Abstract
Aim
Depressive symptoms are common in the early prodromal phase of schizophrenia and other psychotic disorders. The objectives of the present study were to retrospectively examine the severity of depressive symptoms and their relationship to positive symptoms over the developmental course of adolescent-onset psychosis (AO-PSY).
Methods
The subjects were 62 unmedicated adolescents with DSM-IV psychosis and 104 normal controls from a Pacific island isolate with an elevated prevalence of schizophrenia. We used a modified K-SADS-PL to assess adolescents for a full range of Axis I psychopathology and quantified severity of depressive and positive symptoms over the adolescent’s lifespan.
Results
Among AO-PSY subjects, 84% reported abnormal levels of depressive symptoms with mean onset 1.3 years prior to transition to psychosis. In 60% of the AO-PSY subjects with depressive symptoms, positive symptoms began first. A continuous linear increase in depressive symptom severity over the developmental course of illness mirrored the steady rise in positive symptom severity as psychosis emerged.
Conclusions
We found that it is typically a combination of positive symptoms and depressive symptoms building in parallel that leads from the prodrome to frank psychosis. These results suggest that depressive symptoms represent more of an integral component of disease progression than an independent risk factor that predicts transition to early onset psychosis.
Keywords: depression, high risk adolescents, isolated population, psychosis, schizophrenia
INTRODUCTION
Depression is a common comorbid disorder in schizophrenia.1,2 In fact, studies of the dimensional structure of schizophrenia symptoms have shown that depression is a major component of the symptom profile, representing a fourth factor that is distinct from positive symptoms, negative symptoms and symptoms of disorganization.3–7 The comorbidity of psychotic symptoms and depression often complicates diagnosis, particularly during the early ‘prodromal’ phase in the developmental course of schizophrenia when symptoms are relatively non-specific.8,9
Prodromal schizophrenia was initially studied retrospectively in first episode patients (FEPs) using assessment instruments covering a broad range of both specific and non-specific symptoms. Yung and McGorry’s10 review of these studies identified the five most commonly experienced prodromal symptoms as reduced concentration/attention, reduced motivation/drive, depressed mood, sleep disturbances and anxiety. More recent retrospective studies of FEPs have confirmed that the earliest symptoms of the prepsychotic phase are relatively non-specific psychological disturbances reflecting a dysphoric state.
Hafner et al.8,11,12 conducted a study of 130 FEPs using the Instrument for the Retrospective Assessment of the Onset of Schizophrenia to examine the ‘hidden’ course of illness back to initial symptom onset.13 In 73% of cases, schizophrenia began with a prodromal phase of non-specific affective and negative symptoms, which lasted an average of 5 years. Only 7% of cases began with positive symptoms and 20% of cases began with both positive and depressive symptoms. One of the main findings was the prominence of depressive symptoms in the early course of illness. Over 80% of FEPs had experienced depressed mood prior to their first admission, and the most frequent initial symptom of schizophrenia was depressed mood.2,8,11,14
Another large-scale study of 99 FEPs was conducted in England using the Nottingham Onset Schedule (NOS),15 which was developed to define the chronology and components of psychosis onset. The NOS identified several distinct stages of the psychosis prodrome beginning with an initial prodromal period of ‘unease’, followed by a middle stage prodrome consisting of ‘non-diagnostic’ symptoms, and a later prodromal period when psychotic symptoms began to appear and build up until they reached threshold for a definite diagnosis. According to this chronological course, depressive symptoms that first appear in the initial prodromal period of ‘unease’ are one of the earliest symptoms signalling the subsequent onset of a DSM-IV psychotic disorder.
In the late 1990s, interest in the prodromal phase of psychosis as a potential target for preventive intervention prompted a major shift in research to prospective studies of symptomatic young people at high risk (HR) for developing a psychotic disorder. In an effort to identify symptoms that predict transition from prodromal to frank psychosis, these prospective studies developed assessment instruments and diagnostic criteria for the prodrome that focused on the positive symptoms specific to psychotic disorders and de-emphasized the non-specific symptoms like emotional disturbances that are common to a broad spectrum of psychiatric disorders. Despite their emphasis on subthreshold psychotic symptoms, prospective studies confirmed the presence of depressive symptoms in the early course of illness.
