotein E with microtubule-associated protein tau: implications for Alzheimer disease. Proc Natl Acad Sci USA. 1994;91:11183–11186. doi: 10.1073/pnas.91.23.11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Harris FM, Brecht WJ, Xu Q, et al. Carboxyl-terminal-truncated apolipoprotein E4 causes Alzheimer’s disease-like neurodegeneration and behavioral deficits in transgenic mice. Proc Natl Acad Sci USA. 2003;100:10966–10971. doi: 10.1073/pnas.1434398100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Huang Y, Liu XQ, Wyss-Coray T, Brecht WJ, Sanan DA, Mahley RW. Apolipoprotein E fragments present in Alzheimer’s disease brains induce neurofibrillary tangle-like intracellular inclusions in neurons. Proc Natl Acad Sci USA. 2001;98:8838–8843. doi: 10.1073/pnas.151254698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Brecht WJ, Harris FM, Chang S, et al. Neuron-specific apolipoprotein ε4 proteolysis is associated with increased tau phosphorylation in brains of transgenic mice. J Neurosci. 2004;24:2527–2534. doi: 10.1523/JNEUROSCI.4315-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hayashi H, Igbavboa U, Hamanaka H, et al. Cholesterol is increased in the exofacial leaflet of synaptic plasma membranes of human apolipoprotein E4 knock-in mice. Neuroreport. 2002;13:383–386. doi: 10.1097/00001756-200203250-00004. [DOI] [PubMed] [Google Scholar]
- 186.Cataldo AM, Peterhoff CM, Troncoso JC, Gomez-Isla T, Hyman BT, Nixon RA. Endocytic pathway abnormalities precede amyloid β deposition in sporadic Alzheimer’s disease and Down syndrome: differential effects of APOE genotype and presenilin mutations. Am J Pathol. 2000;157:277–286. doi: 10.1016/s0002-9440(10)64538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Cataldo AM, Barnett JL, Pieroni C, Nixon RA. Increased neuronal endocytosis and protease delivery to early endosomes in sporadic Alzheimer’s disease: neuropathologic evidence for a mechanism of increased β-amyloidogenesis. J Neurosci. 1997;17:6142–6151. doi: 10.1523/JNEUROSCI.17-16-06142.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Strittmatter WJ, Saunders AM, Schmechel D, et al. Apolipoprotein E: high-avidity binding to β-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ma J, Yee Brewer HB, Jr, Das S, Potter H. Amyloid-associated proteins α1-antichymotrypsin and apolipoprotein E promote assembly of Alzheimer β-protein into filaments. Nature. 1994;372:92–94. doi: 10.1038/372092a0. [DOI] [PubMed] [Google Scholar]
- 190.Wisniewski T, Castano EM, Golabek A, Vogel T, Frangione B. Acceleration of Alzheimer’s fibril formation by apolipoprotein E in vitro. Am J Pathol. 1994;145:1030–1035. [PMC free article] [PubMed] [Google Scholar]
- 191.LaDu MJ, Falduto MT, Manelli AM, Reardon CA, Getz GS, Frail DE. Isoform-specific binding of apolipoprotein E to β-amyloid. J Biol Chem. 1994;269:23403–23406. [PubMed] [Google Scholar]
- 192.Holtzman DM, Bales KR, Tenkova T, et al. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2000;97:2892–2897. doi: 10.1073/pnas.050004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Irizarry MC, Cheung BS, Rebeck GW, Paul SM, Bales KR, Hyman BT. Apolipoprotein E affects the amount, form, and anatomical distribution of amyloid β-peptide deposition in homozygous APP(V717F) transgenic mice. Acta Neuropathol (Berl) 2000;100:451–458. doi: 10.1007/s004010000263. [DOI] [PubMed] [Google Scholar]
- 194.Bales KR, Verina T, Cummins DJ, et al. Apolipoprotein E is essential for amyloid deposition in the APP(V717F) transgenic mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 1999;96:15233–15238. doi: 10.1073/pnas.96.26.15233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Saavedra L, Mohamed A, Ma V, Kar S, de Chaves EP. Internalization of β-amyloid peptide by primary neurons in the absence of apolipoprotein E. J Biol Chem. 2007;282:35722–35732. doi: 10.1074/jbc.M701823200. [DOI] [PubMed] [Google Scholar]
- 196•.Ghribi O, Larsen B, Schrag M, Herman MM. High cholesterol content in neurons increases BACE, β-amyloid, and phosphorylated tau levels in rabbit hippocampus. Exp Neurol. 2006;200:460–467. doi: 10.1016/j.expneurol.2006.03.019. Demonstrates that cholesterol-enriched diets can cause an increase in the levels of β-site amyloid precursor protein-clearing enzyme, the enzyme involved in amyloid β (Aβ) generation, Aβ and of phosphorylated tau. [DOI] [PubMed] [Google Scholar]
