Abstract
TGF-β isoforms are key modulators of a broad range of biological pathways and increasingly are exploited as therapeutic targets. Here, we describe the crystal structures of a pan-TGF-β neutralizing antibody, GC-1008, alone and in complex with TGF-β3. The antibody is currently in clinical evaluation for idiopathic pulmonary fibrosis, melanoma, and renal cell cancer. GC-1008 recognizes an asymmetric binding interface across the TGF-β homodimer with high affinity. Whereas both cognate receptors, TGF-β-receptor types I and II, are required to recognize all 3 TGF-β isoforms, GC-1008 has been engineered to bind with high affinity to TGF-β1, 2, and 3 via a single interaction surface. Comparison with existing structures and models of TGF-β interaction with its receptors suggests that the antibody binds to a similar epitope to the 2 receptors together and is therefore a structurally different but functionally identical mimic of the binding mode of both receptors.
Keywords: cancer, fibrotic diseases, TGF-β/antibody complex, TGF-β signaling, X-ray structure
The transforming growth factor β (TGF-β) superfamily of cytokines comprises >30 structurally related proteins that are involved in the regulation of a wide variety of biological processes such as cell proliferation, differentiation, and expression of extracellular matrix proteins (1, 2). Members of this family are ≈25-kDa homodimeric molecules with a similar structural framework in which the 2 monomers are covalently linked via a disulfide bridge (3–8). Three different isoforms of TGF-β are known in mammals that share a sequence identity of 70–82% (2). All 3 isoforms of TGF-β are expressed as latent or inactive propeptides that can be activated by a diverse number of physiologically and pathophysiologically associated mechanisms such as thrombospondin-1, integrin αvβ6, reactive oxygen species, and low pH (9, 10). Expression of TGF-β isoforms and the activation of latent TGF-β to mature active protein are tightly regulated in normal physiology, and a dysregulation of this process has been described in many pathological conditions leading to an enhanced activity of TGF-β in diseases such as fibrotic disease and some malignancies. Gene-deletion studies in vivo indicate that the 3 mammalian isoforms of TGF-β have nonoverlapping activities essential for vascular development and the regulation of immune cell function (11–13). However, in vitro, the biological activities of the 3 isoforms of TGF-β are almost identical. TGF-β1 and TGF-β3 trigger the cellular signaling cascade upon binding to the extracellular domains of 2 transmembrane receptors, known as TGF-β receptor types I and II, forming a ternary complex. This complex assembly occurs first through high affinity binding of the cytokines to their TGF-β receptor type II, followed by the recruitment of the TGF-β receptor type I (14, 15). The binding potency of TGF-β to its type II receptor is isoform-dependent, with the highest binding affinities for TGF-β1 and 3 and a 10- to 20-fold-smaller binding affinity for TGF-β2 (16, 17). The formation of a ternary complex after interaction with TGF-β2 is thought also to require a further interaction with an accessory receptor; TGF-β receptor type III (18). The formation of the ternary complex leads to the transphosphorylation of the C-terminal kinase domain of the type I receptor by the adjacent serine–threonine kinase of the type II receptor (19). The type I kinase in turn phosphorylates nuclear translocating SMAD proteins, which leads to the activation of further downstream signaling events (20, 21). Many severe diseases are linked to malfunctions of the TGF-β-induced signaling pathway. For instance, an increased tissue level of TGF-β is believed to be a key factor in the development of idiopathic pulmonary fibrosis (IPF), chronic obstructive pulmonary disease (COPD), renal disease, or myocardial fibrosis, because TGF-β, among other cytokines, is involved in the activation of fibroblast proliferation (22, 23) leading to the progressive accumulation of extracellular matrix (ECM) regardless of the underlying etiology. This points to up-regulation of the activity of TGF-β as a final common pathway for fibrotic disease (24, 25). Furthermore, TGF-β was found to be involved in cancer development and progression. TGF-β signaling plays a complex role in carcinogenesis because it has the potential to act as either a tumor suppressor or a prooncogenic pathway; TGF-β can act as a tumor suppressor and a potent inhibitor of cell proliferation (26, 27). However, during the past few years, several studies have also shown that high local tissue levels of TGF-β are crucial for the maintenance and progression of some types of cancer cells. The down-regulation of TGF-β signaling can therefore decisively reduce the viability of such tumor cells (28–30). More recently, the role of TGF-β acting as an immune modulator, suppressing immune surveillance of tumors has began to be elucidated (31, 32). To date, numerous TGF-β neutralizing antibodies that show the potential of efficacy in these severe diseases have been described in the literature. GC-1008 belongs to this class of antibodies. It is a pan-specific human monoclonal antibody that targets TGF-β (28). It has been shown to inhibit the effects of overproduction of TGF-β and is in Phase I clinical trials to assess its efficacy in patients with IPF, melanoma, renal cell carcinoma, and focal segmental glomerulosclerosis (33). Although there is an increasing effort to develop new antibodies for the neutralization of cytokines, only a little structural information is available about the interaction and epitope recognition of such antibodies with their antigens.
