Abstract
The Mst1 and Mst2 protein kinases are the mammalian homologs of hippo, a major inhibitor of cell proliferation in Drosophila. Mst1 is most abundant in lymphoid tissues. Mice lacking Mst1 exhibit markedly reduced levels of the Mst1 regulatory protein Nore1B/RAPL in lymphoid cells, whereas Mst2 abundance is unaltered. Mst1-null mice exhibit normal T cell development but low numbers of mature naïve T cells with relatively normal numbers of effector/memory T cells. In vitro, the Mst1-deficient naïve T cells exhibit markedly greater proliferation in response to stimulation of the T cell receptor whereas the proliferative responses of the Mst1-null effector/memory T cell cohort is similar to wild type. Thus, elimination of Mst1 removes a barrier to the activation and proliferative response of naïve T cells. The levels of Mst1 and Nore1B/RAPL in wild-type effector/memory T cells are approximately 10% those seen in wild-type naïve T cells, which may contribute to the enhanced proliferative responses of the former. Freshly isolated Mst1-null T cells exhibit high rates of ongoing apoptosis, a likely basis for their low numbers in vivo; they also exhibit defective clustering of LFA-1, as previously observed for Nore1B/RAPL-deficient T cells. Among known Mst1 substrates, only the phosphorylation of the cell cycle inhibitory proteins MOBKL1A/B is lost entirely in TCR-stimulated, Mst1-deficient T cells. Mst1/2-catalyzed MOBKL1A/B phosphorylation slows proliferation and is therefore a likely contributor to the anti-proliferative action of Mst1 in naïve T cells. The Nore1B/RAPL-Mst1 complex is a negative regulator of naïve T cell proliferation.
Keywords: Mob1, Mst1 knockout, RAPL, hippo, Stk4
Mst1 and Mst2 are closely related mammalian class II GC protein (ser/thr) kinases (1) whose overexpression induces apoptosis in many transformed cell lines through a combination of p53- and Jnk-dependent pathways (2–4). Regarding the physiological roles of these kinases, Kinashi and colleagues demonstrated that the ability of αCD3/αCD28 and chemokines to promote the Rap1-GTP-dependent integrin clustering necessary for T cell adhesion and migration (5) is strongly dependent on Mst1 (6), which is proposed to be activated through the Rap1-GTP recruitment of a Nore1B (also called RAPL)/Mst1 complex to the immunological synapse (7). The Mst1 substrate(s) that mediate this response is (are) not known. Mst1 and Mst2 are found in constitutive complexes with members of the Rassf polypeptide family (8, 9), at least two of which, RASSF1A and Nore1A/RASSF5, are well established tumor suppressors (10). Nevertheless, direct evidence in support of a tumor suppressor function for Mst1 and/or Mst2 is not yet available.
Mst1 and Mst2 resemble most closely the drosophila protein kinase hippo and human Mst2 can complement hippo deficiency (11). Loss of hippo function results in massive overgrowth of the fly eye, due to an acceleration of cell proliferation together with a deficit in developmental apoptosis (11–14). The pathway downstream of hippo has been extensively described (15–17). Hippo phosphorylates the protein kinase warts/LATS, which is facilitated by the binding of both kinases to the noncatalytic scaffold protein salvador/shar-pei; hippo also phosphorylates the noncatalytic protein MATS, which then binds to warts/LATS, promoting its autophosphorylation and activation. Activated warts, in turn, phosphorylates and negatively regulates the transcriptional coactivator yorkie. Loss of hippo, warts/LATS, Salvador, or MATS each results in overgrowth of a similar nature, whereas yorkie loss-of-function reduces growth and is epistatic to the other components. Studies in mammalian systems indicate that this pathway is largely conserved; LATS1 (18) and MOBKL1A/B (19), the mammalian orthologs of warts/LATS and MATS, are Mst1/2 substrates and negative regulators of cell proliferation (19, 20). Moreover, mice lacking LATS1 develop soft tissue sarcomas and ovarian tumors (21). Reciprocally, YAP, a mammalian homolog of yorkie, is phosphorylated and negatively regulated by LATS1 (22–24). YAP is amplified in a wide variety of murine and human tumors (25); overexpression of YAP transforms mammary epithelial cells in vitro (25) and produces hyperplasia and cancer in mouse liver (22, 26). As regards other outputs, Mst1 activated by oxidant stress phosphorylates the Foxo1/3a polypeptides in their forkhead domain, disrupting 14–3-3 binding and inhibiting the ability of Akt to promote Foxo1/3a nuclear exit (27).
To define more fully the physiologic roles of Mst1, we generated mice lacking Mst1 polypeptide expression.
Results
Mst1-Deficient Lymphoid Cells Exhibit Much Lower Nore1B Polypeptide but Unaltered Mst2.
Mice lacking Mst1 polypeptide expression were generated from an ES cell line bearing a gene trap inserted between the first and second exons (see supporting information (SI) Fig. S1). Mst1-deficient mice survive development, are fertile, and appear unremarkable. Mst1 is expressed at greatest abundance in lymphoid organs (Fig. 1A); lymphocytes from the Mst1 trap/trap mice (henceforth called Mst1−/−) lack Mst1 polypeptide but show unaltered levels of Mst2 (Fig. 1B Left). Mst1 can be coprecipitated with rassf family polypeptides (9); in lymphocytes, the Rassf5 isoform Nore1B/RAPL, a Ras-Rap-binding protein, is a major Mst1 partner (6). Surprisingly, the level of the Nore1B/RAPL polypeptide is greatly diminished in the Mst1-null lymphocytes (Fig. 1B Left) despite unaltered Nore1B mRNA levels (Fig. 1B Right), whereas the homologous protein Rassf1C is unaltered. The decrease in Nore1B polypeptide in the Mst1−/− cells is probably due to the lack of Mst1 polypeptide per se, inasmuch as transient coexpression of Nore1B with either wild-type Mst1 or kinase-dead Mst1(K59R) in HEK293 cells results in higher steady-state levels of Nore1B polypeptide than when Nore1B is expressed alone (Fig. 1C).