In the Cologne Early Recognition Study, 70% of the 110 patients with prodromal symptoms transitioned to psychosis over the 9-year follow-up period.16 Initial intake interview data based on the Bonn Scale for the Assessment of Basic Symptoms showed that attenuated/transient psychotic symptoms had the highest positive predictive value, but disorders of emotion and affect including depressed mood had the highest sensitivity and negative predictive value.17 A 3-year prospective study of a Dutch population-based sample found that risk for transition to a psychotic disorder in subjects with psychotic symptoms at baseline increased from 4.0% to 21.7% if depressed mood was also present at baseline.18 The Melbourne study of 104 young people with an ‘At Risk Mental State’ identified 36 subjects (34.6%) who transitioned to frank psychosis within 12 months, and baseline level of depression as measured by the Hamilton Rating Scale for Depression was a significant predictor of future transition to psychosis.19 Cornblatt et al.20 and Lencz et al.,21 reporting on the New York Recognition and Prevention Clinic sample of 82 clinical HR adolescents and young adults, identified non-specific symptoms such as social isolation and depression/anxiety as common features of the symptom profile, and major depression was a frequent comorbid diagnosis. Similarly, Meyer et al.9 found that major depression was the most common DSM-IV diagnosis for patients at entry into the Los Angeles Center for the Assessment and Prevention of Prodromal States study.
In summary, both retrospective and prospective studies of the early prepsychotic or ‘prodromal’ phase of illness have consistently found high levels of depressive symptoms prior to transition to DSM-IV schizophrenia and other psychotic disorders. However, the chronology of depressive symptom onset and course relative to positive symptom onset and course in those individuals who do transition to psychosis remains unclear.
We have been conducting a study of HR adolescents from the Republic of Palau that can further elucidate the role of depressive symptoms in the developmental course of psychosis. The isolated island nation of Palau in Western Micronesia has an elevated prevalence of schizophrenia and strong familial aggregation of cases.22 The Palau Early Psychosis Study (PEPS) was designed to be a prospective study of 300 adolescents with either genetic or clinical HR for developing psychotic disorders such as schizophrenia. However, our baseline clinical assessment identified 62 Palauan adolescents who already met DSM-IV criteria for psychosis yet had never sought treatment. The present study reports on an in-depth retrospective assessment of symptoms in these 62 drug-naïve adolescent-onset cases of DSM-IV psychosis that specifically addressed the role of depressive symptoms in the developmental course of illness.
The objectives of the present study were to identify and quantify the severity of depressive symptoms at each phase of illness and to evaluate the relationship between depressive symptoms and the positive symptoms that define psychosis over the developmental course of illness. Based on the results of prior studies, we hypothesized that depressive symptoms would be common in adolescent-onset psychosis (AO-PSY) subjects during the prodromal phase, would predate the onset of positive symptoms and would increase in severity over the developmental course of illness as positive symptoms increased in severity.
METHODS
Subjects
The sample for the PEPS has been described in detail previously.23 Briefly, a community-based sample of 404 Palauan adolescents 14–19 years of age (mean age =16.5 years), who were not help-seeking and were medication-free, participated in the study. We assessed 121 genetically high risk (GHR) adolescents who were either the offspring of a parent with a psychotic illness or nieces/nephews with two or more affected uncles/aunts. These GHR adolescents were identified during the original ascertainment of adult cases and their families for our genetic epidemiological study of schizophrenia in Palau.22 The remaining adolescent subjects were identified by surveying Palauan high school students using a self-report questionnaire, the Y-PARQ (Youth Psychosis At Risk Questionnaire), as described in Ord et al.24 We recruited 221 students with no affected first, second or third degree relatives who were designated as ‘Genetically Low Risk’ (GLR). These GLR adolescents were drawn from the upper and lower extremes of the Y-PARQ positive symptom score distribution and were subsequently identified by the clinical assessment as either symptomatic (i.e. clinically high risk (CHR)) or non-symptomatic normal controls.
Among the 404 research participants, we identified 62 adolescents who met DSM-IV criteria for schizophrenia or other psychotic disorder at the time of the baseline clinical assessment. These 62 AO-PSY cases were identified by assessing a relatively unique community-based sample of adolescents that may not be directly comparable to other early psychosis samples being studied in other countries throughout the world. The CHR samples that are common in early psychosis research primarily rely on referrals, and age of onset is typically defined as the age at which treatment with antipsychotic medication begins. These Palauan AO-PSY cases, with a significantly earlier age of onset (12.9 years) than previously reported, were not help-seeking individuals, and all adolescents were medication-naïve at the time of the clinical assessment. Furthermore, our Palau sample was drawn from a HR population isolate where all adolescents, even those with no close affected relatives, carry an elevated genetic susceptibility for schizophrenia. The AO-PSY group included 29 GHR subjects with an affected parent or more than one affected aunts/uncles and 33 GLR subjects with no affected first, second, or third degree relatives. All normal control subjects were screened to be GLR.
