Abstract
The liver is known to favor the induction of immunological tolerance rather than immunity. Although Kupffer cells (KC) have been indicated to play a role in liver tolerance to allografts and soluble antigens, the mechanisms involved remain unclear. We hypothesized that KCs could promote immune tolerance by acting as incompetent antigen-presenting cells (APC), as well as actively suppressing T cell activation induced by other potent APCs. The expression of antigen presentation-related molecules by KCs was phenotyped by flow cytometry. The abilities of KCs to act as APCs and to suppress T cell activation induced by splenic dendritic cells (DC) were examined by in vitro proliferation assays using CD4+ OVA-TCR (ovalbumin T cell receptor) transgenic T cells. We found that, compared with DCs, KCs expressed significantly lower levels of major histocompatibility complex (MHC) II, B7-1, B7-2, and CD40. This result is consistent with our observation that KCs were not as potent as DCs in eliciting OVA-specific T cell proliferation. However, KCs isolated from polyinosinic:polycytidylic acid–treated mice expressed significantly higher levels of MHC II and costimulatory molecules than did naïve KCs and could stimulate stronger T cell responses. More importantly, we found that KCs could inhibit DC-induced OVA-specific T cell activation. Further investigation of the underlying mechanism revealed that prostaglandins produced by KCs played an important role. The results ruled out the possible involvement of interleukin-10, nitric oxide, 2,3-dioxygenase, and transforming growth factor β in KC-mediated T cell suppression.
Conclusion
Our data indicate that KCs are a tolerogenic APC population within the liver. These findings suggest that KCs may play a critical role in regulating immune reactions within the liver and contributing to liver-mediated systemic immune tolerance.
Despite the liver’s continuous exposure to pathogens, toxins, and dietary antigens, the adaptive immune response of the liver is known to favor induction of immunological tolerance rather than immunity. This is supported by numerous studies that have demonstrated that: (1) dietary antigens derived from the gastrointestinal tract are tolerized in the liver; (2) allogeneic liver organ transplants are accepted across major histocompatibility complex (MHC) barriers1; (3) preexposure to donor cells through the portal vein of recipient animals increased their acceptance of solid tissue allografts2,3; and (4) preexposure of soluble antigens via the portal vein leads to systemic immune tolerance.4,5 The mechanism of liver-induced systemic immune tolerance is not well understood, although several hypotheses, including T cell apoptosis, immune deviation, and active suppression, have gained some experimental support.
The liver has been dubbed the “elephant’s graveyard” for activated T cells. A number of studies have been conducted using T cell receptor (TCR) transgenic models, in which CD8+T cells express MHC class I-restricted TCR specific for a known antigenic peptide.6,7 These studies demonstrated that, after a series of injections of the specific peptide, the activated T cells undergo apoptosis after a transient accumulation in the liver. This phenomenon coincides with the disappearance of these cells from the peripheral lymphoid organs. However, this hypothesis was recently challenged by the findings that viral-specific CD8+ T cells can accumulate within the liver after an extrahepatic infection, and that these T cells are viable and retain effector functions.8,9
Immune deviation represents another possible mechanism of liver tolerance. It has been demonstrated that liver sinusoidal endothelial cells (LSEC) are capable of selectively suppressing interferon (IFN)-γ–producing T helper (Th)1 cells, but promoting the outgrowth of interleukin (IL)-4 – expressing Th2 cells,10 thereby causing tolerance.
Active suppression of T cell activation can occur within the liver because of its unique anatomy and the composition of “tolerogenic” antigen-presenting cells (APC). The blood flow slows down within the liver because of the narrow sinusoids (7–12 μm) and the temporary obstruction by resident Kupffer cells (KC). Because of the slow blood flow, circulating T cells are forced into contact with endothelium and presented with opportunities to interact with various APCs, including LSECs, KCs, and hepatic dendritic cells (DC). It has been demonstrated that LSECs are capable of presenting antigens to naïve CD4+ and CD8+T cells and inducing tolerance, because LSEC-activated CD8+ cells fail to differentiate into cytotoxic effector cells, and LSEC-activated CD4+ T cells produce immune regulatory cytokines, such as IL-4 and IL-10.11,12 However, a recent study using an improved method for LSEC purification demonstrated that these cells do not express MHC II and costimulatory molecules, suggesting that they cannot act as APCs.13 The tolerogenicity of hepatic DCs has also been investigated, and several studies demonstrated that, compared with spleen DCs, those in the liver exhibit immature phenotypes and that they are ineffective stimulators of antigen-specific T cell responses.14–16 However, because a very low number of DCs reside within the liver, it is not clear whether these cells could play a predominant role in the mechanism of liver tolerance. In contrast, KCs represent the largest population of tissue resident macrophages, and they are ideally situated to encounter circulating T cells in the sinusoidal lumen of the liver.17,18 It has been shown that KCs play a role in liver-mediated tolerance to allografts,19–21 and our previous studies demonstrated their importance in the induction of tolerance against soluble antigens.22 However, the mechanism(s) involved in KC-mediated T cell tolerance have not been clearly defined. The aims of the current study were to (1) characterize the phenotypes of KCs related to antigen presentation, and (2) investigate KCs’ ability to regulate/suppress antigen-specific T cell responses. The data demonstrated that KCs were not effective APCs to elicit T cell activation, and that more importantly, KCs were able to actively suppress T cell proliferation induced by splenic DCs.