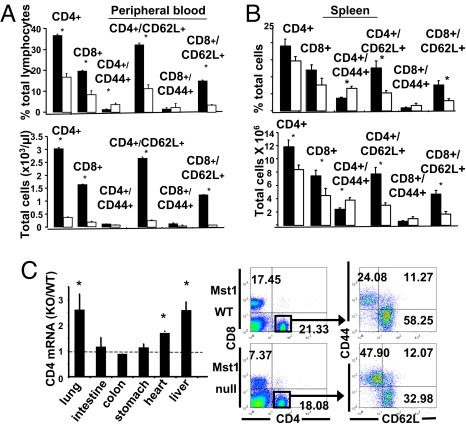
Fig. 1.
Characterization of Mst1-null mice. (A) Expression of Mst1, Mst2 and some other relevant polypeptides in mouse tissues. Mst1/2, Nore1B, and Mob1 expression in mouse organs were analyzed by immunoblot and anti-actin served as a loading control. (B) Polypeptide and mRNA expression in spleen from wild-type and Mst1 gene trap mice. Left, Immunoblot of spleen extracts for the polypeptides indicated. Right, Northern blot of splenic RNA. 18S and 28S ribosomal RNAs serve as internal controls. (C) The Mst1 polypeptide enhances the level of coexpressed Nore1B/RAPL. Vector encoding FLAG-tagged Nore1B was cotransfected in HEK293 cells with empty vector or vector encoding FLAG-Mst1(WT) or FLAG-Mst1(K59R). An immunoblot of the extracts for Mst1, Nore1B, and endogenous actin is shown.
Mst1-Deficient Mice Exhibit Intact T Cell Development but Fewer Naïve Peripheral T Cells and a High Proportion of Effector/Memory T Cells in Liver and Lung.
The thymus in the Mst1−/− mice is normal in size and histologic appearance (Fig. S2A) and the total number of thymocytes is comparable with Mst1+/+ littermates; the fraction of both CD4+ and CD8+ cells is slightly, but not significantly increased (Fig. S2 B and C). In the blood the number of CD4+, CD8+ and total T cells is greatly reduced (Fig. 2A). The Mst1−/− spleen shows a reduction in white pulp (Fig. S3A) and the numbers of CD4+, CD8+, and total T cells are also reduced (Fig. 2B); in addition the number of B220+ cells is diminished (data not shown) and, as observed in mice lacking expression of Nore1B/RAPL (7), marginal zone B cells are essentially absent (Fig. S3 B and C). In the Mst1−/− spleen (Fig. 2B and Fig. S4B) and lymph nodes (Fig. S5) as well as in the circulation (Fig. 2A and Fig. S4A), the fraction of CD4+ and CD8+ exhibiting the CD62Lhi/CD44lo naïve phenotype (28) is markedly reduced, whereas the fraction of CD4+ and CD8+ cells bearing the CD62Llo/CD44hi phenotype (a mixture of activated effector and memory cells) is reciprocally increased; the absolute numbers of CD62Llo/CD44hi cells in the circulation (Fig. 2A) and spleens (Fig. 2B) of the Mst1-null and wild-type mice are generally similar, although significantly higher number of CD4+CD62Llo/CD44hi cells are present in the spleens of Mst1-null mice. The abundance of regulatory T cells (Treg), as reflected by the numbers of CD4+ Foxp3+ cells (29), is similar in the wild-type and Mst1−/− spleens (Fig. S6). We compared some nonlymphoid tissues of wild-type and Mst1-null mice for the abundance of T cells. Notably, the relative abundance of CD4 mRNA in Mst1-null lung and liver is 2–2.5-fold greater than in wild-type tissues (Fig. 2C Left). A cytofluormetric analysis of CD4+ cells in digests of lungs from wild-type or Mst1-null mice (Fig. 2C Right) demonstrates that the CD4+ cells residing in the lung of the Mst1-null mice exhibit a far higher proportion of the CD62Llo/CD44hi effector/memory phenotype than the CD4+ cells resident in the lung of wild-type mice. In summary, the secondary lymphoid organs of the Mst1-null mice contain fewer mature, naïve T cells but similar or slightly higher numbers of effector/memory cells; conversely, the lung, liver and heart of the Mst1-null mice contain higher numbers of CD4+ cells that, in the lung, are predominantly effector/memory cells.
Fig. 2.