The present study compared these 62 AO-PSY cases with the 104 normal control adolescents recruited via the high school survey and identified as non-symptomatic by the subsequent clinical assessment. Given that depressive symptoms are common in the general population, we used the normal control subjects as a comparison group to establish the levels of depressive symptomatology that were typical in Palauan adolescents.
The AO-PSY and normal control groups were equivalent in terms of age (16.7 vs. 17.0 years, t =0.93, P =0.35) and gender (35.5% vs. 48.1% male, χ2 =2.02, P =0.16). It is noteworthy that the 62 AO-PSY cases represent a 2:1 female-to-male ratio, a reversal of the 2:1 male-to-female ratio among Palau’s chronic schizophrenia patients.22 Drug abuse, crime and unemployment, problems that often precipitate referral for treatment, are less prevalent in Palauan females compared with males, and thus females with psychosis are less likely to be treated as schizophrenia patients in Palau.
Study protocols and consent agreements for the PEPS were approved by both the University of Utah and the Palau Institutional Review Boards. All consent agreements were translated into Palauan. All subjects plus one parent or legal guardian gave written informed consent before being admitted into the study. After potential subjects were identified, our Palauan Investigator, Francisca Blailes, sought consent from each adolescent’s parents, and, if consent was granted, she then met with the adolescent to explain the study in the Palauan language and obtain his or her consent.
Clinical assessment
All adolescents were clinically assessed prior to any psychiatric treatment over a 4-year period from 2000 to 2004, as described in Myles-Worsley et al.23 The clinical assessment instrument was a K-SADS-PL (Kiddie-Schedule of Affective Disorders and Schizophrenia-Present and Lifetime),25 which was modified to incorporate prodromal and early psychotic symptomatology as evaluated by the Comprehensive Assessment of At Risk Mental States (CAARMS).26 The modified K-SADS-PL was designed to assess subjects for a full range of Axis I psychopathology including mood and conduct disorders, document the age (and/or school year) when the symptom began, quantify frequency and rate the level of distress associated with the symptom. The subject’s responses were recorded verbatim by an experienced Palauan interviewer who, unless the subject preferred to be interviewed in English, conducted the interview in the Palauan language.
The interview data were summarized by placing each symptom reported by the subject on a timeline representing the adolescent’s lifespan since birth. We acknowledge that data collected retrospectively must be interpreted with caution because of potential recall errors and other types of memory bias. However, several features of our study helped to minimize memory effects. First, our subjects were 14- to 19-year-olds, most of whom were still in high school, and the experiences to be recalled were relatively recent in the adolescent’s life. Second, the AO-PSY subjects were interviewed at a mean age of 16.9 years, an average of 4 years after they reached the diagnostic threshold for psychosis but prior to receiving any neuroleptic treatment that may cloud memory. Third, we established year of onset, rather than a specific date, and used age, grade in school and major life events (e.g. a family death or parental divorce) to anchor symptom onset dates on the subject’s timeline.
Each symptom reported during the interview was assigned a severity rating (ranging from 1 =questionable to 6 =severe) according to the scoring system developed for the CAARMS. Symptom ratings were reviewed by a two-member diagnostic panel in order to reach a consensus diagnosis using PACE (Personal Assistance and Crisis Evaluation) criteria26,27 for subsyndromal psychosis or prodromal schizophrenia and DSM-IV criteria for a psychotic disorder.28 The present study examined only positive and depressive symptoms. Depressive symptoms were selected according to DSM-IV criteria for a major depressive episode.28 We included certain symptoms that some studies have categorized as negative (e.g. ‘fatigue’ could be associated with avolition) and excluded symptoms associated with cognitive disruptions such as attentional difficulties. Symptoms experienced only once were excluded, except in the case of suicidality items.
We summarized the developmental course of illness for each AO-PSY subject by identifying three critical stages or milestones: (i) ‘First Positive Symptom’, defined as the earliest age at which the subject experienced a non-transient or recurrent positive symptom; (ii) ‘Prodrome Onset’, defined as the age when symptoms met PACE criteria for prodromal psychosis; and (iii) ‘Transition to Psychosis’, defined as the age when symptoms met DSM-IV criteria for a psychotic disorder. Total severity scores for positive symptoms and for depressive symptoms were calculated for each subject by summing severity ratings over all symptoms reported at each critical stage and at the time of the clinical assessment. For normal control subjects, severity scores were summed at the mean age of AO-PSY subjects at each critical stage.
