Synopsis
The contraction of hepatic stellate cells has been proposed to mediate fibrosis by regulating sinusoidal blood flow and extracellular matrix remodeling. Abundant data from diverse, yet complementary, experimental methods support a robust model for the regulation of contractile force generation by stellate cells. In this model, soluble factors associated with liver injury, including endothelin-1 and nitric oxide, are transduced primarily through rho signaling pathways that promote the myosin II-powered generation of contractile force by stellate cells. The enhanced knowledge of the role and differential regulation of stellate cell contraction may facilitate the discovery of new and targeted strategies for the prevention and treatment of hepatic fibrosis.
Keywords: hepatic stellate cell, contraction, fibrosis, pericyte, rho-associated kinase, sinusoid
Introduction
Contractile force generation by hepatic stellate cells is recognized to play a key role in the liver’s response to injury. This cellular behavior is consequently believed to contribute to normal healing as well as the development of hepatic fibrosis. Therefore, improved understanding of stellate cell contraction and its regulation would be predicted to facilitate development of clinical strategies for the treatment of liver disease. Despite more than 15 years of study, however, effective therapies based on targeting the generation of contractile force by stellate cells have remained elusive. This chapter will examine the current state of knowledge regarding stellate cell contraction, its role, its regulation, and its potential as a therapeutic target, by addressing three fundamental questions:
What do we believe we know?
What do we really know?
What would we like to know?
What do we believe we know about hepatic stellate cell contraction?
A number of observations in the human liver have led to the concept that stellate cells are contractile and that generation of contractile force by these cells mediates hepatic pathophysiology. Stellate cells express α-smooth muscle actin, a marker of non-muscle cell contractility, in patients with various forms of chronic liver injury [1-6]. Moreover, stellate cells of normal human livers do not express α-smooth muscle actin, suggesting that contractility may be induced by liver injury. Human stellate cells express various receptors for well-known contractile agonists. Studies have demonstrated stellate cell expression of receptors for endothelin-1, arginine-vasopressin, and angiotensin II, all of which induce generation of contractile force by contractile cell types [7-10]. The presence of these receptors suggests that stellate cells are capable of transducing chemical signals into changes in mechanical force. These observations indicate that stellate cells contain the cellular machinery necessary for generation of contractile force.
The anatomic location of stellate cells in the normal human liver also suggests an important role for contraction by these cells in the regulation of sinusoidal blood flow. Immunohistochemical studies of normal human liver show that stellate cells reside in the perisinusoidal space and extend elongate protrusions that run along and encircle one or more sinusoids [3, 4, 11]. This anatomy is similar to that of tissue pericytes, such as the mesangial cells of the kidney, which modulate vascular tone by contracting around their capillaries [12-14]. In the same way, hepatic stellate cells have been theorized to regulate sinusoidal resistance, and consequently blood flow, by contracting around sinusoids.
Studies of the injured human liver have demonstrated that stellate cells appear prominently in fibrotic bands of collagen (scar tissue) remote from their normal location [1-5, 11, 15]. This finding raised the possibility that stellate cells may function similar to cutaneous myofibroblasts that participate in wound healing of the skin by contracting scar tissue as healing and regeneration progresses [16-18]. Hence, it has been proposed that contractile force generation by stellate cells may permit remodeling of extracellular matrix during the liver’s injury response [19].
In summary, conventional wisdom holds that hepatic stellate cells are contractile, and their capacity to generate contractile force mediates the liver’s injury response through modulation of sinusoidal blood flow and scar contracture (Fig. 1). This current understanding of the role of stellate cell contraction is, however, largely based on circumstantial evidence and common logic. In the following section we will dissect the scientific methods that have been employed to study stellate cell contraction, as well as discuss the model (Fig. 2) for the regulation of stellate cell contraction that is best supported by the evidence.
Fig. 1.
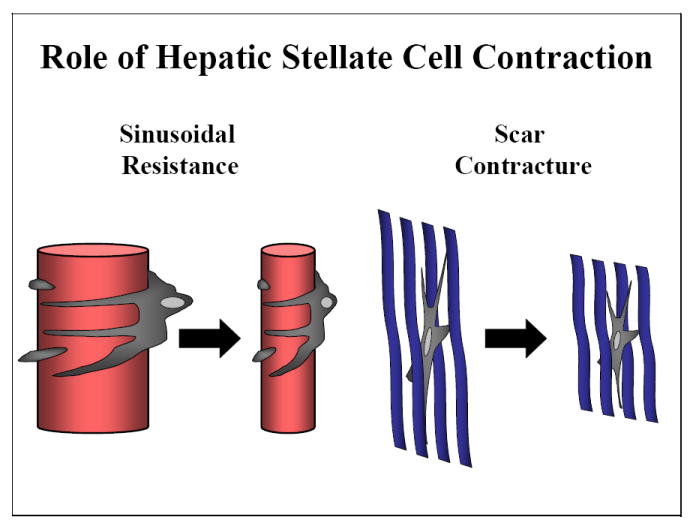
Role of hepatic stellate cell contraction. Stellate cell contractile force generation is thought to mediate the liver’s response to injury through constricting sinusoids and contracting scar.
Fig. 2.
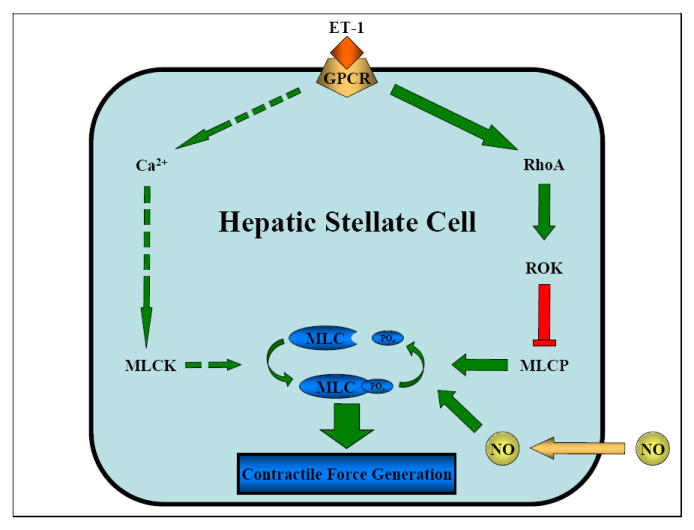
Regulation of hepatic stellate cell contraction. Soluble factors associated with liver injury, such as endothelin-1 and nitric oxide, are transduced primarily through rho signaling pathways that promote the myosin II-powered generation of contractile force by stellate cells. Dashed arrows indicate subordinate Ca2+ signaling pathway. ET-1, endothelin-1; GPCR, G protein coupled receptor; MLCK, myosin light chain kinase; RhoA, rho family GTPase; ROK, rho-associated kinase; MLCP, myosin light chain phosphatase; NO, nitric oxide; MLC, myosin light chain; PO4, phosphate.