Here, we present the structure of GC-1008 in complex with TGF-β3 at a resolution of 3.1 Å, which represents the first structure of a TGF-β-neutralizing antibody in complex with its antigen. The structural information allows a detailed insight into the epitope recognition of GC-1008 and explains its high specificity toward TGF-β. It also highlights that GC-1008 is an artificially evolved mimic of both cognate TGF-β receptors.
Results
Structure Determination.
The GC-1008 Fab fragment used in this crystallographic study was generated by proteolytic digest from a clinical batch of the antibody in human IgG4 format. The variable sequences of GC-1008 were designed to be closest to human germ-line sequences DP-10 (VH1) and DPK22 (Vκ3) as described in Kabat et al. (34). Extensive crystallization trials were carried out with GC-1008 in the presence of a 0.5 molar ratio of all 3 human TGF-β isoforms, assuming an antibody-to-antigen composition of 2:1. For the cocrystallization with TGF-β3, 2 different crystal forms of identical morphology, belonging to the tetragonal space group P43212 and the orthorhombic space group P212121, appeared under the same crystallization condition. Crystals of the orthorhombic form diffracted to 3.1 Å and turned out to be superior to the tetragonal crystal form. The structure of the complex was solved by molecular replacement using the published structure of TGF-β3 (5) and the crystal structure of the unbound GC-1008 Fab fragment as templates. The structure of free GC-1008 could be determined to a resolution of 1.75 Å before the complex crystallization experiments (see supporting information (SI) Text and Fig. S1). The asymmetric unit contained 2 identical complex molecules that were refined to the final model using strict noncrystallographic symmetry (NCS) restraints.
Overall Structure of GC-1008–TGF-β3 Complex.
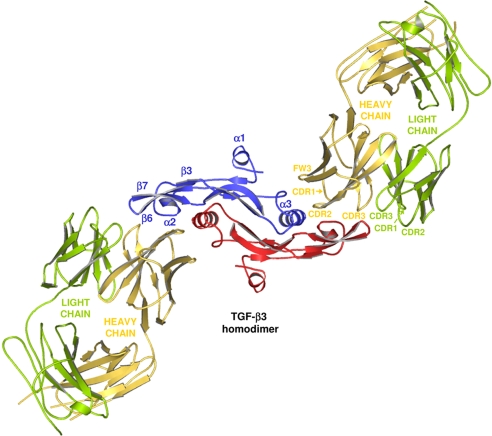
The complex consists of 1 TGF-β3 homodimer flanked by 2 GC-1008 Fab fragments (Fig. 1). The whole structure of the complex exhibits a 2-fold symmetry with its origin in the center of the TGF-β3 homodimer, which forms the core of the complex. The antibody-bound ligand is well-defined, with no disruptions or large structural rearrangements compared with the unbound form. All CDR loops of the heavy chain of GC-1008 are involved in the binding interactions, whereas the CDR loops of the light chain play only a minor role. The refined model comprises all residues of the TGF-β3 homodimer and all residues of the light chain, as well as most of the heavy-chain residues (1–134 and 139–220) of GC-1008. A temperature factor (B-factor) analysis revealed low-to-medium B values for the well-defined core and the binding interface (49.5 Å2 for the TGF-β3 homodimer and 64.4 Å2 for the variable part of GC-1008). In contrast to this, high B values (117.4 Å2 on average) were observed for the outer part of the complex containing the constant parts of the 2 bound Fab fragments, which results in a rather high average B value of 80.7 Å2 for the overall structure. These high B values at the rim of the complex are mainly a result of less-defined electron density caused by flexibility due to missing crystal contacts. As a consequence, the heavy-chain residues 135–138 belonging to a generally flexible loop part of GC-1008 are disordered and therefore absent in the complex structure.
Fig. 1.