Statistical analysis
We used analysis of variance (ANOVA) to test for group differences (AO-PSY vs. normal controls) in symptom severity scores and χ2 for group comparisons of frequency data. In the AO-PSY group, we examined the effects of genetic risk level (high vs. low), and gender on depressive symptom severity scores and age when each of the three critical stages of illness were reached using a mixed design ANOVA. When F-values were significant, we conducted planned comparisons. In AO-PSY subjects, we used linear regression to examine the relationship between positive and depressive symptoms over the developmental course of illness and to evaluate depressive symptom severity as a predictor of age of Prodrome Onset and age of Transition to Psychosis. For all analyses, the alpha level was set at 0.05.
RESULTS
Prevalence of depressive symptoms
Based on a mean severity score for depressive symptoms in the normal control group of 4.8 (SD =2.9) at the time of the clinical assessment, we defined a symptom severity score greater than 5 as an abnormal level of depressive symptomatology in Palauan adolescents. Abnormal levels of depressive symptoms were reported by 52 (83.9%) of the 62 AO-PSY subjects compared with only 26.9% of normal control adolescents (χ2 =50.44, P <0.001) at assessment. In the AO-PSY group, clinically significant depressive symptoms were reported by a similar proportion of females (87.5%) and males (77.3%) (χ2 =1.10, not significant).
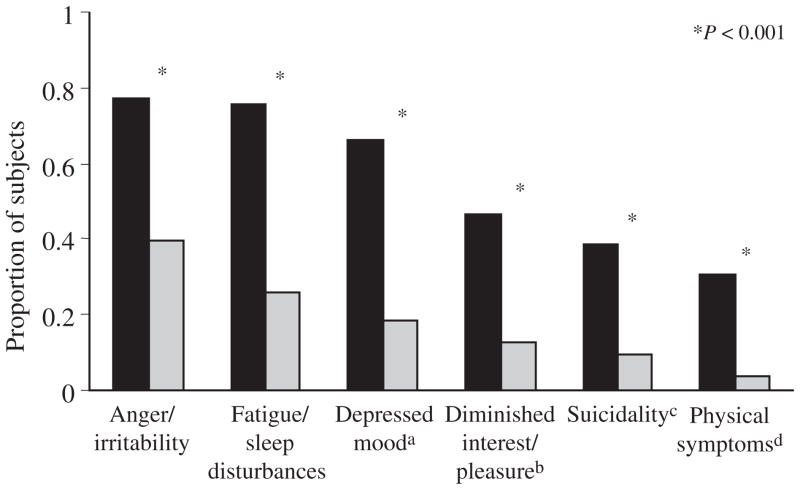
We identified six distinct types of depressive symptoms and compared the proportion of AO-PSY and normal control subjects reporting each type of symptom. As shown in Figure 1, each symptom type was reported more frequently by AO-PSY than by control subjects. However, the rank order of symptom frequency was similar in the two groups, indicating that the prevalence rate rather than the type of depressive symptoms distinguished cases from controls. Both AO-PSY and normal control subjects reported anger/irritability most frequently (77.4% and 39.4%), followed by fatigue/sleep disturbances (75.8% and 26.0%), and then depressed mood (66.1% and 18.3%). Suicidal thoughts, threats, or actions (including single occurrences) were reported by 38.7% of AO-PSY subjects compared with only 9.6% of normal controls. Symptoms reported more frequently by females than by males in the AO-PSY group included anger/irritability (χ2 =6.55, P <0.05) and depressed mood (χ2 =3.96, P <0.05).
FIGURE 1.
Prevalence of depressive symptoms by group. aIncludes feelings of sadness, hopelessness, worthlessness, guilt and the inability to feel happy. bIncludes anhedonia, emotional numbness and social withdrawal. cIncludes acts of self harm, suicidal thoughts, threats, or actions. dIncludes coordination problems, and changes in weight or appetite. (■) Adolescent-onset psychosis, (
 ) normal subjects.
) normal subjects.