What do we really know about hepatic stellate cell contraction?
Current understanding of hepatic stellate cell contraction has been advanced largely through use of in vitro experimental methods. Several methods have been employed to study stellate cell contraction, each with its own inherent limitations in spatial and temporal resolution, accuracy and precision, and fidelity to what occurs in vivo. Hence, to properly appreciate what we really know about stellate cell contraction it is worthwhile to appraise the strengths and limitations of the main assays used to study this important stellate cell behavior.
An early technique for the study of stellate cell contraction examined the decrease in two-dimensional cell surface area of cultured cells as a surrogate marker for generation of contractile force [20-23]. In this assay stellate cells in culture were grown to sub-confluence on a glass cover slip and visualized with transmission light video-microscopy. Reductions in stellate cell surface area could be measured in response to exposure to various agonists and inhibitors. Although this assay permitted quantitative measurements with subcellular spatial resolution and temporal resolution in seconds, changes in stellate cell surface area were not a direct gauge of contractile force. Indeed, shrinkage of stellate cell surface area could result from a number of non-contractile events, including changes in cellular adhesion or volume, three-dimensional shape changes, or even focusing artifacts, making it difficult to discern how accurate a measure of the contractile force generated by stellate cells this method provided.
Hepatic stellate cell contraction has also been evaluated using a model in which stellate cells in culture are grown in a monolayer on silicone rubber-coated coverslips [24-27]. In this assay the silicone rubber substrate, upon which stellate cells were grown, was visualized with transmission light microscopy and wrinkling of the substrate was examined. This method permitted semi-quantitative determination of substrate wrinkling, as a surrogate measure of the tension developed by the population of stellate cells across the silicone rubber, within seconds of exposure to various agonists and inhibitors. Increases in substrate wrinkling likely reflect gross changes in stellate cell contractile force generation, but the tightness of that correlation was unknown. Although determination of substrate wrinkling was subjective and imprecise, this assay did permit examination of the formation and loss of wrinkles, putative correlates of contractile force generation and relaxation, respectively.
Another method for examining stellate cell contraction employed a model in which stellate cells in culture were grown on top of or within gel lattices composed of type-1 collagen [27-35]. In this assay shrinkage of the collagen gels by the population of stellate cells was employed as a surrogate measure of cellular contraction. After release of the gel from a supporting culture dish and exposure to different agonists and inhibitors changes in gel diameter could be measured with a ruler after at least an over night incubation. This technique did not permit measurement of relaxation nor acute changes in contractile force development. In addition, reductions in gel area were not reversible. It is uncertain how closely changes in lattice area correlate with alterations in contractile force generation, and this model may not differentiate between active contraction and passive changes in stellate cell tension (e.g., passive tension across the lattice generated by cellular spreading or increases in stellate cell number).
High resolution intravital videomicroscopy of sinusoids within the isolated rat liver has been used as a model for the study of stellate cell contraction [36-39]. In this assay changes in sinusoidal diameter were visualized adjacent to stellate cell bodies demarcated by retinoid autofluorescence as a surrogate measure of contractile force generation by stellate cells encircling these vessels. These sinusoidal changes could be observed in real time within minutes of exposure to different agonists and inhibitors. By finely measuring and controlling input and output pressures across the major hepatic vessels and estimating sinusoidal blood flow, estimates of increases and decreases in sinusoidal resistance could be derived. This technique, however, did not permit direct measurement of contractile force generation by stellate cells, nor did it rule out the possibility that other cell types (e.g., co-localized endothelial cells or upstream or downstream vascular smooth muscle) or noncontractile events (e.g., cellular swelling) might contribute to the observed changes in sinusoidal diameter.
The contraction of hepatic stellate cells in culture has been directly and quantitatively measured [40-42]. In this assay stellate cells were grown within a three-dimensional type-1 collagen gel lattice, which was placed in an organ bath and attached to a sensitive force transducer. This model permitted real time measurement in actual force units of the contractile tension generated by stellate cells within the gel in response to various agonists and inhibitors. The method exhibited temporal resolution in the range of seconds, and it permitted precise quantification of both contraction and relaxation within the same sample. Although this technique allowed direct measurement of the contractile forces exerted by stellate cells populating a collagen gel, determination of the contractile force generated by a single stellate cell could only be estimated.
When evaluating the methods employed to study hepatic stellate cell contraction, a critical consideration is how closely the assay is likely to reflect what actually occurs within the human liver, in other words the fidelity of the experimental model. Despite the diverse experimental models and substantial efforts to better understand stellate cell contraction important factors limit what we really know about stellate cell contraction in vivo. Except for intravital microscopy, all of the methods used to study stellate cell contraction employed cells in culture. This raises the concern that isolated stellate cells do not function in an identical fashion to stellate cells in the liver. This is particularly true in studies that employed secondary cultures of stellate cells [7, 33, 41, 43]. Thus, it is generally recognized that the results of experiments performed using stellate cells in primary culture may have greater clinical relevance. Another point is that experiments performed on stellate cells in monolayer do not replicate the normal three-dimensional environment in which stellate cells reside in vivo. Therefore, studies of stellate cells grown within collagen gels more likely replicate the authentic milieu within the liver. However, intravital microscopy of hepatic sinusoids in intact liver, both in vivo and ex vivo [36-39], are most likely to be relevant to human pathophysiology. Despite the shared and unique limitations of each of the different methods used to study stellate cell contraction, together these assays complement each other and have contributed to a robust understanding of stellate cell contraction.
A number of chemicals have been demonstrated to stimulate stellate cell contraction, including endothelin-1, arginine-vasopressin, angiotensin-II, thrombin, eicosanoids, and α1-adrenergic agonists [9, 10, 20, 24, 35, 40-42]. The best-studied and most prominent agonist for stellate cell contraction is endothelin-1. Circulating levels of this peptide are elevated in patients with liver disease [7, 44, 45], and increased in animal models of liver injury [46, 47]. Endothelin-1 can induce markers of stellate cell contraction in every one of the assays discussed earlier [20, 25, 29, 36, 40]. In particular, the magnitude and speed of the contractile force generated by stellate cells in response to endothelin-1 has been predicted to be sufficient to regulate sinusoidal resistance to blood flow [40]. Even more significant, perfusion of isolated rodent livers with endothelin-1 caused a reduction in sinusoidal diameter colocalized with stellate cells that was paralleled by an increase in portal pressure [36, 48-51]. Moreover, administration of endothelin-1 receptor antagonists decreased portal pressure in portal hypertensive rats [52]. These experimental findings indicate that endothelin-1 is a potent agonist of stellate cell contraction and suggest an important contribution of this mediator to the regulation of hepatic blood flow.