Peripheral T cell cohorts in wild-type and Mst1-deficient mice. (A) T lymphocytes in the blood of wild-type and Mst1-null mice. The number of CD4+, CD8+, and naïve CD4+ and CD8+ T cells in the peripheral blood of Mst1-null mice compared with wild-type mice (n = 4 pairs of Mst1-null and wild-type littermates). Asterisks in this and all subsequent figures indicates a P < 0.05 or lower calculated from a two-tailed Student t test. Black bars are Mst1+/+ and white bars are Mst1−/− in this and all later figures. (B) T cell populations in the Mst1-null and wild-type spleen. Splenocytes isolated from Mst1-null and littermate wild-type mice were stained with antibodies to CD4, CD8, CD62L, and CD44 and analyzed by cytofluorimetry. There is a significant reduction in the total numbers of CD4+ and CD8+ T cells in the Mst1-null spleens that is due largely to a reduction in the total numbers of naïve CD4+/CD62Lhi and CD8+/CD62Lhi subsets. There is a reciprocal increase in the percentage of effector/memory CD4+ and CD8+ cells (labeled CD44+) but the total cell numbers in those subsets is comparable or slightly increased in the Mst1-null (n = 6 pairs of Mst1-null and wild-type littermates). (C) The relative abundance of CD4+ mRNA and CD4+ T cell subsets in some nonlymphoid tissues. Left, Total RNA was isolated from the indicated tissues of wild-type and Mst1-null mice. The amount of CD4 mRNA relative to that of β-actin was measured by Q-PCR and the value for the Mst1-null (KO) was divided by the wild-type value. The abundance of CD4 mRNA in the lung, heart, and liver of Mst1-null mice is significantly higher than that in the corresponding wild-type tissue. Right, the CD4+ cells present in digests of the lungs of wild-type and Mst1-null mice were analyzed for expression of CD62L and CD44 by cytofluorimetry. An equal number of cells from each digest was counted.
Mst1-Deficient Naïve T Cells Exhibit Much Stronger Proliferative Responses to αCD3.
We next examined some functional responses of purified peripheral T cells. The incorporation of 3H-thymidine in response to both α-CD3 and to α-CD3/α-CD28 in vitro in total T cells from Mst1-null spleens is two- to fivefold greater than in T cells from spleens of wild-type littermates (Fig. 3A). This hyperproliferative response of Mst1-deficient total splenic T cells is also seen in cells stained with CFSE, which undergo some cell division in vitro even in the absence of any added stimulus (Fig. S7). Examining the CD4+ subsets, it is evident that the up-regulated proliferative response is due entirely to a marked hyperproliferation of the Mst1-null CD62Lhi naïve cells (Fig. 3B); furthermore, their high incorporation of 3H-thymidine in response to α-CD3 is only modestly augmented by the additional stimulation with αCD28. In contrast, the Mst1-null CD4+/CD62Llo cells show a proliferative response that is similar to that of the matched wild-type subset. Thus, the deficiency of Mst1 results in a dramatic up-regulation and disinhibition of the proliferative response of naïve T cells to engagement of the T cell receptor but little or no change in the response of the effector/memory cohort.
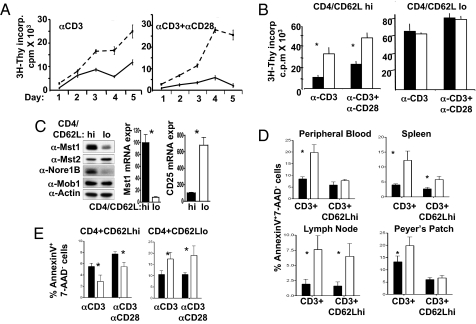
Fig. 3.
Proliferative and apoptotic responses in Mst1-null and wild-type T cells. (A) (3H) thymidine incorporation into Mst1-null and wild-type T cells. Wild-type (solid line) or Mst1-null (dashed line) splenic T cells purified by CD90 (Thy 1.2) microbeads were stimulated with plate-bound anti-CD3 (1 μg/ml) without or with anti-CD28 (1 μg/ml). (3H) thymidine incorporation was determined in triplicate at the times indicated. One of four experiments with similar results is shown. (B) (3H) thymidine incorporation into T cell subsets. Purified CD4+ CD62Lhi (naïve) (Left) and CD62Llo cells (Right) were isolated from the spleens of wild-type (black bars) and Mst1-null (white bars) mice. (3H) thymidine incorporation was determined after incubation for 48 h with anti-CD3 or anti-CD3+ anti-CD28. The results shown are the mean and standard errors of triplicate determinations from one of four independent experiments with similar results. (C) Mst1 polypeptide and mRNA are greatly reduced in wild-type effector/memory compared with wild-type naïve CD4+ cells. The whole cell lysates prepared from naïve and effector/memory CD4+ cells were analyzed by immunoblot with anti-Mst1, -Mst2, -Nore1B, and -Mob1. Actin served as a loading control. RNA was isolated from these cells and real-time PCR performed with specific primers to Mst1 and CD25. (D) Annexin V binding to freshly isolated total and naïve T cells from Mst1-null and wild-type littermates. Annexin V+/7AAD-staining of lymphocytes freshly isolated from spleen, peripheral blood, lymph node, and Peyer's patches of 8- to 9-week wild-type (black bars) and Mst1-null (white bars) mice. The bars represent the mean percentage and SD of Annexin V+/7AAD- cells from four independent experiments. (E) Annexin V binding by T cells after stimulation in vitro. Annexin V+/7AAD-staining of naïve and memory CD4+ T cells stimulated with anti-CD3 or anti-CD3+ anti-CD28 for 48 h. Shown is the mean and SE of triplicate measurements from one of two experiments.
The Mst1-null CD4+ T cells also show enhanced production of the cytokines IL-2, IFN-γ, and IL-4 in response to α-CD3, both in the absence and presence of α-CD28 (Fig. S8). In contrast to the hyperproliferative response, which is observed only in the Mst1-null naïve T cells, both the naïve and effector/memory T cell cohorts from Mst1-null mice exhibit enhanced cytokine production. Elimination of Mst1 does not, however, alter the proclivity of naïve CD4+ cells to differentiate toward a Th1 or Th2 phenotype in vitro in response to the appropriate stimulation (Fig. S9).