Three-dimensional representation of the overall structure of the GC-1008–TGF-β3 complex shown as ribbon diagram. View is down the 2-fold symmetry axis according to Groppe et al. (14). The identical monomers of the TGF-β3 homodimer are depicted in blue and red. The bound Fab fragments are shown in yellow (heavy chain) and green (light chain).
A superposition of the variable part of GC-1008 alone and in complex with TGF-β3 showed that most of the CDR loops undergo only minor conformational changes upon binding to TGF-β3 (Fig. S2). Bigger changes are mainly located in CDR loops 1 and 2 as well as in framework 3 (FW3) of the heavy chain, caused by adaptations to the antigen.
Analysis of the GC-1008–TGF-β3 Interface.
Binding interface.
GC-1008 recognizes both monomers of the TGF-β3 homodimer and not only residues from a single monomer. The total binding area consists of 2 identical binding interfaces on the surface of the TGF-β3 homodimer. Each of them covers a surface area of ≈862 Å2 with a shape complementarity of 0.58. A careful analysis of the binding interface revealed that the binding of GC-1008 to TGF-β3 is predominantly based on hydrophobic interactions because >2/3 of the contact surface atoms are nonpolar. Only 3 hydrogen bonds and no salt bridges could be identified, which confirmed the hydrophobic character of this interaction, although the determination of water-mediated hydrogen bonds was not possible because of the limited resolution of the complex structure. An electron density figure (Fig. S3) and a summary of all important parameters describing the GC-1008–TGF-β3 interface (Table S1) can be found in the SI Text.
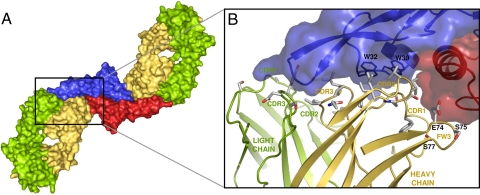
All CDR loops of the heavy chain are involved in the binding to TGF-β3 as well as some residues of the FW3 (Fig. 2 and Fig. S4). In contrast to this, only 7 residues of the light chain are involved. Hydrophobic residues of the CDR2 and CDR3 loop of the heavy chain, which represents ≈50% of the total interface accessible surface area, are mostly responsible for the main binding interaction. They bind to the hydrophobic pocket of the TGF-β3 homodimer, which includes 2 adjacent tryptophans. An additional binding interaction was found between Glu-74, Ser-75, and Ser-77 of FW3 and a large loop belonging to the second TGF-β3 monomer, which connects the N terminus of α-helix 3 to the rest of the molecule.
Fig. 2.
GC-1008–TGF-β3 binding interface. (A) Surface representation of the complex. The main binding interactions of the Fab fragments toward the TGF-β3 homodimer are accomplished by CDR loops of the heavy chains (yellow). (B) Contact residues of GC-1008 (gray) involved in the recognition of TGF-β3. The TGF-β3 homodimer is shown as transparent surface representation.
Epitope recognition.
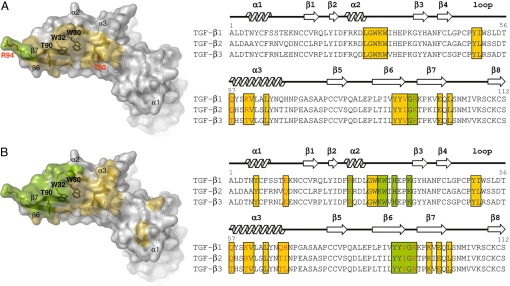
As mentioned above, GC-1008 recognizes parts of both TGF-β3 molecules of the homodimer. Residues of this epitope belong to the concave-shaped part of the homodimer, which is formed by the β-strands 6 and 7, the loop between helix 2 and β-strand 3, and the large α-helix 3 of the second TGF-β3 monomer together with its N-terminal loop (Fig. 3A). Two adjacent tryptophans (Trp-30 and Trp-32) and Tyr-90 represents a large aromatic patch within the inner part of this binding pocket. Although they would be excellent π–π interaction partners for aromatic side chains of an appropriate binding partner like the CDR-loops of an antibody, there are no aromatic residues present in the CDR loops of GC-1008 that take part in the main binding interactions. Instead, binding of these large residues is exclusively achieved by 4 hydrophobic, nonaromatic residues in CDR2 of the heavy chain (Ile-52, Ile-54, Val-55, and Ile-57).
Fig. 3.