Several agents, including nitric oxide, carbon monoxide, and prostaglandins, may counteract the effects of contraction-inducing stimuli by causing stellate cell relaxation [24, 25, 38, 53-55]. Nitric oxide production is reduced in the injured liver [56-58]. In vitro studies have suggested that activation of nitric oxide signaling (through nitric oxide donors or cytokine stimulation of nitric oxide production) causes relaxation in stellate cells and attenuates agonist-induced contraction [10, 25, 53, 56, 59, 60], a process that might occur through cGMP-dependent activation of myosin light chain phosphatase, similar to what has been demonstrated in smooth muscle cells [61-63]. Finally, nitric oxide donors can attenuate elevations in portal pressure in the perfused rodent liver induced by endothelin-1 or other contraction-inducing stimuli [36, 48, 64]. These observations have led to a proposed model in which sinusoidal tone is finely modulated by the net balance of agents that induce stellate cell relaxation, such as nitric oxide, and agonists of stellate cell contraction, such as endothelin-1 [65-67].
It has long been known that the motor protein complex, myosin II, powers contractile force generation in smooth muscle and fibroblasts through its action on the actin cytoskeleton [68, 69]. Numerous studies observed that hepatic stellate cells in culture express both myosin II [31, 41, 42, 70-73] and a fully formed actin cytoskeleton [31, 41-43, 70-74]. Myosin II activation, as assessed by myosin regulatory light chain phosphorylation, correlates with various surrogate measures of stellate cell contraction [31, 43, 71], as well as with the actual contractile force generated by stellate cells [41]. Moreover, antagonism of myosin phosphorylation inhibited contractile force generation by stellate cells [42]. Finally, the myosin regulatory light chain expressed by stellate cells is phosphorylated at serine 19 [73], the consensus activation site for myosin II. Taken together these results indicate that stellate cell contraction is powered by myosin II, which is activated by phosphorylation of its myosin regulatory light chain.
Evidence suggests that Ca2+ signaling pathways regulate stellate cell contraction by activating myosin light chain kinase, which selectively phosphorylates the myosin regulatory light chain [20, 75-77], similar to what has been demonstrated in smooth muscle. This notion was supported by several experimental observations. First, ligands including endothelin-1, thrombin, and angiotensin II, that induced transient increases in cytosolic Ca2+ concentration also stimulated stellate cell contraction [7, 10, 20, 25, 40, 41]. Second, plasma membrane Ca2+ channel expression, Ca2+ influx through these channels, and cytosolic Ca2+ concentration, each correlated with reductions in stellate cell surface area [23, 60, 77]. Third, inhibitors of Ca2+-dependent myosin light chain kinase attenuated the shrinkage of collagen gels populated with stellate cells [35, 43]. Although these findings suggested an important role for Ca2+ signaling in the control of stellate cell contraction, they did not provide any direct evidence to support this model.
In contrast to previously held views, current data indicate that Ca2+ signaling pathways play a subordinate role in the regulation of contractile force generation by stellate cells. The contribution of Ca2+ signaling pathways to the regulation of stellate cell contraction was directly tested by modulating cytosolic Ca2+ and directly measuring the contractile force generated by this cell type [42]. Increases in cytosolic Ca2+ induced by depolarizing the plasma membrane did not provoke contractile force generation. Superphysiological elevations in cytosolic Ca2+ triggered by a calcium ionophore induced minimal increases in contractile force. Eliminating increases in cytosolic Ca2+ with a calcium chelator had no effect on endothelin-1-induced contractile force generation. This study provided surprising evidence indicating that Ca2+ signaling is neither necessary for contractile force generation by stellate cells, nor is it sufficient to provoke stellate cell contraction. This fresh perspective was supported by the recent observation stellate cell contraction was stimulated by the inhibition of myosin phosphatase despite the absence of any changes in cytosolic Ca2+ concentration [35].
Over the past decade substantial data have emerged demonstrating that contractile force generation by certain non-muscle cell types, including fibroblasts and endothelial cells, is predominantly regulated by transduction pathways that signal through the ras-like GTPase, rhoA, rather than Ca2+ [69, 78-80]. Mounting evidence indicate that rho signaling pathways also control stellate cell contraction. Stellate cells express rhoA and rho-associated kinase [31, 70, 71]. Specific inhibition of rhoA caused derangement of the stellate cell actin cytoskeleton [70, 74]. Highly selective antagonism of the rhoA effector protein, rho-associated kinase, impeded shrinkage of collagen gels populated with stellate cells [31, 43, 71], inhibited myosin regulatory light chain phosphorylation [31, 41-43, 71, 73], and blocked contractile force generation by stellate cells [41, 42]. Attenuation of myosin phosphatase also reduced stellate cell contraction as assessed by the shrinkage of stellate cell-populated collagen gels [35]. In combination with studies of the contribution of Ca2+ signaling, these studies support a model in which contractile force generation by stellate cells is mediated primarily by rho signal transduction pathways.
With regards to the putative roles that stellate cell contraction may play in the pathophysiology of the liver, the strongest data pertains to their contribution to the modulation of sinusoidal blood flow. The concept that stellate cells modulate resistance to hepatic blood flow by contracting around sinusoids is supported by several observations. First, stellate cells in situ exhibit a pericyte-like morphology with protrusions encircling the sinusoids [81-83]. Second, the number and spacing of stellate cells and their characteristic protrusions overlay the entire sinusoidal network [84]. Third, ex vivo perfusion of the liver with endothelin-1 induced reductions in sinusoidal caliber colocalized with stellate cells [36, 37, 85]. Fourth, direct measurement of contractile force generation by stellate cells within collagen gels suggests that the magnitude and rate of stellate cell contraction and relaxation are capable of modulating blood flow via sinusoidal constriction [40]. Taken together these findings obtained from several complementary methods indicate that stellate cells contribute to the regulation of sinusoidal blood flow.
What would we like to know about hepatic stellate cell contraction?