Chronology and developmental course
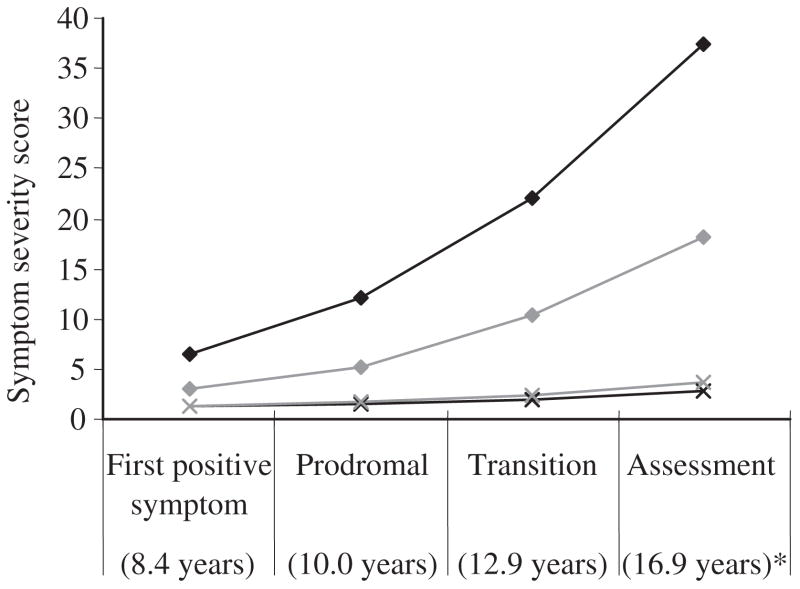
Figure 2 presents mean symptom severity scores for both the AO-PSY subjects and the normal control group at each of the three critical stages plus the time of the clinical assessment. The mean age of AO-PSY subjects at each of the three critical stages was 8.4 years (SD =3.0 years) for the first positive symptom, 10.0 years (SD =3.1 years) for prodrome onset and 12.9 years (SD =2.4 years) for transition to psychosis. A repeated measures ANOVA with group, gender and genetic risk as between-subjects factors and time as a within-subjects factor showed that depressive symptoms were more severe among AO-PSY subjects over the developmental course (F =30.42, P <0.001). The group by time interaction was significant, indicating that depressive symptoms increased over time only in the AO-PSY group (F =10.68, P <0.01). There were no main or interactive effects of gender or level of genetic risk on the developmental course of depressive symptom severity.
FIGURE 2.
Symptom severity at critical stages for adolescent-onset psychosis (AO-PSY) ((
 ) positive, (
) positive, (
 ) depressive) versus normal control subjects ((
) depressive) versus normal control subjects ((
 ) positive, (
) positive, (
 ) depressive). *Mean age of AO-PSY subjects at each critical stage.
) depressive). *Mean age of AO-PSY subjects at each critical stage.
In AO-PSY subjects with a clinically significant depressive syndrome (n =52), the mean onset age of abnormal levels of depressive symptoms was 11.6 years (SD =3.7 years) or an average of 1.6 years after prodrome onset and 1.3 years prior to transition to psychosis. Age of depressive symptom onset did not differ by gender (χ2 =1.10, not significant). Positive symptom onset preceded depressive symptom onset in 30 (59.6%) of the 52 AO-PSY cases with clinically significant depressive symptoms. In the remaining 22 subjects, depressive symptom onset either coincided with positive symptom onset (n =17) or occurred shortly before positive symptom onset (n =5).
We defined three groups of AO-PSY subjects based on onset age of clinically significant depressive symptoms relative to the subject’s age of prodrome onset and age of transition to psychosis. Depressive symptom onset preceded prodrome onset (‘Preprodrome’) in 30.8% of AO-PSY cases, occurred between prodrome onset and transition to psychosis (‘During Prodrome’) in 38.4% of cases and followed transition to psychosis (‘Post-transition’) in 30.8% of cases. Group comparisons, as summarized in Table 1, showed that the ‘During Prodrome’ depression onset group had a longer prodromal phase (F =8.48, P <0.001), a later age of Transition to Psychosis (F =9.69, P <0.001), and a greater increase in positive symptom severity from prodrome onset to transition (F =8.64, P =0.003 for the group–time interaction) relative to the other two groups. Positive symptom severity was similar across the three groups both at prodrome onset (F =2.67, P =0.079) and at transition to psychosis (F =2.48, P =0.094).
TABLE 1.