- 197•.Ehehalt R, Keller P, Haass C, Thiele C, Simons K. Amyloidogenic processing of the Alzheimer β-amyloid precursor protein depends on lipid rafts. J Cell Biol. 2003;160:113–123. doi: 10.1083/jcb.200207113. Demonstrates that both amyloid precursor protein (APP) and β-secretase are co-localized in the cholesterol-rich microdomains, thereby providing an interesting link between high cholesterol and the increased likelihood of developing AD pathology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Fassbender K, Simons M, Bergmann C, et al. Simvastatin strongly reduces levels of Alzheimer’s disease β-amyloid peptides Aβ 42 and Aβ 40 in vitro and in vivo. Proc Natl Acad Sci USA. 2001;98:5856–5861. doi: 10.1073/pnas.081620098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kojro E, Gimpl G, Lammich S, Marz W, Fahrenholz F. Low cholesterol stimulates the nonamyloidogenic pathway by its effect on the α-secretase ADAM 10. Proc Natl Acad Sci USA. 2001;98:5815–5820. doi: 10.1073/pnas.081612998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Simons M, de Strooper B, Multhaup G, Tienari PJ, Dotti CG, Beyreuther K. Amyloidogenic processing of the human amyloid precursor protein in primary cultures of rat hippocampal neurons. J Neurosci. 1996;16:899–908. doi: 10.1523/JNEUROSCI.16-03-00899.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wahrle S, Das Nyborg P, et al. Cholesterol-dependent γ-secretase activity in buoyant cholesterol-rich membrane microdomains. Neurobiol Dis. 2002;9:11–23. doi: 10.1006/nbdi.2001.0470. [DOI] [PubMed] [Google Scholar]
- 202•.Abad-Rodriguez J, Ledesma MD, Craessaerts K, et al. Neuronal membrane cholesterol loss enhances amyloid peptide generation. J Cell Biol. 2004;167:953–960. doi: 10.1083/jcb.200404149. Suggests a novel mechanism wherein the protease complex responsible for Aβ generation is segregated from APP in cholesterol rich-microdomains and that loss of neuronal membrane cholesterol contributes to Aβ accumulation and amyloid pathology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kaether C, Haass C. A lipid boundary separates APP and secretases and limits amyloid β-peptide generation. J Cell Biol. 2004;167:809–812. doi: 10.1083/jcb.200410090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wood WG, Schroeder F, Igbavboa U, Avdulov NA, Chochina SV. Brain membrane cholesterol domains, aging and amyloid β-peptides. Neurobiol Aging. 2002;23:685–694. doi: 10.1016/s0197-4580(02)00018-0. [DOI] [PubMed] [Google Scholar]
- 205.Cutler RG, Kelly J, Storie K, et al. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. Proc Natl Acad Sci USA. 2004;101:2070–2075. doi: 10.1073/pnas.0305799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sparks DL. Coronary artery disease, hypertension, ApoE, and cholesterol: a link to Alzheimer’s disease? Ann NY Acad Sci. 1997;826:128–146. doi: 10.1111/j.1749-6632.1997.tb48466.x. [DOI] [PubMed] [Google Scholar]
- 207.Mori T, Paris D, Town T, et al. Cholesterol accumulates in senile plaques of Alzheimer disease patients and in transgenic APP(SW) mice. J Neuropathol Exp Neurol. 2001;60:778–785. doi: 10.1093/jnen/60.8.778. [DOI] [PubMed] [Google Scholar]
- 208.Gomez-Ramos P, Asuncion Moran M. Ultrastructural localization of intraneuronal Aβ-peptide in Alzheimer disease brains. J Alzheimers Dis. 2007;11:53–59. doi: 10.3233/jad-2007-11109. [DOI] [PubMed] [Google Scholar]
- 209.Kivipelto M, Helkala EL, Laakso MP, et al. Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. Br Med J. 2001;322:1447–1451. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kivipelto M, Helkala EL, Hanninen T, et al. Midlife vascular risk factors and late-life mild cognitive impairment: a population-based study. Neurology. 2001;56:1683–1689. doi: 10.1212/wnl.56.12.1683. [DOI] [PubMed] [Google Scholar]
- 211.Sparks DL, Scheff SW, Hunsaker JC, 3rd, Liu H, Landers T, Gross DR. Induction of Alzheimer-like β-amyloid immunoreactivity in the brains of rabbits with dietary cholesterol. Exp Neurol. 1994;126:88–94. doi: 10.1006/exnr.1994.1044. [DOI] [PubMed] [Google Scholar]
- 212.Hajjar I, Schumpert J, Hirth V, Wieland D, Eleazer GP. The impact of the use of statins on the prevalence of dementia and the progression of cognitive impairment. J Gerontol A Biol Sci Med Sci. 2002;57:M414–M418. doi: 10.1093/gerona/57.7.m414. [DOI] [PubMed] [Google Scholar]
- 213.Jick H, Zornberg GL, Jick SS, Seshadri S, Drachman DA. Statins and the risk of dementia. Lancet. 2000;356:1627–1631. doi: 10.1016/s0140-6736(00)03155-x. [DOI] [PubMed] [Google Scholar]
- 214.Wolozin B, Kellman W, Ruosseau P, Celesia GG, Siegel G. Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch Neurol. 2000;57:1439–1443. doi: 10.1001/archneur.57.10.1439. [DOI] [PubMed] [Google Scholar]
- 215.Li G, Shofer JB, Kukull WA, et al. Serum cholesterol and risk of Alzheimer disease: a community-based cohort study. Neurology. 2005;65:1045–1050. doi: 10.1212/01.wnl.0000178989.87072.11. [DOI] [PubMed] [Google Scholar]