TGF-β3 epitope recognition by GC-1008 and TGF-β receptor types I and II, highlighting the similarities for both epitopes. (A) Surface representation of TGF-β3 (Left) showing the epitope recognized by GC-1008. (Right) A sequence alignment of the 3 human TGF-β isoforms where the residues involved in binding to GC-1008 are highlighted in yellow (recognized by heavy chain) and green (recognized by light chain). (B) (Left) Surface representation of TGF-β3 showing the epitope recognized by TGF-β receptor type I (yellow) and TGF-β receptor type II (green). (Right) A sequence alignment of the 3 human TGF-β isoforms where the residues involved in binding to both receptors are highlighted. Red letters in the sequence alignments indicate residues from the interfaces that are nonconserved among the 3 different TGF-β isoforms.
Because GC-1008 is a panspecific antibody that recognizes all 3 isoforms of TGF-β, a detailed analysis was carried out for the epitope recognition of all 3 human isoforms. They are structurally very similar and share a high sequence identity. An alignment of the 3 different TGF-β isoforms revealed that only 4 residues involved in the binding interaction to GC-1008 are different among the 3 isoforms (Fig. 3A). Therefore, GC-1008 will most likely recognize the same epitope for all isoforms with highly comparable binding interactions. With the help of a proliferation assay with MLEC cells, the IC50 values for GC-1008 against the different TGF-β isoforms were determined and found to be in the low nanomolar range, showing only marginal variations with the lowest values for TGF-β1, followed by TGF-β3 and TGF-β2 (Table 1). Superimposing the crystal structures of TGF-β1 and 2 onto TGF-β3 in complex with GC-1008 revealed that the differences for the interacting residues within the TGF-β isoforms are very subtle, with the most notable differences at residues 60 and 94 (Fig. 3A). A lysine at position 60, as in TGF-β1, appears to result in a slightly stronger binding interaction. A reason for this could be the possible formation of a salt bridge between Lys-60 and a Glu-74 of FW3, whereas in the case of TGF-β3, Glu-74 is only involved in weak hydrophobic interactions. The formation of such a salt bridge would also be possible for TGF-β2, which has an arginine at that same position. However, the arginine of TGF-β2 is bulkier than the lysine of TGF-β1 and would, therefore, disfavor a tight interaction with the residues of FW3. Another significant difference can be found for residues 94 at the outermost edge of the TGF-β molecules. The Arg-94 of TGF-β1 and TGF-β3 allows interactions with the backbone of the distant CDR1 loop of the heavy chain of GC-1008 as well as some additional hydrophobic interactions with residues of the CDR3 loop. It covers 93 Å2 of the interface area, which represents >10% of the total interface accessible surface area and >50% of the interface area of the light chain. For TGF-β2, the Arg-94 is replaced by a lysine that most probably reduces this large contribution to the light-chain interaction, resulting in the observed lower binding affinity of TGF-β2 to GC-1008.
Table 1.
IC50 values for GC-1008 against different TGF-β isoforms (MLEC proliferation assay)
| TGF-β isoform | GC-1008 (n = 6) |
|---|---|
| TGF-β1 (40 pM) | 1 ± 2 |
| TGF-β2 (40 pM) | 14 ± 5 |
| TGF-β3 (40 pM) | 7 ± 2 |
IC50 values are given in nM. KD values for GC-1008 against TGF-β 1 and 3 have been determined in a competition assay with TGF-β receptor type II to be 50 pM and 250 pM, respectively.
Discussion
Comparison with TGFβ-Receptor Type I and II Binding.
Recently, the structure of the ternary signaling complex consisting of TGF-β3 bound to its type I and II receptors was published (14) (Fig. 4A). Because binding of GC-1008 to the different TGF-β isoforms is competitive with receptor binding, we compared the binding mode and epitope recognition for both structures.
Fig. 4.
Comparison of the GC-1008 binding mode with type I and type II TGF-β receptor binding. (A) Structure of the ternary TGF-β signaling complex consisting of TGF-β3 bound to its type I and II receptors. Structure is shown as depicted in ref. 14. (B) Schematic comparison of GC-1008 binding versus receptor binding.