Sample characteristics by chronology of depressive symptom onset
| Total AO-PSY Sample(n =62) | Depressive symptom onset
|
Comparison of three Chronological Groups | ||||
|---|---|---|---|---|---|---|
| Sample with depressive symptoms(n =52) | Preprodrome(n =16) | During prodrome(n =20) | Post-transition(n =16) | |||
| Mean age in years (SD) of | ||||||
| Depressive symptom onset | not available | 11.62 (3.68) | 7.81 (2.64) | 12.35 (3.03) | 14.56 (1.75) | F =28.74, P <0.001 |
| Prodrome onset | 10.02 | 10.19 (3.30) | 11.44 (2.56) | 9.45 (3.90) | 9.88 (2.94) | F =1.78, P =0.180 |
| Transition to psychosis | 12.89 | 12.90 (2.28) | 13.00 (2.25) | 14.15 (1.23) | 11.25 (2.38) | F =9.69, P =0.0003 |
| Duration of prodrome | 2.88 | 2.71 (3.13) | 1.56 (2.28) | 4.70 (3.63) | 1.38 (1.71) | F =8.48, P =0.0007 |
| Positive symptom severity (SD) | ||||||
| At prodrome onset | 12.23 (5.96) | 12.67 (6.16) | 14.94 (6.97) | 10.40 (4.03) | 13.25 (6.88) | F =2.67, P =0.079 |
| At transition | 22.19 (10.05) | 21.90 (9.46) | 20.56 (9.45) | 25.40 (8.76) | 18.88 (9.48) | F =2.48, P =0.094 |
The relationship of depressive symptom severity to positive symptom severity and to age of Prodrome Onset and Transition in AO-PSY subjects was examined using linear regression. The correlation between positive and depressive symptom severity scores was significant both at Prodrome Onset (r =0.32, P =0.010) and at Transition to Psychosis (r =0.39, P =0.002). Severity of depressive symptoms at Prodrome Onset predicted age of Prodrome Onset (r =0.27, P =0.036), and severity of depressive symptoms at Transition predicted age of Transition (r =0.32, P =0.010). However, prediction was not in the expected direction: the greater the severity of depressive symptoms the later the age at which AO-PSY subjects reached each of these critical stages.
DISCUSSION
The present study used a timeline approach to retrospectively examine the onset and course of depressive symptoms over the developmental course of adolescent-onset psychosis (AO-PSY) in Palau. Overall, 84% of AO-PSY subjects reported abnormal levels of depressive symptomatology that appeared prior to transition by an average of 1.3 years, confirming the results of prior retrospective.8,11,12,15 The most common depressive symptoms were anger/irritability, fatigue/sleep disturbances and depressed mood.
Although our findings confirmed the high prevalence of depressive symptoms over the developmental course of illness, we found a more variable chronology of depressive symptom onset relative to positive symptom in our AO-PSY sample than previous studies have described. The retrospective study of German FEPs8,11,12 reported that depressive symptoms typically occur at the earliest stages of symptom development, well before the onset of positive symptoms. Only 7% of the German sample experienced positive symptoms first.14 In our study, positive symptom onset preceded depressive symptom onset in almost 60% of the AO-PSY cases with clinically significant depressive symptoms. Only 30% of these cases had early onset of depressive symptoms prior to the prodrome. Depressive symptom onset occurred during the prodrome in 40% of cases and following transition to psychosis in the remaining 30% of cases. The main characteristics that distinguished these three groups were age of transition to psychosis and duration of the prodrome. The late depressive symptom onset group had the youngest age of transition, and both the early and late groups had a shorter prodromal phase than the ‘During Prodrome’ onset group.
When we examined the full developmental course of AO-PSY, we found that depressive symptom severity increased steadily from prodrome onset to transition to psychosis, mirroring the progression of positive symptom severity as psychosis developed. A similar developmental course was described by Hafner et al.,8,11,12 who have subsequently argued that schizophrenia and depression are very similar during the prodromal phase.2
Prospective studies of both clinical HR18,19 and genetic HR individuals29 have found higher levels of depressive symptoms in subjects who transition to psychosis than in those who do not, indicating that the presence of depressive symptoms in HR individuals may be a predictive risk factor for a subsequent diagnosis of psychosis. However, in our AO-PSY sample, greater severity of depressive symptoms was associated with later prodrome onset and later transition to psychosis, indicating that the more prominent the subject’s depressive symptoms, the longer it takes for positive symptoms to reach diagnostic thresholds. If level of depressive symptomatology were indeed predictive of impending psychosis, critical stages would be reached earlier not later.
In summary, our findings, together with those of Hafner et al.,2,8,11,12 suggest that depressive symptoms represent more of an integral component of disease progression than an independent risk factor that predicts transition to psychosis. Despite the prominence of depressive symptoms over the developmental course of schizophrenia and other psychotic disorders, affective clinical symptoms such as depression have been excluded from widely used diagnostic criteria for the prodrome (PACE criteria,26 COPS or Criteria of Prodromal Syndromes,30 see Olsen and Rosenbaum31 for a review) because of their lack of specificity for psychosis.
