Abstract
Plumatella bushnelli Wood, 2001 is the first reported freshwater bryozoan for Micronesia, and the Guam collections are only the fourth record of this species in the world. In Guam it normally occurs as small colonies on the undersides of duckweed leaves but formed larger colonies on artificial substrate.
Introduction
Bryozoans are among the most common suspension feeding invertebrates in fresh waters, occurring throughout a wide range of habitats. While they are best known from North America (Wood 2001a), Europe (Wood & Okamura 2006), and Japan (Hirose 2006), there are probably few freshwater habitats in the world where these animals do not occur. Nevertheless, reports from the tropical western Pacific consist of just a single cursory bryozoan survey from Indonesia (Vorstman 1928, 1929), one species description from the Philippines (Kraepelin 1887), and a brief report from Taiwan (Toriumi 1942). Despite major advances in bryozoan taxonomy over the past several decades, no further sightings of freshwater bryozoans have been published from any of the Pacific islands, including Guam. Indeed, there has been very little study of any of the small and microscopic freshwater organisms of Guam to date (Lobban & Schefter 2008). Thus the finding of a bryozoan in freshwater marshes on Guam was of interest both for local biodiversity and as a contribution to the knowledge of freshwater bryozoans worldwide.
This report is part of an on-going survey of freshwater micro-invertebrates and protists of Guam, and additional records can be accessed at http://university.uog.edu/botany/474/fw_toc.htm.
Methods
Bryozoan colonies were first found in “Route 4 Marsh,” 13° 25′ 34″ N, 144° 46′ 56″ E, is a seasonal wetland (dry from March to July) in Ordot-Chalan Pago, Guam (Lobban & Schefter 2008). During 2004–2006, duckweed (Lemna aequinoctialis Welwitsch) formed a nearly continuous floating canopy over the water at the roadside edge and provided the only natural substratum to which the bryozoan attached. The pond gradually became overgrown by tall grass (Phragmites) and by the 2007–08 wet season there was no more Lemna between the grass stalks. Subsequently we collected duckweed from two other ponds: Alamagosa Pond on Naval Ordnance Facility, Agat (13° 20′ 50″N, 144° 40′ 43″E) and at LeoPalace Golf Club (Palmer #14), Yona (13° 24′ 12″N, 144° 44′ 31″E).
Samples of the surface Lemna mat were initially collected with a bucket along the roadside edge of the marsh. Small panels of corrugated polyethylene were later floated in open water where they were colonized naturally by bryozoans from germinated floatoblasts. During December 2007 corrugated plastic settling plates were floated on Alamagosa and LeoPalace ponds to trap the bryozoan. Live observations by CL and KQ were made with an Olympus CZ51 trinocular dissection microscope, an Olympus CX41 compound microscope with brightfield, phase, and darkfield illumination, or a Nikon Eclipse E600 with differential interference contrast (DIC). Photographs of live material were taken with an Olympus 7070 camera on all the microscopes. Colonies for preservation were relaxed by floating menthol flakes on the water surface and fixing in 70% ethanol (Wood 2005). Statoblast dimensions were measured with a compound microscope fitted with an ocular micrometer at 100x magnification. The means were calculated at 95% confidence levels using Instat software (GraphPad, San Diego, USA). For scanning electron microscopy statoblasts were cleaned manually, dried at −10° C, sputter coated in gold, and photographed with a JEOL JSM-5600LV microscope. A specimen has been deposited in the U.S. National Museum, No. XXX
Results
Plumatella bushnelli Wood, 2001b formed small colonies, up to four active zooids, on the underside of living or dead duckweed leaves (Figures 1, 3) and on dead grass leaf fragments and similar debris floating on the water surface. It was reliably found in Route 4 Marsh through the 2005–06 and 2006–07 seasons and subsequently found in Alamagosa Pond but not in the LeoPalace pond.
Figures 1–2. Living colonies.
Fig. 1. Typical colony habit and habitat on Guam (Route 4 Pond), on underside of duckweed leaves. The remains of the body wall of the first zooid can be seen on the left. There are 4 active zooids and the youngest (at right) has a bud. Fig. 2. Photo of colony on settling plate (Alamagosa Pond), showing sparse branching, lophophores and floatoblasts.
Figures 3–4. Living specimens.
Fig. 3. Small colony showing branch. Fig. 4. Detail of lophophore in which 47 tentacles can be counted.
On plastic panels (Figure 2) the colonies spread widely, producing as many as 8 zooids in linear series before the appearance of any secondary branches. Tubules were generally attached to the substratum throughout their length, but the zooids bent sharply at their base, extending outwards 1–2.5 mm, not including the lophophore. It appeared that free branches could be formed where substrate was limited, enabling colonies to span small gaps and spread beneath a floating canopy of duckweed.
The ectocyst was often transparent (Figure 3), but in some cases acquired a dusting of particles (Figure 1, oldest zooid) that obliterated any view of internal structures. A faint raphe was often visible on older branches attached firmly to the substratum. The relatively long and slender tentacles numbered about 47 and were arranged in a horseshoe-shaped configuration on the lophophore (Figure 4).