To identify possible structural variations, the bound TGF-β3 molecules of both complexes were superimposed (Fig. S5). The rmsd of all main-chain atoms was calculated to be <1.2 Å, indicating that the ligands are structurally very similar. Both TGF-β3 molecules adopt a closed conformation, as observed in crystal structures of the free ligand (5). The only differences that could be identified are a slight change of the orientation of α-helix 3 and a displacement of β-strands 6 and 7 by 2.5 Å at the tip of TGF-β3. The observed displacement of these 2 β-strands is even more pronounced when compared with the unbound TGF-β3 (5 Å) and is mainly caused by adaptation to the CDR loops of GC-1008. Furthermore, the observed change in the orientation of α-helix 3 of TGF-β3 was not surprising because this helix, together with its connecting loop segment, is supposed to be a rather flexible region (35, 36). It allows a conformational adaptation of the TGF-β3 homodimer to its corresponding binding partners as observed in the TGF-β3–TβRII complex (37), in which this region was even disordered and accompanied by a massive rearrangement of the 2 monomers. In contrast to this, the conformation of α-helix 3 is fixed in both complexes compared here through binding to the receptor type I and the heavy chain of GC-1008, respectively. Beside the hypothesis of Groppe and coworkers (14) that steric hindrance or van der Waals contacts to an appropriate binding partner might restrict TGF-β3 to its closed state, they also proposed that the equilibrium between the open and closed conformations of TGF-β3 could be pH dependent, with the open state predominating at pH 4 and below. However, our observations clearly support the former hypothesis, because we determined the structure of TGF-β3 alone in an open conformation also at a near neutral pH (data not shown). This implies that the closed state of TGF-β3 in both complex structures is, indeed, caused by the presence of an appropriate binding partner that restricts the conformational flexibility of TGF-β3.
GC-1008 recognizes the same 2 β-strands (β6 and β7) at the outer part of the TGF-β3 homodimer as seen for the TGF-β receptor type II (Fig. 4). Additionally, the heavy chain of GC-1008 interacts with approximately the same surface area of TGF-β3 -the concave side of the TGF-β3 homodimer—as does TGF-β receptor type I. This indicates a similar binding mode for both complexes.
Epitope Recognition.
Seven of the 10 residues that are recognized by the TGF-β receptor type II are also involved in binding to GC-1008 (Fig. 3). Binding to these residues is accomplished by CDR loops 1 and 3 of the light chain and CDR loop 3 of the heavy chain of GC-1008. In contrast to GC-1008 binding, 2 residues of TGF-β3 (Arg-25 and Arg-94) are forming hydrogen-bonded ion pairs with 2 acidic residues of the TGF-β receptor type II (37). These interactions are suggested to be the main factor for the binding specificity of the different TGF-β isoforms toward the type II receptor. Because Arg-94 also plays a significant role in the binding interaction with GC-1008, this might be the reason for the similar binding preference observed for GC-1008, although not to such a large extent. Ten of the 15 residues that are involved in the binding interaction to TGF-β receptor type I are identical to residues belonging to the epitope recognized by the CDR1 and 2 and FW3 of the heavy chain. TβR1 recognizes 2 additional residues of α-helix 3 and has further binding interactions with residues that are located close to or within α-helix 1. These interactions are not observed for GC-1008. A detailed comparison of all important properties of both binding interfaces is given in Table S1.
Overall, the epitope recognition for both complexes is astonishingly similar. The structure of the TGF-β3–GC-1008 complex is, therefore, a structural mimetic of TGF-β type I and II receptor binding that reflects the competitive character of GC-1008 against TGF-β receptor binding. Although similar residues of TGF-β are recognized by both GC-1008 and the cognate receptors, the precise type of atomic interactions is very different for both binding events. GC-1008 appears to have found a parallel, ex vivo-derived solution on the one generated by in vivo evolution.
Conclusions
The structural studies together with the biological data presented in this article reveal that GC-1008 represents a potent TGF-β-neutralizing antibody. The binding mode of GC-1008 to TGF-β3 is very similar to that of TGF-β3 bound to both of its receptors (types I and II). GC-1008 is, therefore, a mechanistically different but functionally identical structural mimetic for TGF-β receptor binding. From the determined complex structure, detailed information of the binding interactions could be extracted, which allows for the explanation of the different binding affinities of GC-1008 toward the 3 TGF-β isoforms. The crystal structure of the panspecific antibody GC-1008 in complex with TGF-β3 provides structural insight into the binding mode of a TGF-β neutralizing antibody to its target. It therefore provides a mechanistic understanding of the mode of action of this exciting therapeutic approach.
Methods
Expression and Purification of GC-1008 Fab Fragment and TGFβ-3.