- 216.Eckert GP, Wood WG, Muller WE. Statins: drugs for Alzheimer’s disease? J Neural Transm. 2005;112:1057–1071. doi: 10.1007/s00702-004-0273-1. [DOI] [PubMed] [Google Scholar]
- 217.Wood WG, Eckert GP, Igbavboa U, Muller WE. Amyloid β-protein interactions with membranes and cholesterol: causes or casualties of Alzheimer’s disease. Biochim Biophys Acta. 2003;1610:281–290. doi: 10.1016/s0005-2736(03)00025-7. [DOI] [PubMed] [Google Scholar]
- 218.Ledesma MD, Dotti CG. Amyloid excess in Alzheimer’s disease: What is cholesterol to be blamed for? FEBS Lett. 2006;580:5525–5532. doi: 10.1016/j.febslet.2006.06.038. [DOI] [PubMed] [Google Scholar]
- 219.Eckert GP, Muller WE, Wood WG. Cholesterol-lowering drugs and Alzheimer’s disease. Future Lipidol. 2007;2:423–432. [Google Scholar]
- 220.Lutjohann D, Breuer O, Ahlborg G, et al. Cholesterol homeostasis in human brain: evidence for an age-dependent flux of 24S-hydroxycholesterol from the brain into the circulation. Proc Natl Acad Sci USA. 1996;93:9799–9804. doi: 10.1073/pnas.93.18.9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Bjorkhem I, Lutjohann D, Diczfalusy U, Stahle L, Ahlborg G, Wahren J. Cholesterol homeostasis in human brain: turnover of 24S-hydroxycholesterol and evidence for a cerebral origin of most of this oxysterol in the circulation. J Lipid Res. 1998;39:1594–1600. [PubMed] [Google Scholar]
- 222.Lutjohann D, von Bergmann K. 24S-hydroxycholesterol: a marker of brain cholesterol metabolism. Pharmacopsychiatry. 2003;36(Suppl 2):S102–S106. doi: 10.1055/s-2003-43053. [DOI] [PubMed] [Google Scholar]
- 223.Lutjohann D, Papassotiropoulos A, Bjorkhem I, et al. Plasma 24S-hydroxycholesterol (cerebrosterol) is increased in Alzheimer and vascular demented patients. J Lipid Res. 2000;41:195–198. [PubMed] [Google Scholar]
- 224.Bretillon L, Lutjohann D, Stahle L, et al. Plasma levels of 24S-hydroxycholesterol reflect the balance between cerebral production and hepatic metabolism and are inversely related to body surface. J Lipid Res. 2000;41:840–845. [PubMed] [Google Scholar]
- 225.Bretillon L, Siden A, Wahlund LO, et al. Plasma levels of 24S-hydroxycholesterol in patients with neurological diseases. Neurosci Lett. 2000;293:87–90. doi: 10.1016/s0304-3940(00)01466-x. [DOI] [PubMed] [Google Scholar]
- 226.Papassotiropoulos A, Lutjohann D, Bagli M, et al. 24S-hydroxycholesterol in cerebrospinal fluid is elevated in early stages of dementia. J Psychiatr Res. 2002;36:27–32. doi: 10.1016/s0022-3956(01)00050-4. [DOI] [PubMed] [Google Scholar]
- 227.Leoni V, Masterman T, Mousavi FS, et al. Diagnostic use of cerebral and extracerebral oxysterols. Clin Chem Lab Med. 2004;42:186–191. doi: 10.1515/CCLM.2004.034. [DOI] [PubMed] [Google Scholar]
- 228.Leoni V, Shafaati M, Salomon A, Kivipelto M, Bjorkhem I, Wahlund LO. Are the CSF levels of 24S-hydroxycholesterol a sensitive biomarker for mild cognitive impairment? Neurosci Lett. 2006;397:83–87. doi: 10.1016/j.neulet.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 229.Schonknecht P, Lutjohann D, Pantel J, et al. Cerebrospinal fluid 24S-hydroxycholesterol is increased in patients with Alzheimer’s disease compared to healthy controls. Neurosci Lett. 2002;324:83–85. doi: 10.1016/s0304-3940(02)00164-7. [DOI] [PubMed] [Google Scholar]
- 230.Leoni V, Masterman T, Patel P, Meaney S, Diczfalusy U, Bjorkhem I. Side chain oxidized oxysterols in cerebrospinal fluid and the integrity of blood–brain and blood–cerebrospinal fluid barriers. J Lipid Res. 2003;44:793–799. doi: 10.1194/jlr.M200434-JLR200. [DOI] [PubMed] [Google Scholar]
- 231.Shafaati M, Solomon A, Kivipelto M, Bjorkhem I, Leoni V. Levels of ApoE in cerebrospinal fluid are correlated with Tau and 24S-hydroxycholesterol in patients with cognitive disorders. Neurosci Lett. 2007;425:78–82. doi: 10.1016/j.neulet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 232.Abildayeva K, Jansen PJ, Hirsch-Reinshagen V, et al. 24S-hydroxycholesterol participates in a liver X receptor-controlled pathway in astrocytes that regulates apolipoprotein E-mediated cholesterol efflux. J Biol Chem. 2006;281:12799–12808. doi: 10.1074/jbc.M601019200. [DOI] [PubMed] [Google Scholar]
- 233.Fukumoto H, Deng A, Irizarry MC, Fitzgerald ML, Rebeck GW. Induction of the cholesterol transporter ABCA1 in central nervous system cells by liver X receptor agonists increases secreted Aβ levels. J Biol Chem. 2002;277:48508–48513. doi: 10.1074/jbc.M209085200. [DOI] [PubMed] [Google Scholar]