What we would really like to know about how the emerging pathobiology of stellate cell contraction can be used to develop new strategies for the prevention and treatment of hepatic fibrosis in humans. Despite fifteen years of intensive investigation and a great deal of new information about the role and regulation of stellate cell contraction, no effective stellate cell contraction-targeted therapies for hepatic fibrosis have been validated. In fact, no fibrosis directed treatments of any sort have yet been developed for the treatment of chronic liver disease [86-89]. As discussed in other chapters of this monograph, the only proven therapies for fibrosis so far are directed at the prevention or removal of a specific cause of chronic hepatic injury, such as treatment of hepatitis C or biliary obstruction.
Two logical approaches for developing new treatments for hepatic fibrosis are (1) to destroy stellate cells or disable their function and (2) to modulate specific molecular targets within key signal transduction pathways used by stellate cells. There are, however, serious real and theoretical challenges to these general therapeutic approaches. Stellate cells mediate the response of the liver to both acute and chronic injury. Hence, they are believed to contribute to both the normal wound healing process, as well as to the development of hepatic fibrosis and subsequent cirrhosis. If this is accurate, then destruction or disabling of stellate cells could impair healthy and essential responses of the liver to injury in addition to the anticipated prevention or attenuation of fibrosis. One solution to the paradox that the presence of intact stellate cells may be necessary for both normal wound healing and fibrogenesis might be to selectively target influential signaling pathways used by stellate cells. The problem with this therapeutic strategy is that important signaling pathways are generally shared by amongst different cell types. For example, employment of a mitogen-activated protein kinase antagonist to inhibit stellate cell proliferation would also be predicted to influence the proliferation of hepatocytes, and numerous other cell types. Therefore, it would be especially advantageous to identify and develop treatment strategies precisely and specifically targeted to stellate cell behaviors that mediate hepatic fibrosis.
The contractile force exerted by stellate cells contributes to the regulation of sinusoidal blood flow and the development of fibrosis. As discussed, emerging evidence indicates that generation of contractile force by stellate cells may be differentially regulated by transduction pathways that signal through rho and rho-associated kinase, rather than Ca2+ and myosin light chain kinase as it is in vascular smooth muscle. This differential regulation of stellate cell contraction offers the possibility that novel therapeutic strategies could be developed that selectively target the generation of contractile force by stellate cells. In fact, commercially available highly selective small molecule inhibitors of rho-associated kinase attenuate the increases in intrahepatic vascular resistance and portal hypertension [35, 71, 90, 91] and lessen the development of hepatic fibrosis [91-94] in diverse rodent models of liver injury. These studies provide proof-of-principle that stellate cell contraction can be selectively targeted to treat hepatic fibrosis, at least in rodent models of chronic liver injury.
A significant concern with targeting rho signal transduction pathways as a strategy for treating hepatic fibrosis is that this pathway is ubiquitous in playing vital roles in diverse cell types throughout the body. This concern could be circumvented by delivering inhibitors directly to the liver or stellate cells. Possible methods for directed delivery include portal or hepatic venous injection, coupling drugs to carriers (e.g., antibodies, peptides, lectins, or lipids) with affinity for the liver or stellate cells, and the use of particular viruses to selectively deliver therapeutic genes or ribonucleic acids [71, 95-99] to stellate cells or the liver. By integrating new technologies for liver-directed delivery with pharmaceutical or genetic agents that selectively target stellate cell contraction it may be possible to develop effective strategies for the prevention and treatment of hepatic fibrosis.
Summary
The contractile force generated by stellate cells within the liver may contribute to the development of hepatic fibrosis by modulating sinusoidal blood flow and participating in extracellular matrix remodeling. For more than fifteen years, the role and regulation of stellate cell contraction have been areas of substantial research. Diverse, but complementary, experimental methods have been employed to elucidate the pathophysiology of stellate cell contraction. Although each technique for studying the contraction of stellate cells has its own limitations, taken together the published studies have provided a robust model for the regulation of stellate cell contractile force generation. In this model, soluble factors associated with liver injury, including endothelin-1 and nitric oxide, are transduced primarily through rho signaling pathways that promote the myosin II-powered generation of contractile force by stellate cells. Moreover, compelling data support a role for stellate cells in the control of hepatic blood flow by contracting around sinusoids. Our enhanced understanding of the role and differential regulation of stellate cell contraction may facilitate the discovery of new and targeted strategies for the prevention and treatment of hepatic fibrosis.
Acknowledgments