GC-1008 IgG was expressed in a NS0 cell line and purified from conditioned media by absorption to and elution from ProSep A (Millipore). The purified GC-1008 IgG was incubated with papain (Sigma) in PBS (pH 7.2) containing 30 mM cysteine at a ratio of 0.1 mg of papain to 100 mg of IgG to produce the Fab fragment. After incubation for 6 h, the digest was terminated by addition of iodoacetamide to a final concentration of 50 mM. The Fab fragment was purified by applying the digest to Q-Sepharose at pH 7.2, followed by collection of the unbound Fab fragment. After concentration, the Fab fragment was further purified by gel filtration using a Sephacryl S-200 HR column. For crystallization, the pure GC-1008 was dialyzed and concentrated to 26.6 mg ml−1 in 50 mM Tris (pH 7.5) and 50 mM NaCl. Biologically active human TGF-β3 was prepared and purified as described previously (5, 38).
Preparation, Crystallization, and Structure Determination of the GC-1008–TGF-β3 Complex.
For the preparation of the complex, GC-1008 (at a concentration of 20.0 mg ml−1 in 50 mM Tris (pH 7.5) and 50 mM NaCl) was slowly added to TGF-β3 [at a concentration of 9.1 mg ml−1 in 50 mM Na-citrate (pH 3.5)] until reaching a 2.0-fold molar excess of GC-1008. The mixture was incubated on ice for 30 min and then centrifuged at 20,000 × g for 45 min. The supernatant was directly used for crystallization trials without further purification. The complex was crystallized by vapor diffusion at 4 °C against well solution containing 0.1 M sodium citrate (pH 6.5–7.0), 20–25% tert-Butanol and 200 mM phenoxyacetic acid, by using the sitting-drop technique. The crystals were cryoprotected by using reservoir buffer containing 25% (vol/vol) PEG400. A dataset from a single crystal was collected at the X06SA beamline of the Swiss Light Source (PSI, Villigen, Switzerland) to a resolution of 3.1 Å. The diffraction data were processed with XDS (39). The structure of the complex was solved by molecular replacement with PHASER (40) using the published structure of TGF-β3 (5) (PDB ID code 1TGJ) and the refined structure of GC-1008 as templates. The asymmetric unit contained 2 GC-1008–TGFβ3 complexes, each composed of 2 GC-1008 molecules bound to 1 TGFβ3 homodimer. The model was refined with CNS (41) at a resolution of 3.1 Å by using several rounds of model building and simulated annealing to prevent model bias, resulting in final Rcryst and Rfree values of 25.5% and 27.9%, respectively. Because of the rather low resolution, the B factors were refined by using the group B factor function of CNS to avoid overrefinement. During refinement, the structure was manually corrected by using the program COOT (42). Model geometry and stereochemistry were checked with PROCHECK (43). Data collection and structure refinement statistics are summarized in Table S2.
Mink Lung Epithelial Cell (MLEC) Proliferation Assay.
The IC50 values of GC-1008 against the 3 different TGFβ isoforms were determined by using the MLEC proliferation assay described by Danielpour et al. (44). In principle, this assay is based on the inhibition of the serum-induced MLEC proliferation in response to TGF-β1, TGF-β2, or TGF-β3. Antibodies (scFvs or IgGs) were tested for neutralization of TGF-β1, TGF-β2, or TGF-β3, resulting in the restoration of the cell proliferation. Proliferation was measured by the uptake of [3H]thymidine. The potency of the antibody was defined as the concentration of the antibody that neutralized a single concentration of TGF-β1, TGF-β2, or TGF-β3 at a level of 50% (IC50) in nanomoles.
Supplementary Material
Acknowledgments.
We thank the Swiss National Science Foundation for financial support.
Footnotes
Conflict of interest statement: C.G. and M.G.G. declare no conflict of interest; T.W., R.T., S.P., D.F., M.M., and L.J. are employees of MedImmune; and S.L. is an employee of Genzyme.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 3EO0 and 3EO1).
This article contains supporting information online at www.pnas.org/cgi/content/full/0807200106/DCSupplemental.