In Normal Mice, Mst1 and Nore1B Polypeptide Levels Are Greatly Reduced in Effector/Memory Compared With Naïve T Cells.
The marked disinhibition of the proliferative response engendered by Mst1 removal led us to inquire whether down-regulation of Mst1 expression is a normal part of the program for the transition of T cells from a naïve to an activated effector or memory phenotype. Strongly in support of this view, the abundance of the Mst1 polypeptide (Fig. 3C Left) and mRNA (Fig. 3C Center) in wild-type CD4+/CD62Llo effector/memory cells is reduced approximately 10-fold compared with that in the wild-type CD4+/CD62Lhi naïve cells, whereas the content of Mst2 polypeptide (Fig. 3C Left) is similar in these two subsets. Moreover, the abundance of the Nore1B polypeptide (Fig. 3C Left) in the CD4+/CD62Llo subset is reduced in parallel to Mst1 and to a similar extent. These data identify Mst1 as a likely determinant of the threshold for activation of naïve T cells (30). Naïve cells lacking Mst1 gene expression entirely, as well as wild-type memory cells, which exhibit greatly reduced levels of Mst1 and Nore1B, both exhibit a much more robust proliferative response to TCR stimulation compared with wild-type naïve cells. The augmented cytokine responses of the Mst1-null effector/memory CD4+ cohort (Fig. S8), unaccompanied by an augmented proliferative response (Fig. 3B Right) implies that the low level of Mst1 that prevails in differentiated CD4+ cells no longer exerts an antiproliferative effect but continues to restrain T cell cytokine production.
Freshly Isolated Mst1-Deficient T Cells Exhibit High Rates of Ongoing Apoptosis.
The occurrence of a hyperproliferative response of Mst1-null naïve T cells to stimulation of the TCR in vitro together with low numbers of total T cells in the spleen and lymph nodes in vivo raises the possibility that the naïve cells may become activated in vivo even without exogenous immunization and lost thereafter through apoptosis (31). We therefore measured the Annexin V surface reactivity of freshly isolated total T cells, an indicator of ongoing apoptosis. Regardless of the source, the prevalence of Annexin V reactivity is much higher in the freshly isolated Mst1-null T cells than the wild-type T cells (Fig. 3D), consistent with an increased rate of T cell apoptosis in vivo. Overexpression of Mst1 per se is well known to promote apoptosis (8), so that the higher rate of in vivo apoptosis in the Mst1-null T cells appeared paradoxical. We therefore examined the occurrence of apoptosis in vitro in response to αCD3 and αCD3+αCD28 (Fig. 3E). In response to TCR stimulation in vitro, the Mst1-null naïve T cells do show less apoptosis compared with wild-type naïve cells; in contrast, the Mst1-null effector/memory cells continue to exhibit in vitro the higher rate of apoptosis seen in vivo, indicating that Mst1 does not exert an appreciable proapoptotic effect in this subset.
Elimination of Mst1 Inhibits αCD3-Induced LFA-1 Clustering.
The low numbers of peripheral CD4/CD62L hi cells in the Mst1-null mice led us to examine the expression of various chemokine and adhesion receptors on these cells compared with wild type (Fig. S10). The abundance of mRNA encoding KLF2, a transcription factor critical for maintenance of the chemokine receptor pattern of naïve T cells (32), and the mRNAs encoding CCR7 and CXCR4, the dominant chemokine receptors in naïve T cells (33), is similar in wild-type and Mst1-null CD4/CD62Lhi cells (Fig. S10 Upper). Although their absolute abundance is very low, modest up-regulation of CCR6 and CXCR3 mRNA is evident in the Mst1-null cells (Fig. S10 Lower), perhaps reflecting some admixture of central memory cells (33). These data indicate that the low numbers of peripheral CD4/CD62Lhi cells in the Mst1-null mice are not due to altered expression of their chemokine or adhesion receptors.
Elimination of Nore1B/RAPL (7) or depletion of Mst1 (6) is reported to interfere with inside-out signaling to T cell integrins. In confirmation, we find that elimination of Mst1 reduces the ability of TCR activation and chemokines to promote T cell adhesion to ICAM-1 whereas the response to PMA is unaffected (Fig. 4A Left). In addition, the migration of Mst1-null T cells toward CCL21/SLC is also substantially reduced, although in comparing CD4+ naïve cells from Mst1 and wild-type spleens, the magnitude of this reduction, although significant, is less (Fig. 4A Right). The ability of α-CD3 or SLC to induce the clustering of LFA-1 is also diminished in the Mst1-null T cells (Fig. 4B). Thus, as with the deletion of Nore1B/RAPL itself, the elimination of Mst1, which is accompanied by a marked decrease in Nore1B/RAPL, impairs LFA-1 clustering and adhesion.
Fig. 4.
The effect of Mst1 deletion on T cell adhesion and migration. (A) Left, Mst1 deficiency impairs inside-out activation of T cell binding to ICAM-1. CD3+ T cells from Mst1-null and wild-type littermates were stimulated in ICAM-1-coated wells (3 μg/ml) for 30 min at 37°C with carrier, anti-CD3, 100 nM SLC, or 10 ng/ml PMA. After washing the number of retained cells were counted and expressed as % of input. The mean and SE of triplicate determinations from one of two independent experiments is shown. Right, Transwell migration of T cells toward SLC. CD3+ or CD4+CD62Lhi cells (1 × 106) from Mst1-null and wild-type littermates were placed in a 24-transwell migration chamber. Cells were permitted to migrate through a 5-μm filter for 3 h into a lower chamber containing 0 or 200 ng/ml SLC. The mean and SE of triplicate measurements from one of two independent experiments are shown. (B) LFA-1 clustering in Mst1-null and wild-type cells. CD3+ T cells from Mst1-null and wild-type littermate mice were stimulated with 100 nM SLC or 10 μg/ml anti-CD3 (145–2C11) for 15 min. followed by fixation in 4% paraformaldehyde. The fixed cells were stained with anti-LFA-1 α (M17/4), followed by Texas red conjugated anti-Rat IgG. Original magnification was 100×; a single cell is shown in the Inset. Similar results were obtained in two independent experiments.