This work was supported in part by NIH, R01 DK61532 and the Technical Training Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schmitt-Graff A, Kruger S, Bochard F, et al. Modulation of alpha smooth muscle actin and desmin expression in perisinusoidal cells of normal and diseased human livers. Am J Pathol. 1991;138(5):1233–42. [PMC free article] [PubMed] [Google Scholar]
- 2.Nouchi T, Tanaka Y, Tsukada T, et al. Appearance of alpha-smooth-muscle-actin-positive cells in hepatic fibrosis. Liver. 1991;11(2):100–5. doi: 10.1111/j.1600-0676.1991.tb00499.x. [DOI] [PubMed] [Google Scholar]
- 3.Yamaoka K, Nouchi T, Marumo F, et al. Alpha-smooth-muscle actin expression in normal and fibrotic human livers. Dig Dis Sci. 1993;38(8):1473–9. doi: 10.1007/BF01308606. [DOI] [PubMed] [Google Scholar]
- 4.Enzan H, Himeno H, Iwamura S, et al. Immunohistochemical identification of Ito cells and their myofibroblastic transformation in adult human liver. Virchows Arch. 1994;424(3):249–56. doi: 10.1007/BF00194608. [DOI] [PubMed] [Google Scholar]
- 5.Enzan H, Himeno H, Iwamura S, et al. Sequential changes in human Ito cells and their relation to postnecrotic liver fibrosis in massive and submassive hepatic necrosis. Virchows Arch. 1995;426(1):95–101. doi: 10.1007/BF00194703. [DOI] [PubMed] [Google Scholar]
- 6.Baroni GS, Pastorelli A, Manzin A, et al. Hepatic stellate cell activation and liver fibrosis are associated with necroinflammatory injury and Th1-like response in chronic hepatitis C. Liver. 1999;19(3):212–9. doi: 10.1111/j.1478-3231.1999.tb00038.x. [DOI] [PubMed] [Google Scholar]
- 7.Pinzani M, Milani S, De Franco R, et al. Endothelin 1 is overexpressed in human cirrhotic liver and exerts multiple effects on activated hepatic stellate cells. Gastroenterology. 1996;110(2):534–48. doi: 10.1053/gast.1996.v110.pm8566602. [DOI] [PubMed] [Google Scholar]
- 8.Yokomori H, Oda M, Yasogawa Y, et al. Enhanced expression of endothelin B receptor at protein and gene levels in human cirrhotic liver. Am J Pathol. 2001;159(4):1353–62. doi: 10.1016/S0002-9440(10)62522-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bataller R, Nicolas JM, Gines P, et al. Arginine vasopressin induces contraction and stimulates growth of cultured human hepatic stellate cells. Gastroenterology. 1997;113(2):615–24. doi: 10.1053/gast.1997.v113.pm9247484. [DOI] [PubMed] [Google Scholar]
- 10.Bataller R, Gines P, Nicolas JM, et al. Angiotensin II induces contraction and proliferation of human hepatic stellate cells. Gastroenterology. 2000;118(6):1149–56. doi: 10.1016/s0016-5085(00)70368-4. [DOI] [PubMed] [Google Scholar]
- 11.Hautekeete ML, Geerts A. The hepatic stellate (Ito) cell: its role in human liver disease. Virchows Arch. 1997;430(3):195–207. doi: 10.1007/BF01324802. [DOI] [PubMed] [Google Scholar]
- 12.Sims DE. The pericyte--a review. Tissue Cell. 1986;18(2):153–74. doi: 10.1016/0040-8166(86)90026-1. [DOI] [PubMed] [Google Scholar]
- 13.Sims DE. Recent advances in pericyte biology--implications for health and disease. Can J Cardiol. 1991;7(10):431–43. [PubMed] [Google Scholar]
- 14.Pallone TL, Silldorff EP. Pericyte regulation of renal medullary blood flow. Exp Nephrol. 2001;9(3):165–70. doi: 10.1159/000052608. [DOI] [PubMed] [Google Scholar]
- 15.Novo E, Cannito S, Zamara E, et al. Proangiogenic cytokines as hypoxia-dependent factors stimulating migration of human hepatic stellate cells. Am J Pathol. 2007;170(6):1942–53. doi: 10.2353/ajpath.2007.060887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997;276(5309):75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 17.Nedelec B, Ghahary A, Scott PG, et al. Control of wound contraction. Basic and clinical features. Hand Clin. 2000;16(2):289–302. [PubMed] [Google Scholar]
- 18.Darby IA, Hewitson TD. Fibroblast differentiation in wound healing and fibrosis. Int Rev Cytol. 2007;257:143–79. doi: 10.1016/S0074-7696(07)57004-X. [DOI] [PubMed] [Google Scholar]
- 19.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88(1):125–72. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinzani M, Failli P, Ruocco C, et al. Fat-storing cells as liver-specific pericytes. Spatial dynamics of agonist-stimulated intracellular calcium transients. J Clin Invest. 1992;90(2):642–6. doi: 10.1172/JCI115905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakamoto M, Ueno T, Kin M, et al. Ito cell contraction in response to endothelin-1 and substance P. Hepatology. 1993;18(4):978–83. doi: 10.1002/hep.1840180432. [DOI] [PubMed] [Google Scholar]
- 22.Tao J, Mallat A, Gallois C, et al. Biological effects of C-type natriuretic peptide in human myofibroblastic hepatic stellate cells. J Biol Chem. 1999;274(34):23761–9. doi: 10.1074/jbc.274.34.23761. [DOI] [PubMed] [Google Scholar]
- 23.Bataller R, Gasull X, Gines P, et al. In vitro and in vivo activation of rat hepatic stellate cells results in de novo expression of L-type voltage-operated calcium channels. Hepatology. 2001;33(4):956–62. doi: 10.1053/jhep.2001.23500. [DOI] [PubMed] [Google Scholar]
- 24.Kawada N, Klein H, Decker K. Eicosanoid-mediated contractility of hepatic stellate cells. Biochem J. 1992;285(Pt 2):367–71. doi: 10.1042/bj2850367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawada N, Tran-Thi TA, Klein H, et al. The contraction of hepatic stellate (Ito) cells stimulated with vasoactive substances. Possible involvement of endothelin 1 and nitric oxide in the regulation of the sinusoidal tonus. Eur J Biochem. 1993;213(2):815–23. doi: 10.1111/j.1432-1033.1993.tb17824.x. [DOI] [PubMed] [Google Scholar]
- 26.Kawada N, Inoue M. Effect of adrenomedullin on hepatic pericytes (stellate cells) of the rat. FEBS Lett. 1994;356(1):109–13. doi: 10.1016/0014-5793(94)01178-8. [DOI] [PubMed] [Google Scholar]
- 27.Kawada N, Kuroki T, Kobayashi K, et al. Action of endothelins on hepatic stellate cells. J Gastroenterol. 1995;30(6):731–8. doi: 10.1007/BF02349639. [DOI] [PubMed] [Google Scholar]