References
- 1.Massague J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- 2.Roberts AB, Sporn MB. In: Peptide Growth Factors and their Receptors. Roberts AB, Sporn MB, editors. Heidelberg: Springer; 1990. pp. 421–472. [Google Scholar]
- 3.Daopin S, Li M, Davies DR. Crystal structure of TGF-beta 2 refined at 1.8 A resolution. Proteins. 1993;17:176–192. doi: 10.1002/prot.340170207. [DOI] [PubMed] [Google Scholar]
- 4.Hinck AP, et al. Transforming growth factor beta 1: Three-dimensional structure in solution and comparison with the X-ray structure of transforming growth factor beta 2. Biochemistry. 1996;35:8517–8534. doi: 10.1021/bi9604946. [DOI] [PubMed] [Google Scholar]
- 5.Mittl PR, et al. The crystal structure of TGF-beta 3 and comparison to TGF-beta 2: Implications for receptor binding. Protein Sci. 1996;5:1261–1271. doi: 10.1002/pro.5560050705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nickel J, Kotzsch A, Sebald W, Mueller TD. A single residue of GDF-5 defines binding specificity to BMP receptor IB. J Mol Biol. 2005;349:933–947. doi: 10.1016/j.jmb.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 7.Scheufler C, Sebald W, Hulsmeyer M. Crystal structure of human bone morphogenetic protein-2 at 2.7 A resolution. J Mol Biol. 1999;287:103–115. doi: 10.1006/jmbi.1999.2590. [DOI] [PubMed] [Google Scholar]
- 8.Schlunegger MP, Grutter MG. Refined crystal structure of human transforming growth factor beta 2 at 1.95 A resolution. J Mol Biol. 1993;231:445–458. doi: 10.1006/jmbi.1993.1293. [DOI] [PubMed] [Google Scholar]
- 9.Koli K, et al. Latency, activation, and binding proteins of TGF-beta. Microsc Res Tech. 2001;52:354–362. doi: 10.1002/1097-0029(20010215)52:4<354::AID-JEMT1020>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 10.Munger JS, et al. Latent transforming growth factor-beta: Structural features and mechanisms of activation. Kidney Int. 1997;51:1376–1382. doi: 10.1038/ki.1997.188. [DOI] [PubMed] [Google Scholar]
- 11.Bartram U, et al. Double-outlet right ventricle and overriding tricuspid valve reflect disturbances of looping, myocardialization, endocardial cushion differentiation, and apoptosis in TGF-beta(2)-knockout mice. Circulation. 2001;103:2745–2752. doi: 10.1161/01.cir.103.22.2745. [DOI] [PubMed] [Google Scholar]
- 12.Kulkarni AB, Karlsson S. Transforming growth factor-beta 1 knockout mice. A mutation in one cytokine gene causes a dramatic inflammatory disease. Am J Pathol. 1993;143:3–9. [PMC free article] [PubMed] [Google Scholar]
- 13.Proetzel G, et al. Transforming growth factor-beta 3 is required for secondary palate fusion. Nat Genet. 1995;11:409–414. doi: 10.1038/ng1295-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groppe J, et al. Cooperative assembly of TGF-beta superfamily signaling complexes is mediated by two disparate mechanisms and distinct modes of receptor binding. Mol Cell. 2008;29:157–168. doi: 10.1016/j.molcel.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 15.Wrana JL, et al. Cell. 1992;71:1003–1014. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- 16.Cheifetz S, et al. Distinct transforming growth factor-beta (TGF-beta) receptor subsets as determinants of cellular responsiveness to three TGF-beta isoforms. J Biol Chem. 1990;265:20533–20538. [PubMed] [Google Scholar]
- 17.Zuniga JE, et al. Assembly of TbetaRI:TbetaRII:TGFbeta ternary complex in vitro with receptor extracellular domains is cooperative and isoform-dependent. J Mol Biol. 2005;354:1052–1068. doi: 10.1016/j.jmb.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 18.Sankar S, et al. Expression of transforming growth factor type III receptor in vascular endothelial cells increases their responsiveness to transforming growth factor beta 2. J Biol Chem. 1995;270:13567–13572. doi: 10.1074/jbc.270.22.13567. [DOI] [PubMed] [Google Scholar]
- 19.Wrana JL, et al. Mechanism of activation of the TGF-beta receptor. Nature. 1994;370:341–347. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- 20.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 21.ten Dijke P, Miyazono K, Heldin CH. Signaling inputs converge on nuclear effectors in TGF-beta signaling. Trends Biochem Sci. 2000;25:64–70. doi: 10.1016/s0968-0004(99)01519-4. [DOI] [PubMed] [Google Scholar]