Our findings suggest that the early course of psychosis is characterized by a synergistic combination of positive symptoms and depressive symptoms building up in parallel until the DSM-IV threshold for a psychotic disorder is reached. This combination may be particularly apparent in the case of psychotic disorders with a strong affective component such as schizoaffective disorder, depressive type. Considering the important role of depressive symptoms in the developmental course of psychotic disorders, additional emphasis on affective clinical symptoms in the criteria used to define the prodrome may be warranted.
Acknowledgments
The authors thank all members of the Palauan community who participated in this research, which was supported by US Public Health Service grant MH54186 and a Grable Foundation NARSAD award.
References
- 1.Siris SG, Bench C. Depression and schizophrenia. In: Hirsch SR, Weinberger DR, editors. Schizophrenia. 2. Oxford: Blackwell Publishing; 2003. pp. 142–67. [Google Scholar]
- 2.Hafner H, Maurer K, Trendler G, an der Heiden W, Schmidt M, Konnecke R. Schizophrenia and depression: challenging the paradigm of two separate diseases-a controlled study of schizophrenia, depression and healthy controls. Schizophr Res. 2005;77:11–24. doi: 10.1016/j.schres.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Kitamura T, Okazaki Y, Fujinawa A, Yoshino M, Kasahara Y. Symptoms of psychoses: a factor-analytic study. Br J Psychiatry. 1995;166:236–40. doi: 10.1192/bjp.166.2.236. 1995. [DOI] [PubMed] [Google Scholar]
- 4.Lenzenweger MF, Dworkin RH. The dimensions of schizophrenia phenomenology: not one or two, at least three, perhaps four. Br J Psychiatry. 1996;68:432–40. doi: 10.1192/bjp.168.4.432. [DOI] [PubMed] [Google Scholar]
- 5.McGorry PD, Bell RC, Dudgeon PL, Jackson HJ. The dimensional structure of first episode psychosis: an exploratory factor analysis. Psychol Med. 1998;28:935–47. doi: 10.1017/s0033291798006771. [DOI] [PubMed] [Google Scholar]
- 6.Van Os J, Gilvarry C, Bale R, et al. A comparison of the utility of dimensional and categorical representations of psychosis. Psychol Med. 1999;29:595–606. doi: 10.1017/s0033291798008162. [DOI] [PubMed] [Google Scholar]
- 7.Blanchard JJ, Cohen AS. The structure of negative symptoms within schizophrenia: implications for assessment. Schizophr Bull. 2006;32:238–45. doi: 10.1093/schbul/sbj013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hafner H, Loffler W, Maurer K, Hambrecht M, an der Heiden W. Depression, negative symptoms, social stagnation and social decline in the early course of schizophrenia. Acta Psychiatr Scand. 1999;100:105–18. doi: 10.1111/j.1600-0447.1999.tb10831.x. [DOI] [PubMed] [Google Scholar]
- 9.Meyer SE, Bearden CE, Lux SR, et al. The psychosis prodrome in adolescent patients viewed through the lens of DSM-IV. J Child Adol Psychopharmacol. 2005;15:434–51. doi: 10.1089/cap.2005.15.434. [DOI] [PubMed] [Google Scholar]
- 10.Yung AR, McGorry PD. The prodromal phase of first-episode psychosis: past and current conceptualizations. Schizophr Bull. 1996;22:353–70. doi: 10.1093/schbul/22.2.353. [DOI] [PubMed] [Google Scholar]
- 11.Hafner H, Maurer K, Loffler W, et al. The ABC Schizophrenia Study: a preliminary overview of the results. Soc Psychiatry Psychiatr Epidemiol. 1998;33:380–6. doi: 10.1007/s001270050069. [DOI] [PubMed] [Google Scholar]
- 12.Hafner H. Onset and early course as determinants of the further course of schizophrenia. Acta Psychiatr Scand. 2000;407:44–8. [PubMed] [Google Scholar]
- 13.Hafner H, Riecher-Rossler A, Hambrecht M, et al. IRAOS: an instrument for the assessment of onset and early course of schizophrenia. Schizophr Res. 1992;6:209–23. doi: 10.1016/0920-9964(92)90004-o. [DOI] [PubMed] [Google Scholar]