Materials and Methods
Animals
Six-week-old to 8-week-old male C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and kept in the Center for Laboratory Animal Care at the University of Colorado Health Sciences Center for 1 week before being used for isolation of various APCs. In some experiments, the mice were injected intraperitoneally with polyinosinic:polycytidylic acid (poly I:C, 50 μg/mouse, Amersham Biosciences, Piscataway, NJ) 12 hours before KC isolation. Mice transgenic for TCR that recognizes OVA323-339 peptide in the context of I-Ad (OTII mice) on a C57BL/6J background were provided by Dr. Philippa Marrack (National Jewish Medical and Research Center, Denver, CO). An OTII breeding colony was set up and maintained in pathogen-free conditions in the Center for Laboratory Animal Care. All animal handlings were performed in accordance with guidelines from the University of Colorado Health Sciences Center Institutional Animal Care and Use Committee.
KC Isolation and Purification
Mice were anesthetized with isoflurane and livers perfused in situ with a Ca++-free Hanks’ balanced salt solution (HBSS) containing 1 mM ethylene glycol tetra-acetic acid for 4 minutes at 37°C followed by a 0.05% collagenase solution (type IV, Sigma, St. Louis, MO) for 20 minutes. After digestion, the liver was disrupted in HBSS containing 0.5% of fetal bovine serum and 0.6% citrate-dextrose (Sigma). Single-cell suspension was filtered through a 100-μm cell strainer (BD Falcon, Bedford, MA), and the cells centrifuged at 30g for 3 minutes to pellet hepatocytes. The supernatant enriched of non-parenchymal cells (NPCs) was centrifuged and washed twice at 500g for 7 minutes. The pellet was resuspended in 1.5 mL RPMI 1640 containing 10% fetal bovine serum, and subsequently mixed with 3 mL 30% Nycodenz (Accurate Chemical & Scientific Co., Westbury, NY). The resulting suspension was layered under 5 mL HBSS and centrifuged at 500g for 15 minutes. KC-enriched NPCs located in the interface were harvested and washed twice. The cells were stained with fluorescein isothiocyanate–conjugated anti-CD45 (clone 30-F11, eBioscience, San Diego, CA) and allophycocyanin-conjugated anti-F4/80 (clone BM8, eBioscience) antibodies for 30 minutes on ice. To prevent nonspecific binding, cells were blocked with normal rat serum (Sigma) and anti-mouse CD16/32 (clone 93, eBio-science) antibody for 5 minutes on ice. Subsequently, CD45+F4/80+ KCs were sorted using a MoFlo High Performance Cell Sorter (Cytomation Inc., Fort Collins, CO).
Isolation of Resident Peritoneal Macrophages and Splenic DCs
Male C57BL/6J mice were injected intraperitoneally with 8 mL cold HBSS and lavaged three times. The collected cells were plated on a fetal bovine serum pre-coated culture dish for 60 minutes, and the adherent cells harvested as peritoneal macrophages (PM). To isolate splenic DCs, mouse spleens were cut into small pieces and incubated in HBSS containing collagenase D (1 mg/mL, Roche Applied Science, Indianapolis, IN) for 30 minutes at 37°C. The tissue pieces were homogenized and filtered through a 100-μm cell strainer. Single-cell suspension was centrifuged at 300g for 5 minutes. CD11c+ DCs were purified by magnetic cell sorting using MACS CD11c MicroBeads (Milltenyi Biotec Inc., Auburn, CA).
Isolation of CD4+ T Cells from C57BL/6J WT and OVA-TCR Transgenic OTII Mice
The spleens of OTII or C57BL/6J mice were removed and homogenized. Single-cell suspension was filtered through a 100-μm cell strainer. CD4+T cells were purified by negative selection using a CD4+T cell isolation kit (Milltenyi Biotec Inc).