- 234.Famer D, Meaney S, Mousavi M, Nordberg A, Bjorkhem I, Crisby M. Regulation of α- and β-secretase activity by oxysterols: cerebrosterol stimulates processing of APP via the α-secretase pathway. Biochem Biophys Res Commun. 2007;359:46–50. doi: 10.1016/j.bbrc.2007.05.033. [DOI] [PubMed] [Google Scholar]
- 235.Vaya J, Schipper HM. Oxysterols, cholesterol homeostasis, and Alzheimer disease. J Neurochem. 2007;102:1727–1737. doi: 10.1111/j.1471-4159.2007.04689.x. [DOI] [PubMed] [Google Scholar]
- 236.Weisgraber KH, Pitas RE, Mahley RW. Lipoproteins, neurobiology and Alzheimer’s disease: structure and function of apolipoprotein E. Curr Opin Struct Biol. 1994;4:507–515. [Google Scholar]
- 237.Wilson C, Wardell MR, Weisgraber KH, Mahley RW, Agard DA. Three-dimensional structure of the LDL receptor-binding domain of human apolipoprotein E. Science. 1991;252:1817–1822. doi: 10.1126/science.2063194. [DOI] [PubMed] [Google Scholar]
- 238.Hatters DM, Peters-Libeu CA, Weisgraber KH. Apolipoprotein E structure: insights into function. Trends Biochem Sci. 2006;31:445–454. doi: 10.1016/j.tibs.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 239.Mahley RW, Rall SC., Jr Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet. 2000;1:507–537. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- 240.Dong LM, Wilson C, Wardell MR, et al. Human apolipoprotein E. Role of arginine 61 in mediating the lipoprotein preferences of the E3 and E4 isoforms. J Biol Chem. 1994;269:22358–22365. [PubMed] [Google Scholar]
- 241.Dong LM, Weisgraber KH. Human apolipoprotein E4 domain interaction. Arginine 61 and glutamic acid 255 interact to direct the preference for very low density lipoproteins. J Biol Chem. 1996;271:19053–19057. doi: 10.1074/jbc.271.32.19053. [DOI] [PubMed] [Google Scholar]
- 242.Raffai RL, Dong LM, Farese RV, Jr, Weisgraber KH. Introduction of human apolipoprotein E4 ‘domain interaction’ into mouse apolipoprotein E. Proc Natl Acad Sci USA. 2001;98:11587–11591. doi: 10.1073/pnas.201279298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Hatters DM, Zhong N, Rutenber E, Weisgraber KH. Amino-terminal domain stability mediates apolipoprotein E aggregation into neurotoxic fibrils. J Mol Biol. 2006;361:932–944. doi: 10.1016/j.jmb.2006.06.080. [DOI] [PubMed] [Google Scholar]
- 244•.Zhang Y, Vasudevan S, Sojitrawala R, et al. monomeric A, biologically active, full-length human apolipoprotein E. Biochemistry. 2007;46:10722–10732. doi: 10.1021/bi700672v. NMR structural analysis of an apoE3 variant that is monomeric in solution in lipid-free state; provides a model for future structural analysis of the lipid-free tetrameric protein. [DOI] [PubMed] [Google Scholar]
- 245.Drury J, Narayanaswami V. Examination of lipid-bound conformation of apolipoprotein E4 by pyrene excimer fluorescence. J Biol Chem. 2005;280:14605–14610. doi: 10.1074/jbc.M414019200. [DOI] [PubMed] [Google Scholar]
- 246.Hatters DM, Peters-Libeu CA, Weisgraber KH. Engineering conformational destabilization into mouse apolipoprotein E. A model for a unique property of human apolipoprotein E4. J Biol Chem. 2005;280:26477–26482. doi: 10.1074/jbc.M503910200. [DOI] [PubMed] [Google Scholar]
- 247•.Peters-Libeu CA, Newhouse Y, Hatters DM, Weisgraber KH. Model of biologically active apolipoprotein E bound to dipalmitoylphosphatidylcholine. J Biol Chem. 2006;281:1073–1079. doi: 10.1074/jbc.M510851200. This x-ray structural analysis is a description of the lipid associated conformation of apoE4 at 10 Å resolution, wherein the molecule adopts a helical hairpin fold with the binding sites for the LDL receptor (LDLr) located at the apex of the bend; it provides a model for future structural studies of lipoprotein-bound apoE. [DOI] [PubMed] [Google Scholar]
- 248.Narayanaswami V, Maiorano JN, Dhanasekaran P, et al. Helix orientation of the functional domains in apolipoprotein e in discoidal high density lipoprotein particles. J Biol Chem. 2004;279:14273–14279. doi: 10.1074/jbc.M313318200. [DOI] [PubMed] [Google Scholar]
- 249.Narayanaswami V, Ryan RO. Molecular basis of exchangeable apolipoprotein function. Biochim Biophys Acta. 2000;1483:15–36. doi: 10.1016/s1388-1981(99)00176-6. [DOI] [PubMed] [Google Scholar]
- 250.Saito H, Dhanasekaran P, Baldwin F, Weisgraber KH, Lund-Katz S, Phillips MC. Lipid binding-induced conformational change in human apolipoprotein E. Evidence for two lipid-bound states on spherical particles. J Biol Chem. 2001;276:40949–40954. doi: 10.1074/jbc.M106337200. [DOI] [PubMed] [Google Scholar]