- 14.An der Heiden W, Hafner H. The epidemiology of onset and course of schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2000;250:292–303. doi: 10.1007/s004060070004. [DOI] [PubMed] [Google Scholar]
- 15.Singh SP, Cooper JE, Fisher HL, et al. Determining the chronology and components of psychosis onset: the Nottingham Onset Schedule (NOS) Schizophr Res. 2005;80:117–30. doi: 10.1016/j.schres.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 16.Klosterkotter J, Hellmich M, Steinmeyer EM, Schultze-Lutter F. Diagnosing schizophrenia in the initial prodromal phase. Arch Gen Psychiatry. 2001;58:158–64. doi: 10.1001/archpsyc.58.2.158. [DOI] [PubMed] [Google Scholar]
- 17.Gross G, Huber G, Klosterkotter J, Linz M. BSABS: Bonn Scale for the Assessment of Basic Symptoms. Berlin, Heidelberg, NY: Springer; 1997. [Google Scholar]
- 18.Krabbendam L, Myin-Germeys I, Hanssen M, et al. Development of depressed mood predicts onset of psychotic disorder in individuals who report hallucinatory experiences. Br J Clin Psychol. 2005;44:113–25. doi: 10.1348/014466504X19767. [DOI] [PubMed] [Google Scholar]
- 19.Yung AR, Phillips LJ, Yuen HP, McGorry PD. Risk factors for psychosis in an ultra high-risk group: psychopathology and clinical features. Schizophr Res. 2004;67:131–42. doi: 10.1016/S0920-9964(03)00192-0. [DOI] [PubMed] [Google Scholar]
- 20.Cornblatt BA, Lencz T, Smith CW, et al. The schizophrenia prodrome revisited: a neurodevelopmental perspective. Schizophr Bull. 2003;29:633–51. doi: 10.1093/oxfordjournals.schbul.a007036. [DOI] [PubMed] [Google Scholar]
- 21.Lencz T, Smith CW, Auther A, Correll CU, Cornblatt B. Non-specific and attenuated negative symptoms in patients at clinical high-risk for schizophrenia. Schizophr Res. 2004;68:37–48. doi: 10.1016/S0920-9964(03)00214-7. [DOI] [PubMed] [Google Scholar]
- 22.Myles-Worsley M, Coon H, Tiobech J, et al. Genetic epidemiological study of schizophrenia in Palau, Micronesia: prevalence and familiality. Am J Med Genet: Neuropsychiatr Genet. 1999;88:4–10. doi: 10.1002/(sici)1096-8628(19990205)88:1<4::aid-ajmg2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 23.Myles-Worsley M, Blailes F, Ord LM, Weaver S, Dever G, Faraone S. The Palau Early Psychosis Study: distribution of cases by level of genetic risk. Am J Med Genet: Neuropsychiatr Genet. 2007;144:5–9. doi: 10.1002/ajmg.b.30362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ord LM, Myles-Worsley M, Blailes F, Ngiralmau H. Screening for prodromal adolescents in an isolated high-risk population. Schizophr Res. 2004;71:507–8. doi: 10.1016/j.schres.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 25.Kaufman J, Birmaher B, Brent D, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 26.Yung AR, Yuen HP, McGorry PD, et al. Mapping the onset of psychosis: the Comprehensive Assessment of At-Risk Mental States. Aust N Z J Psychiatry. 2005;39:964–71. doi: 10.1080/j.1440-1614.2005.01714.x. [DOI] [PubMed] [Google Scholar]
- 27.McGorry PD, Yung AR, Phillips LJ. The ‘close-in’ or ultra high-risk model: a safe and effective strategy for research and clinical intervention in prepsychotic mental disorder. Schizophr Bull. 2003;29:771–90. doi: 10.1093/oxfordjournals.schbul.a007046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 29.Owens DG, Miller P, Lawrie SM, Johnstone EC. Pathogenesis of schizophrenia: a psychopathological perspective. Br J Psychiatry. 2005;186:386–93. doi: 10.1192/bjp.186.5.386. [DOI] [PubMed] [Google Scholar]
- 30.Miller TJ, McGlashan TH, Rosen J, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, inter-rater reliability, and training to reliability. Schizophr Bull. 2003;29:703–15. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- 31.Olsen KA, Rosenbaum B. Prospective investigations of the prodromal state of schizophrenia: assessment instruments. Acta Psychiatr Scand. 2006;113:273–82. doi: 10.1111/j.1600-0447.2005.00698.x. [DOI] [PubMed] [Google Scholar]