T Cell Tyrosine Phosphorylation, Ca++ Mobilization, and MAPK Activation Are Unimpaired by Mst1 Deficiency.
To gain insight into the molecular basis for the hyperproliferative response exhibited by Mst1−/− naive cells, we examined several of the signal transduction pathways relevant to T cell growth control (34, 35). The increase in tyrosine phosphorylation into total cellular polypeptides and specifically into CD3ζ, ZAP70, Lck, and PLCγ in response to α-CD3+α-CD28 is similar in splenic T cells from wild-type and Mst1-null mice (Fig. S11A). In addition, the increase in cytosolic (Ca2+) in Mst1-null and wild-type CD4+/CD62Lhi naïve T cells in response to cross-linking α-CD3 is identical (Fig. S11B). Examining more distal responses, the ability of αCD3+αCD28 to stimulate the phosphorylation of erk, p38, and Akt at sites necessary for the activation of these kinases, as well as the phosphorylation of IκB is indistinguishable in total splenic T cells from Mst1-null and wild-type mice, whereas TCR activation of Jnk is more robust and sustained in the Mst1-null cells (Fig. S11C Left). A similar pattern is observed in comparing the Mst1-null and wild-type CD4+/CD62Lhi naïve subsets, except that evidence for Jnk hyperactivation in the Mst1-null is lacking (Fig. S11C Right).
Stimulation of the T Cell Receptor Activates Mst1 and Promotes Phosphorylation of a Subset of Mst1-Dependent Substrates.
Mst1 and Mst2 are 76% identical and share an identical activation loop sequence. Mst1/2 activation requires autophosphorylation of the activation loop, which can be monitored as a reflection of the activation state (9). Mammalian Lats1 is directly phosphorylated at a carboxyl-terminal site by Mst2 (18), which together with Lats1 activation loop autophosphorylation results in Lats1 activation. Lats1 in turn phosphorylates and inhibits YAP by promoting nuclear exit (22–24). Mst1/2 phosphorylates the mammalian MATS orthologues, MOBKL1A and MOBKL1B (at Thr-12 and Thr-35), which enables MOBKL1A/B binding to Lats1 and promotes Lats1 autophosphorylation; MOBKL1A/B phosphorylation per se is sufficient to inhibit cell cycle progression in U2OS cells (19). The FoxO1/3a polypeptides are also well documented Mst1 substrates (27). Stimulation of splenic total T cells from wild-type mice with αCD3+αCD28 results in a rapid activation of Mst1/2 activation loop phosphorylation, which is completely lacking in the Mst1-null T cells (Fig. 5A Left), indicating that Mst2 contributes very little to overall Mst1/2 activity in T cells. Concomitant with Mst1 activation, a brisk phosphorylation of MOBKL1A/B is evident in the wild-type T cells, and as with Mst1 autophosphorylation, this is completely absent in the Mst1-null T cells. Stimulation of wild-type T cells with α-CD3+α-CD28 produces a modest increase in Lats1/2 carboxyl-terminal phosphorylation and a somewhat slower increase in Lats1/2 activation loop phosphorylation. Elimination of Mst1 has little effect on the Lats1 carboxyl-terminal phosphorylation and no effect on Lats1/2 autophosphorylation. In response to α-CD3+α-CD28, a slight increase in YAP phosphorylation is evident, which is similar in the wild-type and Mst1-null T cells, whereas Foxo1/3a phosphorylation is unaltered. A similar pattern of Mst1/2 substrate phosphorylation is evident in α-CD3+α-CD28-stimulated CD4+/CD62Lhi naïve T cells except little or no stimulation of YAP phosphorylation is evident (Fig. 5A Right). We also compared wild-type CD4+/CD62Lhi naïve cells with wild-type CD4+/CD62Llo effector/memory cells (Fig. 5B); as expected from the lower content of Mst1 in the effector/memory cells (Fig. 3D), there is a much weaker Mst1/2 autophosphorylation and greatly reduced MOBKL1A/B phosphorylation, despite unaltered MOBKL1A/B polypeptide content.
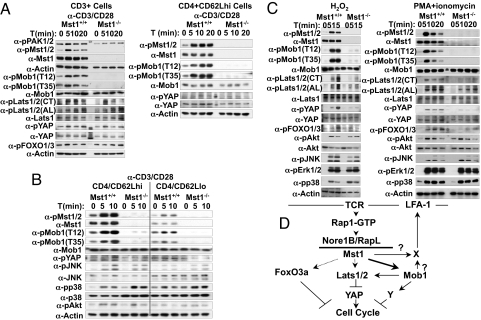
Fig. 5.