- 251.Saito H, Lund-Katz S, Phillips MC. Contributions of domain structure and lipid interaction to the functionality of exchangeable human apolipoproteins. Prog Lipid Res. 2004;43:350–380. doi: 10.1016/j.plipres.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 252.Weers PM, Narayanaswami V, Choy N, et al. Lipid binding ability of human apolipoprotein E N-terminal domain isoforms: correlation with protein stability? Biophys Chem. 2003;100:481–492. doi: 10.1016/s0301-4622(02)00300-9. [DOI] [PubMed] [Google Scholar]
- 253.Morrow JA, Segall ML, Lund-Katz S, et al. Differences in stability among the human apolipoprotein E isoforms determined by the amino-terminal domain. Biochemistry. 2000;39:11657–11666. doi: 10.1021/bi000099m. [DOI] [PubMed] [Google Scholar]
- 254.Morrow JA, Hatters DM, Lu B, et al. Apolipoprotein E4 forms a molten globule. A potential basis for its association with disease. J Biol Chem. 2002;277:50380–50385. doi: 10.1074/jbc.M204898200. [DOI] [PubMed] [Google Scholar]
- 255.Saito H, Dhanasekaran P, Baldwin F, Weisgraber KH, Phillips MC, Lund-Katz S. Effects of polymorphism on the lipid interaction of human apolipoprotein E. J Biol Chem. 2003;278:40723–40729. doi: 10.1074/jbc.M304814200. [DOI] [PubMed] [Google Scholar]
- 256.Sakamoto T, Tanaka M, Vedhachalam C, et al. Contributions of the carboxyl-terminal helical segment to the self-association and lipoprotein preferences of human apolipoprotein E3 and E4 isoforms. Biochemistry. 2008;47:2968–2977. doi: 10.1021/bi701923h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Segall ML, Dhanasekaran P, Baldwin F, et al. Influence of apoE domain structure and polymorphism on the kinetics of phospholipid vesicle solubilization. J Lipid Res. 2002;43:1688–1700. doi: 10.1194/jlr.m200157-jlr200. [DOI] [PubMed] [Google Scholar]
- 258.Acharya P, Segall ML, Zaiou M, et al. Comparison of the stabilities and unfolding pathways of human apolipoprotein E isoforms by differential scanning calorimetry and circular dichroism. Biochim Biophys Acta. 2002;1584:9–19. doi: 10.1016/s1388-1981(02)00263-9. [DOI] [PubMed] [Google Scholar]
- 259.Remaley AT, Stonik JA, Demosky SJ, et al. Apolipoprotein specificity for lipid efflux by the human ABCAI transporter. Biochem Biophys Res Commun. 2001;280:818–823. doi: 10.1006/bbrc.2000.4219. [DOI] [PubMed] [Google Scholar]
- 260.Bortnick AE, Rothblat GH, Stoudt G, et al. The correlation of ATP-binding cassette 1 mRNA levels with cholesterol efflux from various cell lines. J Biol Chem. 2000;275:28634–28640. doi: 10.1074/jbc.M003407200. [DOI] [PubMed] [Google Scholar]
- 261.Cullen P, Cignarella A, Brennhausen B, Mohr S, Assmann G, von Eckardstein A. Phenotype-dependent differences in apolipoprotein E metabolism and in cholesterol homeostasis in human monocyte-derived macrophages. J Clin Invest. 1998;101:1670–1677. doi: 10.1172/JCI119887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Lin CY, Duan H, Mazzone T. Apolipoprotein E-dependent cholesterol efflux from macrophages: kinetic study and divergent mechanisms for endogenous versus exogenous apolipoprotein E. J Lipid Res. 1999;40:1618–1627. [PubMed] [Google Scholar]
- 263.Smith JD, Miyata M, Ginsberg M, Grigaux C, Shmookler E, Plump AS. Cyclic AMP induces apolipoprotein E binding activity and promotes cholesterol efflux from a macrophage cell line to apolipoprotein acceptors. J Biol Chem. 1996;271:30647–30655. doi: 10.1074/jbc.271.48.30647. [DOI] [PubMed] [Google Scholar]
- 264.Vedhachalam C, Narayanaswami V, Neto N, et al. The C-terminal lipid-binding domain of apolipoprotein E is a highly efficient mediator of ABCA1-dependent cholesterol efflux that promotes the assembly of high-density lipoproteins. Biochemistry. 2007;46:2583–2593. doi: 10.1021/bi602407r. [DOI] [PubMed] [Google Scholar]
- 265.Krimbou L, Denis M, Haidar B, Carrier M, Marcil M, Genest J., Jr Molecular interactions between apoE and ABCA1: impact on apoE lipidation. J Lipid Res. 2004;45:839–848. doi: 10.1194/jlr.M300418-JLR200. [DOI] [PubMed] [Google Scholar]
- 266.Huang Y, von Eckardstein A, Wu S, Assmann G. Effects of the apolipoprotein E polymorphism on uptake and transfer of cell-derived cholesterol in plasma. J Clin Invest. 1995;96:2693–2701. doi: 10.1172/JCI118336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Hirsch-Reinshagen V, Zhou S, Burgess BL, et al. Deficiency of ABCA1 impairs apolipoprotein E metabolism in brain. J Biol Chem. 2004;279:41197–41207. doi: 10.1074/jbc.M407962200. [DOI] [PubMed] [Google Scholar]
- 268.Michikawa M, Fan QW, Isobe I, Yanagisawa K. Apolipoprotein E exhibits isoform-specific promotion of lipid efflux from astrocytes and neurons in culture. J Neurochem. 2000;74:1008–1016. doi: 10.1046/j.1471-4159.2000.0741008.x. [DOI] [PubMed] [Google Scholar]