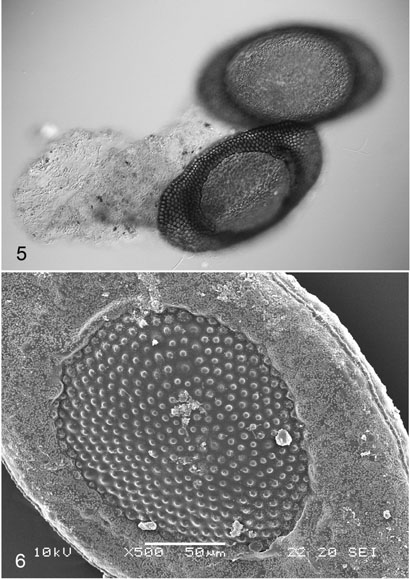
Floatoblasts of the Guam material are distinctive and entirely consistent with those previously described for this species (Figure 5). Floatoblast dimensions are shown in Table 1. Both dorsal and ventral fenestrae bear tubercles that are well-spaced and sharply defined, resembling small beads. This feature is easily seen in light microscopy, but it shows up particularly well with the scanning electron microscope (Figure 6). Also visible by SEM is the dense rash of tiny nodules that cover the floatoblast annulus and spill out onto the fenestra margins. The suture is a prominent, lumpy cord without ribs or tubercles.
Figures 5–6. Floatoblasts.
Fig. 5. Ventral (upper) and dorsal valves of germinated floatoblast, with remnants of first ectocyst. Fig. 6. SEM detail of dorsal valve showing minute nodules on the annulus.
Table 1.
Dimensions of floatoblasts in four species of plumatellid bryozoans: Plumatella bombayensis; Plumatella bushnelli; Plumatella javanica and Plumatella casmiana. Data for Plumatella bushnelli are taken from Guam specimens; data for other species come from Thailand (Wood et al. 2006).
| Overall length |
Overall width |
Overall length/ width |
Dorsal fenestra length |
Dorsal fenestra width |
Dorsal fenestra length/ width |
Ventral fenestra length |
Ventral fenestra width |
Ventral fenestra length/ width |
|
|---|---|---|---|---|---|---|---|---|---|
| PBOM | 386.12 | 175.8 | 2.22 | 94.1 | 71.12 | 1.44 | 201.9 | 150.5 | 1.34 |
| PBUS | 374 ± 12 | 219 ± 6 | 1.72 | 194 ± 3 | 165 ± 3 | 1.18 | 264 ± 3 | 183 ± 3 | 1.45 |
| PJAV | 374 ± 6 | 237±2 | 1.58 | 175±6 | 148±4 | 1.18 | 243±6 | 189±9 | 1.30 |
| PCAS | 372 ± 9 | 218 ± 8 | 1.58 | 201 ± 7 | 132±6 | 1.54 | 244±8 | 143±9 | 1.71 |
Sessoblasts have been reported elsewhere for this species but have not yet been seen in material from Guam.
The taxonomy of Plumatella relies on the fine structure of the resting stages (sessoblasts and floatoblasts). No sessoblasts were found but floatoblasts (Figure 7) were readily obtained from the water surface and from the colonies themselves.
Figures 7. Comparative morphology of the dorsal floatoblast valve in four bryozoan species.
(a) Plumatella bushnelli; (b) Plumatella bombayensis; (c) Plumatella javanica; (d) Plumatella casmiana. Scale bar = 0.01 mm. (Fig. 7a modified from Wood 2001b).
Discussion
The floatoblast of Plumatella bushnelli is longer than that of most other plumatellids. Its external dimensions correspond roughly with those of three other species known from Indonesia: Plumatella bombayensis Annandale, 1910, P. casmiana Oka, 1907, and P. javanica Kraepelin, 1906 (Table 1, Figure 7). However, the differences are clear. The large dorsal annulus of P. bushnelli contrasts sharply with the small fenestra of P. bombayensis. In fact, the latter's dorsal fenestra is so small that its length is less than the width of the dorsal annulus at either pole. This feature of P. bombayensis is also shared with two other east Asian species, P. mukaii Wood, 2001 and P. philippinensis Kraepelin, 1887.
The floatoblast of P. bushnelli is much more similar to that of either P. casmiana or P. javanica. However, the dorsal annulus of P. bushnelli is broader than that of P. casmiana even while its overall statoblast width is less (Table 1); also the fenestra tubercles of P. casmiana are relatively small and weak. Plumatella bushnelli can be distinguished from P. javanica by its widely separated floatoblast tubercles, which contrast with the densely crowded tubercles of P. javanica. A final feature distinguishing P. bushnelli from all these species is the presence of nodules over the entire floatoblast surface (Figure 6), which unfortunately are visible only with scanning electron microscopy.