- 28.Housset C, Rockey DC, Bissell DM. Endothelin receptors in rat liver: lipocytes as a contractile target for endothelin 1. Proc Natl Acad Sci U S A. 1993;90(20):9266–70. doi: 10.1073/pnas.90.20.9266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rockey DC, Housset CN, Friedman SL. Activation-dependent contractility of rat hepatic lipocytes in culture and in vivo. J Clin Invest. 1993;92(4):1795–804. doi: 10.1172/JCI116769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mashiba S, Mochida S, Ishikawa K, et al. Inhibition of hepatic stellate cell contraction during activation in vitro by vascular endothelial growth factor in association with upregulation of FLT tyrosine kinase receptor family, FLT-1. Biochem Biophys Res Commun. 1999;258(3):674–8. doi: 10.1006/bbrc.1999.0611. [DOI] [PubMed] [Google Scholar]
- 31.Yanase M, Ikeda H, Matsui A, et al. Lysophosphatidic acid enhances collagen gel contraction by hepatic stellate cells: association with rho-kinase. Biochem Biophys Res Commun. 2000;277(1):72–8. doi: 10.1006/bbrc.2000.3634. [DOI] [PubMed] [Google Scholar]
- 32.Reynaert H, Vaeyens F, Qin H, et al. Somatostatin suppresses endothelin-1-induced rat hepatic stellate cell contraction via somatostatin receptor subtype 1. Gastroenterology. 2001;121(4):915–30. doi: 10.1053/gast.2001.27971. [DOI] [PubMed] [Google Scholar]
- 33.Kharbanda KK, Rogers DD, 2nd, Wyatt TA, et al. Transforming growth factor-beta induces contraction of activated hepatic stellate cells. J Hepatol. 2004;41(1):60–6. doi: 10.1016/j.jhep.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 34.Perri RE, Langer DA, Chatterjee S, et al. Defects in cGMP-PKG pathway contribute to impaired NO-dependent responses in hepatic stellate cells upon activation. Am J Physiol Gastrointest Liver Physiol. 2006;290(3):G535–42. doi: 10.1152/ajpgi.00297.2005. [DOI] [PubMed] [Google Scholar]
- 35.Laleman W, Van Landeghem L, Severi T, et al. Both Ca2+ -dependent and -independent pathways are involved in rat hepatic stellate cell contraction and intrahepatic hyperresponsiveness to methoxamine. Am J Physiol Gastrointest Liver Physiol. 2007;292(2):G556–64. doi: 10.1152/ajpgi.00196.2006. [DOI] [PubMed] [Google Scholar]
- 36.Zhang JX, Pegoli W, Jr, Clemens MG. Endothelin-1 induces direct constriction of hepatic sinusoids. Am J Physiol. 1994;266(4 Pt 1):G624–32. doi: 10.1152/ajpgi.1994.266.4.G624. [DOI] [PubMed] [Google Scholar]
- 37.Bauer M, Paquette NC, Zhang JX, et al. Chronic ethanol consumption increases hepatic sinusoidal contractile response to endothelin-1 in the rat. Hepatology. 1995;22(5):1565–76. [PubMed] [Google Scholar]
- 38.Suematsu M, Goda N, Sano T, et al. Carbon monoxide: an endogenous modulator of sinusoidal tone in the perfused rat liver. J Clin Invest. 1995;96(5):2431–7. doi: 10.1172/JCI118300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vanheule E, Geerts AM, Reynaert H, et al. Influence of somatostatin and octreotide on liver microcirculation in an experimental mouse model of cirrhosis studied by intravital fluorescence microscopy. Liver Int. 2008;28(1):107–16. doi: 10.1111/j.1478-3231.2007.01629.x. [DOI] [PubMed] [Google Scholar]
- 40.Thimgan MS, Yee HF., Jr Quantitation of rat hepatic stellate cell contraction: stellate cells’ contribution to sinusoidal resistance. Am J Physiol. 1999;277(1 Pt 1):G137–43. doi: 10.1152/ajpgi.1999.277.1.G137. [DOI] [PubMed] [Google Scholar]
- 41.Saab S, Tam SP, Tran BN, et al. Myosin mediates contractile force generation by hepatic stellate cells in response to endothelin-1. J Biomed Sci. 2002;9(6 Pt 2):607–12. doi: 10.1159/000067289. [DOI] [PubMed] [Google Scholar]
- 42.Melton AC, Datta A, Yee HF., Jr Ca2+]i-independent contractile force generation by rat hepatic stellate cells in response to endothelin-1. Am J Physiol Gastrointest Liver Physiol. 2006;290(1):G7–13. doi: 10.1152/ajpgi.00337.2005. [DOI] [PubMed] [Google Scholar]
- 43.Yanase M, Ikeda H, Ogata I, et al. Functional diversity between Rho-kinase- and MLCK-mediated cytoskeletal actions in a myofibroblast-like hepatic stellate cell line. Biochem Biophys Res Commun. 2003;305(2):223–8. doi: 10.1016/s0006-291x(03)00726-5. [DOI] [PubMed] [Google Scholar]
- 44.Moller S, Emmeluth C, Henriksen JH. Elevated circulating plasma endothelin-1 concentrations in cirrhosis. J Hepatol. 1993;19(2):285–90. doi: 10.1016/s0168-8278(05)80584-7. [DOI] [PubMed] [Google Scholar]
- 45.Alam I, Bass NM, Bacchetti P, et al. Hepatic tissue endothelin-1 levels in chronic liver disease correlate with disease severity and ascites. Am J Gastroenterol. 2000;95(1):199–203. doi: 10.1111/j.1572-0241.2000.01684.x. [DOI] [PubMed] [Google Scholar]
- 46.Gandhi CR, Sproat LA, Subbotin VM. Increased hepatic endothelin-1 levels and endothelin receptor density in cirrhotic rats. Life Sci. 1996;58(1):55–62. doi: 10.1016/0024-3205(95)02255-4. [DOI] [PubMed] [Google Scholar]
- 47.Rockey DC, Fouassier L, Chung JJ, et al. Cellular localization of endothelin-1 and increased production in liver injury in the rat: potential for autocrine and paracrine effects on stellate cells. Hepatology. 1998;27(2):472–80. doi: 10.1002/hep.510270222. [DOI] [PubMed] [Google Scholar]
- 48.Tran-Thi TA, Kawada N, Decker K. Regulation of endothelin-1 action on the perfused rat liver. FEBS Lett. 1993;318(3):353–7. doi: 10.1016/0014-5793(93)80544-5. [DOI] [PubMed] [Google Scholar]
- 49.Okumura S, Takei Y, Kawano S, et al. Vasoactive effect of endothelin-1 on rat liver in vivo. Hepatology. 1994;19(1):155–61. doi: 10.1016/0270-9139(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 50.Rockey DC, Weisiger RA. Endothelin induced contractility of stellate cells from normal and cirrhotic rat liver: implications for regulation of portal pressure and resistance. Hepatology. 1996;24(1):233–40. doi: 10.1002/hep.510240137. [DOI] [PubMed] [Google Scholar]