- 22.Chung KF. Cytokines as targets in chronic obstructive pulmonary disease. Curr Drug Targets. 2006;7:675–681. doi: 10.2174/138945006777435263. [DOI] [PubMed] [Google Scholar]
- 23.Lijnen PJ, Petrov VV, Fagard RH. Induction of cardiac fibrosis by transforming growth factor-beta(1) Mol Genet Metab. 2000;71:418–435. doi: 10.1006/mgme.2000.3032. [DOI] [PubMed] [Google Scholar]
- 24.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 25.Yu L, Border WA, Huang Y, Noble NA. TGF-beta isoforms in renal fibrogenesis. Kidney Int. 2003;64:844–856. doi: 10.1046/j.1523-1755.2003.00162.x. [DOI] [PubMed] [Google Scholar]
- 26.Siegel PM, Massague J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer. 2003;3:807–821. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
- 27.Tang B, et al. TGF-beta switches from tumor suppressor to prometastatic factor in a model of breast cancer progression. J Clin Invest. 2003;112:1116–1124. doi: 10.1172/JCI18899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arteaga CL. Inhibition of TGFbeta signaling in cancer therapy. Curr Opin Genet Dev. 2006;16:30–37. doi: 10.1016/j.gde.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 29.Iyer S, et al. Targeting TGFbeta signaling for cancer therapy. Cancer Biol Ther. 2005;4:261–266. doi: 10.4161/cbt.4.3.1566. [DOI] [PubMed] [Google Scholar]
- 30.Saunier EF, Akhurst RJ. TGF beta inhibition for cancer therapy. Curr Cancer Drug Targets. 2006;6:565–578. doi: 10.2174/156800906778742460. [DOI] [PubMed] [Google Scholar]
- 31.Liu VC, et al. Tumor evasion of the immune system by converting CD4+CD25- T cells into CD4+CD25+ T regulatory cells: Role of tumor-derived TGF-beta. J Immunol. 2007;178:2883–2892. doi: 10.4049/jimmunol.178.5.2883. [DOI] [PubMed] [Google Scholar]
- 32.Terabe M, et al. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: Abrogation prevents tumor recurrence. J Exp Med. 2003;198:1741–1752. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Günther A, Markart P, Eickelberg O, Seeger W. Pulmonary fibrosis—A therapeutic dilemma? Med Klin (Munich) 2006;101:308–312. doi: 10.1007/s00063-006-1039-3. [DOI] [PubMed] [Google Scholar]
- 34.Kabat EA, Wu TT. Identical V region amino acid sequences and segments of sequences in antibodies of different specificities. Relative contributions of VH and VL genes, minigenes, and complementarity-determining regions to binding of antibody-combining sites. J Immunol. 1991;147:1709–1719. [PubMed] [Google Scholar]
- 35.Bocharov EV, et al. Sequence-specific 1H and 15N assignment and secondary structure of transforming growth factor beta3. J Biomol NMR. 2000;16:179–180. doi: 10.1023/a:1008315600134. [DOI] [PubMed] [Google Scholar]
- 36.Pellaud J, Schote U, Arvinte T, Seelig J. Conformation and self-association of human recombinant transforming growth factor-beta3 in aqueous solutions. J Biol Chem. 1999;274:7699–7704. doi: 10.1074/jbc.274.12.7699. [DOI] [PubMed] [Google Scholar]
- 37.Hart PJ, et al. Crystal structure of the human TbetaR2 ectodomain—TGF-beta3 complex. Nat Struct Biol. 2002;9:203–208. doi: 10.1038/nsb766. [DOI] [PubMed] [Google Scholar]
- 38.Cerletti N. Novel process for the production of biologically active dimeric protein. EP 95/02718. 1995;MX9700656(A):1–85. [Google Scholar]
- 39.Kabsch W. Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J Appl Crystallogr. 1993;26:795–800. [Google Scholar]
- 40.Read RJ. Pushing the boundaries of molecular replacement with maximum likelihood. Acta Crystallogr D. 2001;57:1373–1382. doi: 10.1107/s0907444901012471. [DOI] [PubMed] [Google Scholar]
- 41.Brunger AT, et al. Crystallography and NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr D. 1998;54(Pt 5):905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 42.Emsley P, Cowtan K. Coot: Model-building tools for molecular graphics. Acta Crystallogr D. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 43.Laskowski RA, McArthur MW, Moss DS, Thornton JM. PROCHECK: A program to check the stereochemical quality of protein structures. J Appl Crystallogr. 1993;26:263–291. [Google Scholar]
- 44.Danielpour D, et al. Immunodetection and quantitation of the two forms of transforming growth factor-beta (TGF-beta 1 and TGF-beta 2) secreted by cells in culture. J Cell Physiol. 1989;138:79–86. doi: 10.1002/jcp.1041380112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.