- 269.Gong JS, Morita SY, Kobayashi M, et al. Novel action of apolipoprotein E (ApoE): ApoE isoform specifically inhibits lipid-particle-mediated cholesterol release from neurons. Mol Neurodegener. 2007;2:9. doi: 10.1186/1750-1326-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Phu MJ, Hawbecker SK, Narayanaswami V. Fluorescence resonance energy transfer analysis of apolipoprotein E C-terminal domain and amyloid β peptide (1–42) interaction. J Neurosci Res. 2005;80:877–886. doi: 10.1002/jnr.20503. [DOI] [PubMed] [Google Scholar]
- 271.Aleshkov SB, Li X, Lavrentiadou SN, Zannis VI. Contribution of cysteine 158, the glycosylation site threonine 194, the amino- and carboxy-terminal domains of apolipoprotein E in the binding to amyloid peptide β (1–40) Biochemistry. 1999;38:8918–8925. doi: 10.1021/bi982002q. [DOI] [PubMed] [Google Scholar]
- 272.Lins L, Thomas-Soumarmon A, Pillot T, Vandekerchkhove J, Rosseneu M, Brasseur R. Molecular determinants of the interaction between the C-terminal domain of Alzheimer’s β-amyloid peptide and apolipoprotein E α-helices. J Neurochem. 1999;73:758–769. doi: 10.1046/j.1471-4159.1999.0730758.x. [DOI] [PubMed] [Google Scholar]
- 273.Golabek AA, Kida E, Walus M, Perez C, Wisniewski T, Soto C. Sodium dodecyl sulfate-resistant complexes of Alzheimer’s amyloid β-peptide with the N-terminal, receptor binding domain of apolipoprotein E. Biophys J. 2000;79:1008–1015. doi: 10.1016/S0006-3495(00)76354-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Pillot T, Goethals M, Najib J, et al. β-amyloid peptide interacts specifically with the carboxy-terminal domain of human apolipoprotein E: relevance to Alzheimer’s disease. J Neurochem. 1999;72:230–237. doi: 10.1046/j.1471-4159.1999.0720230.x. [DOI] [PubMed] [Google Scholar]
- 275.Strittmatter WJ, Weisgraber KH, Huang DY, et al. Binding of human apolipoprotein E to synthetic amyloid β peptide: isoform-specific effects and implications for late-onset Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:8098–8102. doi: 10.1073/pnas.90.17.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Tamamizu-Kato S, Cohen JK, Drake CB, Kosaraju MG, Drury J, Narayanaswami V. Interaction with amyloid β peptide compromises the lipid binding function of apolipoprotein E. Biochemistry. 2008;47(18):5225–5234. doi: 10.1021/bi702097s. [DOI] [PubMed] [Google Scholar]
- 277.Marques MA, Owens PA, Crutcher KA. Progress toward identification of protease activity involved in proteolysis of apolipoprotein E in human brain. J Mol Neurosci. 2004;24:73–80. doi: 10.1385/JMN:24:1:073. [DOI] [PubMed] [Google Scholar]
- 278.Cho HS, Hyman BT, Greenberg SM, Rebeck GW. Quantitation of apoE domains in Alzheimer disease brain suggests a role for apoE in Aβ aggregation. J Neuropathol Exp Neurol. 2001;60:342–349. doi: 10.1093/jnen/60.4.342. [DOI] [PubMed] [Google Scholar]
- 279.Wellnitz S, Friedlein A, Bonanni C, et al. A 13 kDa carboxy-terminal fragment of ApoE stabilizes Aβ hexamers. J Neurochem. 2005;94:1351–1360. doi: 10.1111/j.1471-4159.2005.03295.x. [DOI] [PubMed] [Google Scholar]
- 280.Wisniewski T, Lalowski M, Golabek A, Vogel T, Frangione B. Is Alzheimer’s disease an apolipoprotein E amyloidosis? Lancet. 1995;345:956–958. doi: 10.1016/s0140-6736(95)90701-7. [DOI] [PubMed] [Google Scholar]
- 281.Marques MA, Tolar M, Harmony JA, Crutcher KA. A thrombin cleavage fragment of apolipoprotein E exhibits isoform-specific neurotoxicity. Neuroreport. 1996;7:2529–2532. doi: 10.1097/00001756-199611040-00025. [DOI] [PubMed] [Google Scholar]
- 282.Tolar M, Marques MA, Harmony JA, Crutcher KA. Neurotoxicity of the 22 kDa thrombin-cleavage fragment of apolipoprotein E and related synthetic peptides is receptor-mediated. J Neurosci. 1997;17:5678–5686. doi: 10.1523/JNEUROSCI.17-15-05678.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Sanan DA, Weisgraber KH, Russell SJ, et al. Apolipoprotein E associates with β amyloid peptide of Alzheimer’s disease to form novel monofibrils. Isoform apoE4 associates more efficiently than apoE3. J Clin Invest. 1994;94:860–869. doi: 10.1172/JCI117407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Sadowski MJ, Pankiewicz J, Scholtzova H, et al. Blocking the apolipoprotein E/amyloid-β interaction as a potential therapeutic approach for Alzheimer’s disease. Proc Natl Acad Sci USA. 2006;103:18787–18792. doi: 10.1073/pnas.0604011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Wisniewski T, Frangione B. Apolipoprotein E: a pathological chaperone protein in patients with cerebral and systemic amyloid. Neurosci Lett. 1992;135:235–238. doi: 10.1016/0304-3940(92)90444-c. [DOI] [PubMed] [Google Scholar]