- 51.Kaneda K, Ekataksin W, Sogawa M, et al. Endothelin-1-induced vasoconstriction causes a significant increase in portal pressure of rat liver: localized constrictive effect on the distal segment of preterminal portal venules as revealed by light and electron microscopy and serial reconstruction. Hepatology. 1998;27(3):735–47. doi: 10.1002/hep.510270315. [DOI] [PubMed] [Google Scholar]
- 52.De Gottardi A, Shaw S, Sagesser H, et al. Type A, but not type B, endothelin receptor antagonists significantly decrease portal pressure in portal hypertensive rats. J Hepatol. 2000;33(5):733–7. doi: 10.1016/s0168-8278(00)80303-7. [DOI] [PubMed] [Google Scholar]
- 53.Rockey DC, Chung JJ. Inducible nitric oxide synthase in rat hepatic lipocytes and the effect of nitric oxide on lipocyte contractility. J Clin Invest. 1995;95(3):1199–206. doi: 10.1172/JCI117769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goda N, Suzuki K, Naito M, et al. Distribution of heme oxygenase isoforms in rat liver. Topographic basis for carbon monoxide-mediated microvascular relaxation. J Clin Invest. 1998;101(3):604–12. doi: 10.1172/JCI1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang XE, Watanabe S, Oide H, et al. Hepatic stellate cell contraction is inhibited by lipo-prostaglandin E1 in vitro. J Gastroenterol Hepatol. 1998;13 Suppl:S14–8. [PubMed] [Google Scholar]
- 56.Rockey DC, Chung JJ. Reduced nitric oxide production by endothelial cells in cirrhotic rat liver: endothelial dysfunction in portal hypertension. Gastroenterology. 1998;114(2):344–51. doi: 10.1016/s0016-5085(98)70487-1. [DOI] [PubMed] [Google Scholar]
- 57.Sarela AI, Mihaimeed FM, Batten JJ, et al. Hepatic and splanchnic nitric oxide activity in patients with cirrhosis. Gut. 1999;44(5):749–53. doi: 10.1136/gut.44.5.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu Q, Shao R, Qian HS, et al. Gene transfer of the neuronal NO synthase isoform to cirrhotic rat liver ameliorates portal hypertension. J Clin Invest. 2000;105(6):741–8. doi: 10.1172/JCI7997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sakamoto M, Ueno T, Sugawara H, et al. Relaxing effect of interleukin-1 on rat cultured Ito cells. Hepatology. 1997;25(6):1412–7. doi: 10.1002/hep.510250618. [DOI] [PubMed] [Google Scholar]
- 60.Gasull X, Bataller R, Gines P, et al. Human myofibroblastic hepatic stellate cells express Ca(2+)-activated K(+) channels that modulate the effects of endothelin-1 and nitric oxide. J Hepatol. 2001;35(6):739–48. doi: 10.1016/s0168-8278(01)00198-2. [DOI] [PubMed] [Google Scholar]
- 61.Surks HK, Mochizuki N, Kasai Y, et al. Regulation of myosin phosphatase by a specific interaction with cGMP- dependent protein kinase Ialpha. Science. 1999;286(5444):1583–7. doi: 10.1126/science.286.5444.1583. [DOI] [PubMed] [Google Scholar]
- 62.Etter EF, Eto M, Wardle RL, et al. Activation of myosin light chain phosphatase in intact arterial smooth muscle during nitric oxide-induced relaxation. J Biol Chem. 2001;276(37):34681–5. doi: 10.1074/jbc.M104737200. [DOI] [PubMed] [Google Scholar]
- 63.Nakamura K, Koga Y, Sakai H, et al. cGMP-dependent relaxation of smooth muscle is coupled with the change in the phosphorylation of myosin phosphatase. Circ Res. 2007;101(7):712–22. doi: 10.1161/CIRCRESAHA.107.153981. [DOI] [PubMed] [Google Scholar]
- 64.Laleman W, Van Landeghem L, Van der Elst I, et al. Nitroflurbiprofen, a nitric oxide-releasing cyclooxygenase inhibitor, improves cirrhotic portal hypertension in rats. Gastroenterology. 2007;132(2):709–19. doi: 10.1053/j.gastro.2006.12.041. [DOI] [PubMed] [Google Scholar]
- 65.Racine-Samson L, Rockey DC, Bissell DM. The role of alpha1beta1 integrin in wound contraction. A quantitative analysis of liver myofibroblasts in vivo and in primary culture. J Biol Chem. 1997;272(49):30911–7. doi: 10.1074/jbc.272.49.30911. [DOI] [PubMed] [Google Scholar]
- 66.Rockey DC. New concepts in the pathogenesis of portal hypertension: hepatic wounding and stellate cell contractility. Clin Liver Dis. 1997;1(1):13–29. doi: 10.1016/s1089-3261(05)70252-x. [DOI] [PubMed] [Google Scholar]
- 67.Rockey DC. Cellular pathophysiology of portal hypertension and prospects for management with gene therapy. Clin Liver Dis. 2001;5(3):851–65. doi: 10.1016/s1089-3261(05)70195-1. [DOI] [PubMed] [Google Scholar]
- 68.Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994;372(6503):231–6. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- 69.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83(4):1325–58. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 70.Yee HF., Jr Rho directs activation-associated changes in rat hepatic stellate cell morphology via regulation of the actin cytoskeleton. Hepatology. 1998;28(3):843–50. doi: 10.1002/hep.510280336. [DOI] [PubMed] [Google Scholar]
- 71.Kawada N, Seki S, Kuroki T, et al. ROCK inhibitor Y-27632 attenuates stellate cell contraction and portal pressure increase induced by endothelin-1. Biochem Biophys Res Commun. 1999;266(2):296–300. doi: 10.1006/bbrc.1999.1823. [DOI] [PubMed] [Google Scholar]
- 72.Tangkijvanich P, Tam SP, Yee HF., Jr Wound-induced migration of rat hepatic stellate cells is modulated by endothelin-1 through rho-kinase-mediated alterations in the acto-myosin cytoskeleton. Hepatology. 2001;33(1):74–80. doi: 10.1053/jhep.2001.20677. [DOI] [PubMed] [Google Scholar]
- 73.Melton AC, Yee HF. Hepatic stellate cell protrusions couple platelet-derived growth factor-BB to chemotaxis. Hepatology. 2007;45(6):1446–53. doi: 10.1002/hep.21606. [DOI] [PubMed] [Google Scholar]
- 74.Kato M, Iwamoto H, Higashi N, et al. Role of Rho small GTP binding protein in the regulation of actin cytoskeleton in hepatic stellate cells. J Hepatol. 1999;31(1):91–9. doi: 10.1016/s0168-8278(99)80168-8. [DOI] [PubMed] [Google Scholar]
- 75.Yee HF., Jr Ca2+ and rho signaling pathways: two paths to hepatic stellate cell contraction. Hepatology. 2001;33(4):1007–8. doi: 10.1053/jhep.2001.23901. [DOI] [PubMed] [Google Scholar]