Phosphorylation of Mst1 and its substrates in T cells. (A) Phosphorylation of Mst1/2 activation loop and several Mst1/2 targets in Mst1-null and wild-type T cells. Aliquots of cell extracts were immunoblotted with the antibodies indicated. The antibody used to detect Mst1/2 activation loop phosphorylation in this experiment is generated against the PAK1 activation loop but has been shown to cross-react strongly with Mst1 and Mst2. (B) Phosphorylation responses in wild-type naïve and effector/memory CD4+ T cells. Splenic CD4+/CD62Lhi (naïve, Left) and CD4+/CD62Llo (effector/memory, Right) cells were isolated from wild-type mice. After resting in vitro, cells were stimulated with anti-CD3 + anti-CD28 for the times indicated and extracts were subjected to immunoblot. (C) The effect of H2O2 and PMA + ionomycin on protein phosphorylation in Mst1-null and wild-type T cells. Splenic CD3+ T cells were isolated from Mst1-null and wild-type littermates and after resting were treated with H2O2 (0.5 mM, Left) or PMA (50 ng/ml) plus ionomycin (1 μM) (Right). Aliquots of cells were extracted at the times indicated and subjected to immunoblot. (D) The TCR-Nore1B/Mst1 pathway in naïve T cells. X is an unidentified mediator of Mst1 action on LFA-1 clustering; the question of whether it is a substrate of Mst1, a target of phospho-MOBKL1A/B, or both is indicated by the question marks. Y is an unidentified mediator of Phospho-MOBKL1A/B inhibition of cell cycle progression. The intensity of the arrows emanating from Mst1 reflects the relative strength of Mst1 signaling to each of these substrates elicited by αCD3+αCD28 in wild-type naïve T cells, as indicated by the data in Fig. 5 A–C.
Given the robust αCD3+αCD28-stimulated MOBKL1A/B phosphorylation and its total dependence on Mst1, the relatively modest stimulation of Lats1/2 and YAP phosphorylation and their relative insensitivity to Mst1 elimination is surprising, as is the lack of FoxO1/3a phosphorylation in response to α-CD3+α-CD28. We therefore stimulated total splenic T cells with more vigorous, nonspecific stimuli, that is, H2O2, which is known to activate Mst1/2 (Fig. 5C Left) and PMA+ ionomycin (Fig. 5C Right), which promotes near maximal T cell activation. Both of these treatment result in activation of Mst1 and vigorous phosphorylation of MOBL1A/B, Lats1/2, YAP, and FoxO1/3a, which are each greatly attenuated or (for MOBKL1A/B) abolished in the Mst1-null T cells. Thus, it is clear that although Lats1/2, YAP and FoxO1/3a are, like MOBKL1A/B, downstream targets of Mst1 in T cells (Fig. 5D), they are not significantly phosphorylated in wild-type T cells by stimulation with α-CD3+α-CD28 that is sufficient to promote a robust proliferative response. Consequently, deficient Mst1-catalyzed Lats1/2 or Foxo1/3 phosphorylation cannot account for the hyperproliferative response of Mst1-null naïve T cells to αCD3+αCD28. Thus, although all three of these potential Mst1 substrates have been shown to exert antiproliferative effects (19, 20, 36) only MOBKL1A/B remains a viable candidate as the effector of Mst1's antiproliferative effect in naïve T cells, as its phosphorylation is stimulated by αCD3+αCD28 and lost entirely in the Mst1-null cells. Inasmuch as the best characterized target of phospho-MOBKL1A/B is Lats1 (19, 37), the present data indicates that other Mst1 substrates and/or targets of phospho-MOBKL1A/B remain to be identified.
Discussion
These data identify a key physiological function of Mst1 in T cells; the Nore1B/Mst1 complex is a major negative regulator of the proliferative response of naïve T cells to stimulation of the T cell antigen receptor. This, together with the marked decrease in Nore1B and Mst1 abundance during the progression of wild-type T cells from a naïve to an effector/memory phenotype strongly supports the conclusion that a physiologic reduction in Nore1B/Mst1 is responsible, at least in part, for the greater sensitivity of effector/memory T cells to antigen receptor activation and the decreased requirement for costimulation to elicit a proliferative response. The two clear-cut cell intrinsic actions of Mst1 itself, that is, its antiproliferative action and its promotion of LFA-1 clustering, would appear to be opposing in their effect on the immune response, however, the impact of the enhanced proliferative responsiveness appears to predominate in the Mst1-null mice, at least in the absence of a specific immune challenge. The Mst1-null mice exhibit relatively normal thymocyte development but many fewer splenic naïve T cells, unaltered lymph node size and increased numbers of effector/memory T cells in several tissues; this phenotype, which differs markedly from that of CD11a (i.e., LFA-1)-deficient mice (38), is unlikely to be due primarily to aberrant T cell migration, inasmuch as the expression of mRNAs encoding KLF2 (32), S1P1 and a variety of chemokine receptors is similar in wild-type and Mst1-null CD4+/CD62Lhi T cells (Fig. S8). Rather, the low numbers of naïve T cells in the secondary lymphoid organs of the Mst1-null mice is most simply explained by a lower threshold in the Mst1-null naïve T cells for antigen-stimulated proliferation, resulting in their ready activation, presumably in response to endogenous antigens. The increased apoptosis of all Mst1-null T cell subsets may thus reflect the higher ongoing rate of T cell activation and progression through an immune response (31). This would be expected to be expressed as autoimmunity, however, activation of LFA-1 is required for some immune responses (38), and ongoing autoimmunity can be interrupted by interference with LFA-1 function (39). Thus, the net outcome of these opposing Mst1 outputs on various aspects of the immune response cannot be confidently predicted and will require more detailed analysis.