- 286.Weers PM, Narayanaswami V, Ryan RO. Modulation of the lipid binding properties of the N-terminal domain of human apolipoprotein E3. Eur J Biochem. 2001;268:3728–3735. doi: 10.1046/j.1432-1327.2001.02282.x. [DOI] [PubMed] [Google Scholar]
- 287.Ji ZS, Miranda RD, Newhouse YM, Weisgraber KH, Huang Y, Mahley RW. Apolipoprotein E4 potentiates amyloid β peptide-induced lysosomal leakage and apoptosis in neuronal cells. J Biol Chem. 2002;277:21821–21888. doi: 10.1074/jbc.M112109200. [DOI] [PubMed] [Google Scholar]
- 288.Ji ZS, Mullendorff K, Cheng IH, Miranda RD, Huang Y, Mahley RW. Reactivity of apolipoprotein E4 and amyloid β peptide: lysosomal stability and neurodegeneration. J Biol Chem. 2006;281:2683–2692. doi: 10.1074/jbc.M506646200. [DOI] [PubMed] [Google Scholar]
- 289.Heeren J, Grewal T, Laatsch A, et al. Recycling of apoprotein E is associated with cholesterol efflux and high density lipoprotein internalization. J Biol Chem. 2003;278:14370–14378. doi: 10.1074/jbc.M209006200. [DOI] [PubMed] [Google Scholar]
- 290.Heeren J, Grewal T, Laatsch A, et al. Impaired recycling of apolipoprotein E4 is associated with intracellular cholesterol accumulation. J Biol Chem. 2004;279:55483–55492. doi: 10.1074/jbc.M409324200. [DOI] [PubMed] [Google Scholar]
- 291.Rudenko G, Deisenhofer J. The low-density lipoprotein receptor: ligands, debates and lore. Curr Opin Struct Biol. 2003;13:683–689. doi: 10.1016/j.sbi.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 292.Motoi Y, Aizawa T, Haga S, Nakamura S, Namba Y, Ikeda K. Neuronal localization of a novel mosaic apolipoprotein E receptor LR11, in rat and human brain. Brain Res. 1999;833:209–215. doi: 10.1016/s0006-8993(99)01542-5. [DOI] [PubMed] [Google Scholar]
- 293.Irizarry MC. Modulation of brain apolipoprotein E levels by the low-density lipoprotein receptor. Neurobiol Lipids. 2005;4:2. [Google Scholar]
- 294.Nykjaer A, Willnow TE. The low-density lipoprotein receptor gene family: a cellular Swiss army knife? Trends Cell Biol. 2002;12:273–280. doi: 10.1016/s0962-8924(02)02282-1. [DOI] [PubMed] [Google Scholar]
- 295.Ruiz J, Kouiavskaia D, Migliorini M, et al. The apoE isoform binding properties of the VLDL receptor reveal marked differences from LRP and the LDL receptor. J Lipid Res. 2005;46:1721–1731. doi: 10.1194/jlr.M500114-JLR200. [DOI] [PubMed] [Google Scholar]
- 296.Narita M, Holtzman DM, Fagan AM, et al. Cellular catabolism of lipid poor apolipoprotein E via cell surface LDL receptor-related protein. J Biochem. 2002;132:743–749. doi: 10.1093/oxfordjournals.jbchem.a003282. [DOI] [PubMed] [Google Scholar]
- 297.Narayanaswami V, Szeto SS, Ryan RO. Lipid association-induced N- and C-terminal domain reorganization in human apolipoprotein E3. J Biol Chem. 2001;276:37853–37860. doi: 10.1074/jbc.M102953200. [DOI] [PubMed] [Google Scholar]
- 298.Lu B, Morrow JA, Weisgraber KH. Conformational reorganization of the four-helix bundle of human apolipoprotein E in binding to phospholipid. J Biol Chem. 2000;275:20775–20781. doi: 10.1074/jbc.M003508200. [DOI] [PubMed] [Google Scholar]
- 299.Knouff C, Hinsdale ME, Mezdour H, et al. Apo E structure determines VLDL clearance and atherosclerosis risk in mice. J Clin Invest. 1999;103:1579–1586. doi: 10.1172/JCI6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Mamotte CD, Sturm M, Foo JI, van Bockxmeer FM, Taylor RR. Comparison of the LDL-receptor binding of VLDL and LDL from apoE4 and apoE3 homozygotes. Am J Physiol. 1999;276:E553–E557. doi: 10.1152/ajpendo.1999.276.3.E553. [DOI] [PubMed] [Google Scholar]
- 301.Bohnet K, Pillot T, Visvikis S, Sabolovic N, Siest G. Apolipoprotein (apo) E genotype and apoE concentration determine binding of normal very low density lipoproteins to HepG2 cell surface receptors. J Lipid Res. 1996;37:1316–1324. [PubMed] [Google Scholar]
- 302.Mahley RW. Heparan sulfate proteoglycan/low density lipoprotein receptor-related protein pathway involved in type III hyperlipoproteinemia and Alzheimer’s disease. Isr J Med Sci. 1996;32:414–429. [PubMed] [Google Scholar]
- 303.Bellosta S, Nathan BP, Orth M, Dong LM, Mahley RW, Pitas RE. Stable expression and secretion of apolipoproteins E3 and E4 in mouse neuroblastoma cells produces differential effects on neurite outgrowth. J Biol Chem. 1995;270:27063–27071. doi: 10.1074/jbc.270.45.27063. [DOI] [PubMed] [Google Scholar]
- 304.Holtzman DM, Pitas RE, Kilbridge J, et al. Low density lipoprotein receptor-related protein mediates apolipoprotein E-dependent neurite outgrowth in a central nervous system-derived neuronal cell line. Proc Natl Acad Sci USA. 1995;92:9480–9484. doi: 10.1073/pnas.92.21.9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.DeMattos RB, Curtiss LK, Williams DL. A minimally lipidated form of cell-derived apolipoprotein E exhibits isoform-specific stimulation of neurite outgrowth in the absence of exogenous lipids or lipoproteins. J Biol Chem. 1998;273:4206–4212. doi: 10.1074/jbc.273.7.4206. [DOI] [PubMed] [Google Scholar]