- 76.Reynaert H, Thompson MG, Thomas T, et al. Hepatic stellate cells: role in microcirculation and pathophysiology of portal hypertension. Gut. 2002;50(4):571–81. doi: 10.1136/gut.50.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bataller R, Nicolas JM, Ginees P, et al. Contraction of human hepatic stellate cells activated in culture: a role for voltage-operated calcium channels. J Hepatol. 1998;29(3):398–408. doi: 10.1016/s0168-8278(98)80057-3. [DOI] [PubMed] [Google Scholar]
- 78.Kolodney MS, Thimgan MS, Honda HM, et al. Ca2+-independent myosin II phosphorylation and contraction in chicken embryo fibroblasts. J Physiol. 1999;515(Pt 1):87–92. doi: 10.1111/j.1469-7793.1999.087ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Parizi M, Howard EW, Tomasek JJ. Regulation of LPA-promoted myofibroblast contraction: role of Rho, myosin light chain kinase, and myosin light chain phosphatase. Exp Cell Res. 2000;254(2):210–20. doi: 10.1006/excr.1999.4754. [DOI] [PubMed] [Google Scholar]
- 80.Yee HF, Jr, Melton AC, Tran BN. RhoA/rho-associated kinase mediates fibroblast contractile force generation. Biochem Biophys Res Commun. 2001;280(5):1340–5. doi: 10.1006/bbrc.2001.4291. [DOI] [PubMed] [Google Scholar]
- 81.Blomhoff R, Wake K. Perisinusoidal stellate cells of the liver: important roles in retinol metabolism and fibrosis. FASEB J. 1991;5(3):271–7. doi: 10.1096/fasebj.5.3.2001786. [DOI] [PubMed] [Google Scholar]
- 82.Ekataksin W, Kaneda K. Liver microvascular architecture: an insight into the pathophysiology of portal hypertension. Semin Liver Dis. 1999;19(4):359–82. doi: 10.1055/s-2007-1007126. [DOI] [PubMed] [Google Scholar]
- 83.Oikawa H, Masuda T, Kawaguchi J, et al. Three-dimensional examination of hepatic stellate cells in rat liver and response to endothelin-1 using confocal laser scanning microscopy. J Gastroenterol Hepatol. 2002;17(8):861–72. doi: 10.1046/j.1440-1746.2002.02831.x. [DOI] [PubMed] [Google Scholar]
- 84.Wake K. In: Liver Perivascular Cells Revealed by Gold and Silver Impregnation Methods and Electron Microscopy. Motta P, editor. Dordrecht, Netherlands: Kluwer; 2001. pp. 23–6. [Google Scholar]
- 85.Zhang JX, Bauer M, Clemens MG. Vessel- and target cell-specific actions of endothelin-1 and endothelin-3 in rat liver. Am J Physiol. 1995;269(2 Pt 1):G269–77. doi: 10.1152/ajpgi.1995.269.2.G269. [DOI] [PubMed] [Google Scholar]
- 86.Tangkijvanich P, Yee HF., Jr Cirrhosis--can we reverse hepatic fibrosis? Eur J Surg Suppl. 2002;(587):100–12. [PubMed] [Google Scholar]
- 87.Murphy F, Arthur M, Iredale J. Developing strategies for liver fibrosis treatment. Expert Opin Investig Drugs. 2002;11(11):1575–85. doi: 10.1517/13543784.11.11.1575. [DOI] [PubMed] [Google Scholar]
- 88.Rockey DC. Hepatic fibrosis, stellate cells, and portal hypertension. Clin Liver Dis. 2006;10(3):459–79. vii–viii. doi: 10.1016/j.cld.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 89.Friedman SL, Bansal MB. Reversal of hepatic fibrosis -- fact or fantasy? Hepatology. 2006;43(2 Suppl 1):S82–8. doi: 10.1002/hep.20974. [DOI] [PubMed] [Google Scholar]
- 90.Zhou Q, Hennenberg M, Trebicka J, et al. Intrahepatic upregulation of RhoA and Rho-kinase signalling contributes to increased hepatic vascular resistance in rats with secondary biliary cirrhosis. Gut. 2006;55(9):1296–305. doi: 10.1136/gut.2005.081059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kitamura K, Tada S, Nakamoto N, et al. Rho/Rho kinase is a key enzyme system involved in the angiotensin II signaling pathway of liver fibrosis and steatosis. J Gastroenterol Hepatol. 2007;22(11):2022–33. doi: 10.1111/j.1440-1746.2006.04735.x. [DOI] [PubMed] [Google Scholar]
- 92.Tada S, Iwamoto H, Nakamuta M, et al. A selective ROCK inhibitor, Y27632, prevents dimethylnitrosamine-induced hepatic fibrosis in rats. J Hepatol. 2001;34(4):529–36. doi: 10.1016/s0168-8278(00)00059-3. [DOI] [PubMed] [Google Scholar]
- 93.Murata T, Arii S, Nakamura T, et al. Inhibitory effect of Y-27632, a ROCK inhibitor, on progression of rat liver fibrosis in association with inactivation of hepatic stellate cells. J Hepatol. 2001;35(4):474–81. doi: 10.1016/s0168-8278(01)00169-6. [DOI] [PubMed] [Google Scholar]
- 94.Murata T, Arii S, Mori A, et al. Therapeutic significance of Y-27632, a Rho-kinase inhibitor, on the established liver fibrosis. J Surg Res. 2003;114(1):64–71. doi: 10.1016/s0022-4804(03)00202-6. [DOI] [PubMed] [Google Scholar]
- 95.Beljaars L, Molema G, Schuppan D, et al. Successful targeting to rat hepatic stellate cells using albumin modified with cyclic peptides that recognize the collagen type VI receptor. J Biol Chem. 2000;275(17):12743–51. doi: 10.1074/jbc.275.17.12743. [DOI] [PubMed] [Google Scholar]
- 96.Gonzalo T, Beljaars L, van de Bovenkamp M, et al. Local inhibition of liver fibrosis by specific delivery of a platelet-derived growth factor kinase inhibitor to hepatic stellate cells. J Pharmacol Exp Ther. 2007;321(3):856–65. doi: 10.1124/jpet.106.114496. [DOI] [PubMed] [Google Scholar]
- 97.Kinoshita K, Iimuro Y, Fujimoto J, et al. Targeted and regulable expression of transgenes in hepatic stellate cells and myofibroblasts in culture and in vivo using an adenoviral Cre/loxP system to antagonise hepatic fibrosis. Gut. 2007;56(3):396–404. doi: 10.1136/gut.2005.085704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schoemaker MH, Rots MG, Beljaars L, et al. PDGF-Receptor beta-Targeted Adenovirus Redirects Gene Transfer from Hepatocytes to Activated Stellate Cells. Mol Pharm. 2008 doi: 10.1021/mp700118p. [DOI] [PubMed] [Google Scholar]
- 99.Sato Y, Murase K, Kato J, et al. Resolution of liver cirrhosis using vitamin A-coupled liposomes to deliver siRNA against a collagen-specific chaperone. Nat Biotechnol. 2008;26:431–42. doi: 10.1038/nbt1396. [DOI] [PubMed] [Google Scholar]