Aside from its morphology little is known about Plumatella bushnelli. It was first noticed in 1983 at a small barrier island pond in North Carolina, where a small population still persists (Wood 2001b). A second isolated population was reported (but not identified) from the Mangaone River near Kaitawa, New Zealand (Wood et al. 1998). Most recently, floatoblasts were recovered with a plankton net from Lake Apopka, 20 km WNW of Orlando, Florida (Taticchi, M., E. Concetta, L. Battoe & K. Havens, unpubl. ms. 2007). All of these sites and the pond on Guam occur in relatively warm climates, suggesting a warmtemperate to tropical distribution for the species.
The sparse and widely distributed occurrence of Plumatella bushnelli is not surprising. Apparent scarcity may simply reflect the general lack of distribution data for tropical freshwater bryozoans. The widely disjunct distribution for this and many other bryozoan species is attributed to passive dispersal by migrating waterfowl (Bilton et al. 2001). Such effective dispersal, combined with a highly fragmented habitat, often leads to the occurrence of bryozoan metapopulations (Okamura 1997), where small, isolated population units appear and disappear abruptly from any particular site.
A population center for Plumatela bushnelli will eventually be found, possibly in China or Australia, neither of which has ever been targeted in any systematic bryozoan survey. Additional field work might also be fruitful in southeastern United States and New Zealand where the species has already been reported.
Acknowledgements
CL thanks María Schefter for her encouragement in pursuing this study and for her collaboration in obtaining funding for the microscopy and science communications curriculum development projects that facilitated the observations on Guam. We also thank Ann Marie Gawel and COMNAVMAR for access to the Naval Base. Funding was from U.S. Dept. Education Minority Science and Engineering Improvement Project (ED-MSEIP) grant P120A-040092, with additional funding for the Nikon E600 microscope was from an NIH-NIGMS RISE grant GM063682.
TW thanks Maria Illuminata Taticchi (University of Perugia) for sharing photos and information from her Florida collection.
References
- Bilton D, Freeland JR, Okamura B. Dispersal in freshwater invertebrates. Annual Reviews in Ecology and Systematics. 2001;32:159–181. [Google Scholar]
- Hirose M. A revision of Japanese Plumatellidae (Bryozoa: Phylactolaemata) based on a mtDNA phylogeny and statoblast micromorphology. Division of Biological Science, Hokkaido University; Sapporo, Japan: 2007. p. 147. Master's thesis. [Google Scholar]
- Kraepelin K. Eine Susswasser Bryozoe (Plumatella) aus Java. Mitteilungen aus dem Naturhistoriches Museum in Hamburg. 1906;23:143–146. [Google Scholar]
- Lobban CS, Schefter M. Freshwater biodiversity of Guam. 1. Introduction and new records of ciliates and a heliozoan. Micronesica. 40:273–293. [PMC free article] [PubMed] [Google Scholar]
- Okamura B. The ecology of subdivided populations of a clonal freshwater bryozoan in southern England. Archiv für Hydrobiologie. 1997;141:13–34. [Google Scholar]
- Paulay G, editor. Marine Biodiversity of Guam and the Mariana Islands. Micronesica. 2003a;35-36:682. [Google Scholar]
- Paulay G. Miscellaneous marine invertebrates and protists from the Mariana Islands. Micronesica. 2003b;35-36:676–682. [Google Scholar]
- Toriumi M. Studies on freshwater Bryozoa IV. Freshwater Bryozoa of Formosa. Vol. 17. Science Reports of the Tôhoku Imperial University; 1942. pp. 207–214. (Series 4). [Google Scholar]
- Vorstman A. Some freshwater Bryozoa of West Java. Treubia. 1928a;10:1–14. [Google Scholar]
- Vorstman A. Freshwater Bryozoa from E. Java. Treubia. 1928b;10:1–14. [Google Scholar]
- Wood TS. Bryozoans. In: Thorp J, Covich A, editors. Ecology and Classification of North American Freshwater Invertebrates. 2nd. Academic Press; New York: 2001a. pp. 505–525. [Google Scholar]
- Wood TS. Three new species of plumatellid bryozoans (Ectoprocta: Phylactolaemata) defined by statoblast nodules. Journal of the North American Benthological Society. 2001b;20:133–143. [Google Scholar]
- Wood TS. Study methods for freshwater bryozoans. Denisia. 2005;16:103–110. [Google Scholar]
- Wood TS, Wood L, Geimer G, Massard J. Freshwater bryozoans of New Zealand: a preliminary survey. New Zealand Journal of Marine and Freshwater Research. 1998;32:639–648. [Google Scholar]
- Wood T, Okamura B. The freshwater bryozoans of Britain, Ireland, and Continental Europe. Scientific Publication No, 63, Freshwater Biology Association of the United Kingdom; Swansholme, UK: 2005. p. 113. [Google Scholar]
- Wood T, Anurakpongsatorn P, Mahujchariyawong J. Freshwater bryozoans of Thailand (Ectoprocta and Entoprocta) Natural History Journal of Chulalongkorn University. 2006;6:83–119. [Google Scholar]