- 306.Crutcher KA, Clay MA, Scott SA, Tian X, Tolar M, Harmony JA. Neurite degeneration elicited by apolipoprotein E peptides. Exp Neurol. 1994;130:120–126. doi: 10.1006/exnr.1994.1191. [DOI] [PubMed] [Google Scholar]
- 307.Hara M, Matsushima T, Satoh H, et al. Isoform-dependent cholesterol efflux from macrophages by apolipoprotein E is modulated by cell surface proteoglycans. Arterioscler Thromb Vasc Biol. 2003;23:269–274. doi: 10.1161/01.atv.0000054199.78458.4b. [DOI] [PubMed] [Google Scholar]
- 308.Mahley RW, Ji ZS. Remnant lipoprotein metabolism: key pathways involving cell-surface heparan sulfate proteoglycans and apolipoprotein E. J Lipid Res. 1999;40:1–16. [PubMed] [Google Scholar]
- 309.Futamura M, Dhanasekaran P, Handa T, Phillips MC, Lund-Katz S, Saito H. Two-step mechanism of binding of apolipoprotein E to heparin: implications for the kinetics of apolipoprotein E–heparan sulfate proteoglycan complex formation on cell surfaces. J Biol Chem. 2005;280:5414–5422. doi: 10.1074/jbc.M411719200. [DOI] [PubMed] [Google Scholar]
- 310.Libeu CP, Lund-Katz S, Phillips MC, et al. New insights into the heparan sulfate proteoglycan-binding activity of apolipoprotein E. J Biol Chem. 2001;276:39138–39144. doi: 10.1074/jbc.M104746200. [DOI] [PubMed] [Google Scholar]
- 311.Shuvaev VV, Laffont I, Siest G. Kinetics of apolipoprotein E isoforms-binding to the major glycosaminoglycans of the extracellular matrix. FEBS Lett. 1999;459:353–357. doi: 10.1016/s0014-5793(99)01285-5. [DOI] [PubMed] [Google Scholar]
- 312.Shuvaev VV, Siest G. Heparin specifically inhibits binding of apolipoprotein E to amyloid β-peptide. Neurosci Lett. 2000;280:131–134. doi: 10.1016/s0304-3940(00)00764-3. [DOI] [PubMed] [Google Scholar]
- 313.Fukuchi K, Hart M, Li L. Alzheimer’s disease and heparan sulfate proteoglycan. Front Biosci. 1998;3:D327–D337. doi: 10.2741/a277. [DOI] [PubMed] [Google Scholar]
- 314.Feyzi E, Saldeen T, Larsson E, Lindahl U, Salmivirta M. Age-dependent modulation of heparan sulfate structure and function. J Biol Chem. 1998;273:13395–13398. doi: 10.1074/jbc.273.22.13395. [DOI] [PubMed] [Google Scholar]
- 315.Ikewaki K, Zech LA, Brewer HB, Jr, Rader DJ. Comparative in vivo metabolism of apolipoproteins E2 and E4 in heterozygous apoE2/4 subjects. J Lab Clin Med. 2002;140:369–374. doi: 10.1067/mlc.2002.129066. [DOI] [PubMed] [Google Scholar]
- 316.Fukumoto H, Ingelsson M, Garevik N, et al. APOE ε3/ε4 heterozygotes have an elevated proportion of apolipoprotein E4 in cerebrospinal fluid relative to plasma, independent of Alzheimer’s disease diagnosis. Exp Neurol. 2003;183:249–253. doi: 10.1016/s0014-4886(03)00088-8. [DOI] [PubMed] [Google Scholar]
- 317.Fryer JD, McCormick DR, O’Dell LM, et al. The low density lipoprotein receptor regulates the level of central nervous system human and murine apolipoprotein E but does not modify amyloid plaque pathology in PDAPP mice. J Biol Chem. 2005;280:25754–25759. doi: 10.1074/jbc.M502143200. [DOI] [PubMed] [Google Scholar]
- 318.Sing CF, Davignon J. Role of the apolipoprotein E polymorphism in determining normal plasma lipid and lipoprotein variation. Am J Hum Genet. 1985;37:268–285. [PMC free article] [PubMed] [Google Scholar]
- 319.Gregg RE, Zech LA, Schaefer EJ, Brewer HB., Jr Apolipoprotein E metabolism in normolipoproteinemic human subjects. J Lipid Res. 1984;25:1167–1176. [PubMed] [Google Scholar]
- 320.Gregg RE, Zech LA, Schaefer EJ, Stark D, Wilson D, Brewer HB., Jr Abnormal in vivo metabolism of apolipoprotein E4 in humans. J Clin Invest. 1986;78:815–821. doi: 10.1172/JCI112645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Larson IA, Ordovas JM, DeLuca C, Barnard JR, Feussner G, Schaefer EJ. Association of apolipoprotein (Apo)E genotype with plasma apo E levels. Atherosclerosis. 2000;148:327–335. doi: 10.1016/s0021-9150(99)00280-4. [DOI] [PubMed] [Google Scholar]
- 322.Schiele F, De Bacquer D, Vincent-Viry M, et al. Apolipoprotein E serum concentration and polymorphism in six European countries: the ApoEurope Project. Atherosclerosis. 2000;152:475–488. doi: 10.1016/s0021-9150(99)00501-8. [DOI] [PubMed] [Google Scholar]
- 323.Dong LM, Parkin S, Trakhanov SD, et al. Novel mechanism for defective receptor binding of apolipoprotein E2 in type III hyperlipoproteinemia. Nat Struct Biol. 1996;3:718–722. doi: 10.1038/nsb0896-718. [DOI] [PubMed] [Google Scholar]