The coordinate decrease in Nore1B and Mst1 polypeptide abundance emphasizes the operation of Nore1B and Mst1 as a complex in these cells, functioning in a manner analogous to the regulatory and catalytic subunits of, for example, PKA. Nore1 binds in a GTP-dependent way in vitro or in a 2-hybrid assay to several Ras-like GTPases, including Ki- and Ha-Ras, RRas, MRas/RRas3, Rap1, and Rap2a (40); it is likely that more than one of these serves as a physiologic partner depending on the cellular context. Nore1B, the isoform expressed primarily in lymphoid cells, was retrieved in a two-hybrid screen using activated Rap1 (and therefore named RAPL) and endogenous Nore1B can be coprecipitated from T cells with endogenous activated Rap1 (41). Previous work has shown that a complex of Mst1 and Nore1B mediates the clustering of the integrin LFA-1 in T cells elicited by receptor activation of Rap-1 (6, 7). Thus, although a specific identification of the ras-like GTPase that underlies the antiproliferative action of the Nore1B/Mst1 complex in naïve T cells remains to be accomplished, Rap1-GTP is the leading candidate.
Materials and Methods
The methods used for the generation and genotyping of Mst1-deficient mice, for the isolation of mouse immune cells, and their characterization by flow cytometry, immunofluorescence, and immunoblotting, as well as methods for Northern blot and for the measurement of T cell Ca++ flux, proliferation, apoptosis, cytokine production, and adhesion and migration are provided in detail in SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank R. Gerzsten and S. Pillai for discussion and advice and H. Lu, E. Mizoguchi, Y. Yin, R. Tyszkowski, and S. Sinha for technical support. J.A. is grateful to Brian Seed for important suggestions. Support from the National Institutes of Health (to R.S.B., V.B., and J.A.) is acknowledged.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810773105/DCSupplemental.
References
- 1.Dan I, Wantanable NM, Kusumi A. The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol. 2001;11:220–230. doi: 10.1016/s0962-8924(01)01980-8. [DOI] [PubMed] [Google Scholar]
- 2.Graves JD, et al. Caspase-mediated activation and induction of apoptosis by the mammalian Ste20-like MST1. EMBO J. 1998;17:2224–2234. doi: 10.1093/emboj/17.8.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee K-K, Ohyama T, Yajima N, Tsubuki S, Yonehara S. MST, a physiological caspase substrate, highly sensitizes apoptosis both upstream and downstream of caspase activation. J Biol Chem. 2001;276:19276–19285. doi: 10.1074/jbc.M005109200. [DOI] [PubMed] [Google Scholar]
- 4.Lin Y, Khoklatchev A, Figeys D, Avruch J. Death-associated protein 4 binds MST1 and augments MST1-induced apoptosis. J Biol Chem. 2002;277:47991–48001. doi: 10.1074/jbc.M202630200. [DOI] [PubMed] [Google Scholar]
- 5.Katagiri K, Hattori M, Minato N, Irie S, Takatsu K, Kinashi T. Rap1 is a potent activation signal for leukocyte function-associated antigen 1 distinct from protein kinase C and phosphatidylinositol-3-OH kinase. Mol Cell Biol. 2000;20:1956–1969. doi: 10.1128/mcb.20.6.1956-1969.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katagiri K, Imamura M, Kinashi T. Spatiotemporal regulation of the kinase Mst1 by binding protein RAPL is critical for lymphocyte polarity and adhesion. Nat Immunol. 2006;7:919–928. doi: 10.1038/ni1374. [DOI] [PubMed] [Google Scholar]
- 7.Katagiri K, et al. Crucial functions of the Rap1 effector molecule RAPL in lymphocyte and dendritic cell trafficking. Nat Immunol. 2004;5:1045–1051. doi: 10.1038/ni1111. [DOI] [PubMed] [Google Scholar]
- 8.Khokhlatchev A, et al. Identification of a novel Ras-regulated proapoptotic pathway. Curr Biol. 2002;12:253–265. doi: 10.1016/s0960-9822(02)00683-8. [DOI] [PubMed] [Google Scholar]
- 9.Praskova M, Khoklatchev A, Ortiz-Vega S, Avruch J. Regulation of the MST1 kinase by autophosphorylation, by the growth inhibitory proteins, RASSF1 and NORE1 and by ras. Biochem J. 2004;381:453–462. doi: 10.1042/BJ20040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Weyden L, Adams DJ. The Ras-association domain family (RASSF) members and their role in human tumourigenesis. Biochim Biophys Acta. 2007;1776:58–85. doi: 10.1016/j.bbcan.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu S, Huang J, Dong J, Pan D. hippo Encodes a Ste-20 Family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell. 2003;114:445–456. doi: 10.1016/s0092-8674(03)00549-x. [DOI] [PubMed] [Google Scholar]
- 12.Hamaratoglu F, et al. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat Cell Biol. 2003;10:914–920. doi: 10.1038/ncb1050. [DOI] [PubMed] [Google Scholar]
- 13.Harvey KF, Pfleger CM, Iswar K, Hariharan The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell. 2003;114:457–467. doi: 10.1016/s0092-8674(03)00557-9. [DOI] [PubMed] [Google Scholar]
- 14.Jia J, Zhang W, Wang B, Trinko R, Jiang J. The Drosophila Ste20 family kinase dMST functions as a tumor suppressor by restricting cell proliferation and promoting apoptosis. Genes Dev. 2003;17:2514–2519. doi: 10.1101/gad.1134003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harvey K, Tapon N. The Salvador-Warts-Hippo pathway - an emerging tumour-suppressor network. Nat Rev Cancer. 2007;7:182–191. doi: 10.1038/nrc2070. [DOI] [PubMed] [Google Scholar]
- 16.Pan D. Hippo signaling in organ size control. Genes Dev. 2007;21:886–897. doi: 10.1101/gad.1536007. [DOI] [PubMed] [Google Scholar]
- 17.Saucedo LJ, Edgar BA. Filling out the hippo pathway. Nat Rev Mol Cell Biol. 2007;8:613–621. doi: 10.1038/nrm2221. [DOI] [PubMed] [Google Scholar]
- 18.Chan EH, Nousiainen M, Chalamalasetty RB, Schäfer A, Nigg EA, Silljé HH. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene. 2005;24:2076–2086. doi: 10.1038/sj.onc.1208445. [DOI] [PubMed] [Google Scholar]
- 19.Praskova M, Xia F, Avruch J. MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation. Curr Biol. 2008;18:311–321. doi: 10.1016/j.cub.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang X, Li DM, Chen W, Xu T. Human homologue of Drosophila lats, LATS1, negatively regulate growth by inducing G(2)/M arrest or apoptosis. Oncogene. 2001;20:6516–6523. doi: 10.1038/sj.onc.1204817. [DOI] [PubMed] [Google Scholar]
- 21.St John MA, et al. Mice deficient of Lats1 develop soft-tissue sarcomas, ovarian tumours and pituitary dysfunction. Nat Genet. 1999;21:182–186. doi: 10.1038/5965. [DOI] [PubMed] [Google Scholar]
- 22.Dong J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1123. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao B, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hao Y, Chun A, Cheung K, Rashidi B, Yang X. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J Biol Chem. 2008;283:5496–5509. doi: 10.1074/jbc.M709037200. [DOI] [PubMed] [Google Scholar]
- 25.Overholtzer M, et al. Transforming properties of YAP, a candadate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci. 2006;103:12405–12410. doi: 10.1073/pnas.0605579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carmargo FD, et al. Yap1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 27.Lehtinen MK, et al. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell. 2006;125:987–1001. doi: 10.1016/j.cell.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 28.Moulton VR, Farber DL. Committed to memory: Lineage choices for activated T cells. Trends Immunol. 2006;27:261–267. doi: 10.1016/j.it.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 29.Ramsdell F. Foxp3 and natural regulatory T cells: Key to a cell lineage? Immunity. 2003;19:165–168. doi: 10.1016/s1074-7613(03)00207-3. [DOI] [PubMed] [Google Scholar]
- 30.Rogers PR, Dubey C, Swain SL. Qualitative changes accompany memory T cell generation: Faster, more effective responses at lower doses of antigen. J Immunol. 2000;164:2338–2346. doi: 10.4049/jimmunol.164.5.2338. [DOI] [PubMed] [Google Scholar]
- 31.Krammer PH, Arnold R, Lavrik IN. Life and death in peripheral T cells. Nat Rev Immunol. 2007;7:532–542. doi: 10.1038/nri2115. [DOI] [PubMed] [Google Scholar]
- 32.Sebdza E, Zou Z, Lee JS, Wang T, Kahn ML. Transcription factor KLF2 regulates the migration of naive T cells by restricting chemokine receptor expression patterns. Nat Immunol. 2008;9:292–300. doi: 10.1038/ni1565. [DOI] [PubMed] [Google Scholar]
- 33.Sallusto F, Lanzavecchia A. Understanding dendritic cell and T-lymphocyte traffic through the analysis of chemokine receptor expression. Immunol Rev. 2000;177:134–140. doi: 10.1034/j.1600-065x.2000.17717.x. [DOI] [PubMed] [Google Scholar]
- 34.Kane LP, Lin J, Weiss A. Signal transduction by the TCR for antigen. Curr Opin Immunol. 2000;12:242–249. doi: 10.1016/s0952-7915(00)00083-2. [DOI] [PubMed] [Google Scholar]
- 35.Cronin SJ, Penninger JM. From T-cell activation signals to signaling control of anti-cancer. Immunol Rev. 2007;220:151–168. doi: 10.1111/j.1600-065X.2007.00570.x. [DOI] [PubMed] [Google Scholar]
- 36.Lin L, Hron JD, Peng SL. Regulation of NF-kappaB, Th activation, and autoinflammation by the forkhead transcription factor Foxo3a. Immunity. 2004;21:203–213. doi: 10.1016/j.immuni.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 37.Wei X, Shimizu T, Lai ZC. Mob as tumor suppressor is activated by Hippo kinase for growth inhibition in Drosophila. EMBO J. 2007;26:1772–1781. doi: 10.1038/sj.emboj.7601630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmits R, et al. LFA-1-deficient mice show normal CTL responses to virus but fail to reject immunogenic tumor. J Exp Med. 1996;183:1415–1426. doi: 10.1084/jem.183.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bertry-Coussot L, et al. Long-term reversal of established autoimmunity upon transient blockade of the LFA-1/intercellular adhesion molecule-1 pathway. J Immunol. 2002;168:3641–3648. doi: 10.4049/jimmunol.168.7.3641. [DOI] [PubMed] [Google Scholar]
- 40.Ortiz-Vega S, et al. The putative tumor suppressor RASSF1A homodimerizes and heterodimerizes with the ras-GTP binding protein Nore1. Oncogene. 2002;21:1381–1390. doi: 10.1038/sj.onc.1205192. [DOI] [PubMed] [Google Scholar]
- 41.Katagiri K, Maeda A, Shimonaka M, Kinashi T. RAPL, a Rap1-binding molecule that mediates Rap1-induced adhesion through spatial regulation of LFA-1. Nat Immunol. 2003;4:741–748. doi: 10.1038/ni950. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.