Flow Cytometric Analysis
Liver NPCs, splenocytes, and lavaged peritoneal cells were stained with one or more of the following antibodies, including anti-CD45, anti-F4/80, anti-CD11b (clone M1/70, eBioscience), and anti-CD11c (clone N418, eBioscience) for the identification of KCs (CD45+F4/80+), PMs (CD11b+F4/80+), and splenic and liver DCs (CD45+CD11c+). Phenotypical analysis of various APCs was performed by staining the cells with the following phycoerythrin-conjugated antibodies (BD Biosciences, San Jose, CA), which include anti-I-Ab (clone AF6-120.1), anti-B7-1 (clone 16-10A1), anti-B7-2 (clone GL1), anti-CD40 (clone 3/23) and anti-intercellular adhesion molecule (ICAM)-1 (clone 3E2). To examine the status of T cell activation, the cells were collected after 12 hours’ incubation and stained with allophycocyanin-conjugated anti-CD4 (clone GK1.5, eBioscience), fluorescein isothiocyanate–conjugated CD69 (clone H1.2F3, eBioscience), and phycoerythrin-conjugated CD25 (clone PC61.5, eBioscience). After antibody staining, the cells were analyzed on a FACSCalibur using CellQuest software (BD Biosciences). The data were further analyzed using FlowJo software (Tree Star Inc., Ash-land, OR).
T Cell Stimulation by Various APCs In Vitro
Purified CD4+ OVA-TCR T cells (5 × 104) were incubated with 2.5 × 104 PMs, DCs, KCs, or the combination of DCs and KCs. The cells were cultured in 96-well plates in RPMI 1640 medium containing 10% fetal bovine serum (200 μL/well [Invitrogen, Carlsbad, CA]). After 3 days, various concentrations of OVA323-339 (ISQAVHAAHAEINEAGR, Molecular Resource Center, National Jewish Medical Research Center, Denver, CO) were included in the cultures. T cell proliferation was measured by [3H]-thymidine (0.5 μCi/well [Amersham Biosciences]) incorporation during the last 16 hours of incubation. In some experiments, the naïve CD4+ OVA-TCR T cells were labeled with 0.5 μM carboxy-fluorescein diacetate succinimidyl ester (CFSE, Molecular Probes, Eugene, OR) before incubation with various APCs. After 3 days, the CFSE fluorescence intensity was measured by flow cytometry to determine the degree of T cell differentiation. For IL-2 measurement, the supernatants were collected 12 hours after culturing T cell alone, or T cells plus DCs or KCs. IL-2 levels were determined by sandwich enzyme-linked immunosorbent assay using capture and detection antibody pairs according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN). In some experiments, CD4+ T cells (2 × 105/well) isolated from C57BL/6J mice were cocultured with DCs or KCs for 3 days in wells of 96-well plates precoated with anti-CD3 antibody (5 μg/mL, clone 145-2C11, eBioscience). T cell proliferation was determined by [3H]-thymidine incorporation during the last 16 hours of incubation.
To examine the involvement of various soluble factors in mediating KC-induced T cell suppression, 1-methyl-d,l-trytophan (1-MT, 1 mM, Sigma), NG-monomethyl-L-arginine (L-NMMA, 0.1 mM, Sigma), anti–IL-10 or anti–transforming growth factor (TGF) β antibody (10 μg/mL, R&D Systems) was included in some DC/KC cocultures for 16 hours before the addition of T cells. To determine the effect of prostaglandin (PG)E2 and 15-deoxy-delta12,14-PGJ2 (15d-PGJ2) in mediating KC-induced T cell suppression, indomethacin (1 μM, Sigma) was added to DC/KC cocultures overnight before the addition of T cells. In some of these cultures, a combination of PGE2 (3 ng/mL) and PGJ2 (3 ng/mL) was included. Alternatively, the combination of PGE2 and PGJ2 was added to T/KC/DC cocultures the same time as the addition of T cells.
Measurement of PGE2, 15d-PGJ2, and Nitric Oxide
After 48 hours of incubation, the supernatants were collected from the cultures of T cell alone, or T cells plus DCs, KCs, or DCs and KCs. In some experiments, KCs (2.5 × 104/well) were cultured in the absence and presence of various concentration of indomethacin. The levels of PGE2 and 15d-PGJ2 were measured using enzyme immunoassay kits (Assay Designs, Ann Arbor, MI) according to the manufacturer’s instructions. For nitric oxide (NO) measurement, the supernatants of PM/T cell and KC/T cell cocultures were collected at 48 hours. Total nitrite/nitrate levels were determined by the use of a Griess Reagent System kit (Promega Co., Madison, WI).
Results
Purification and Characterization of Hepatic KCs
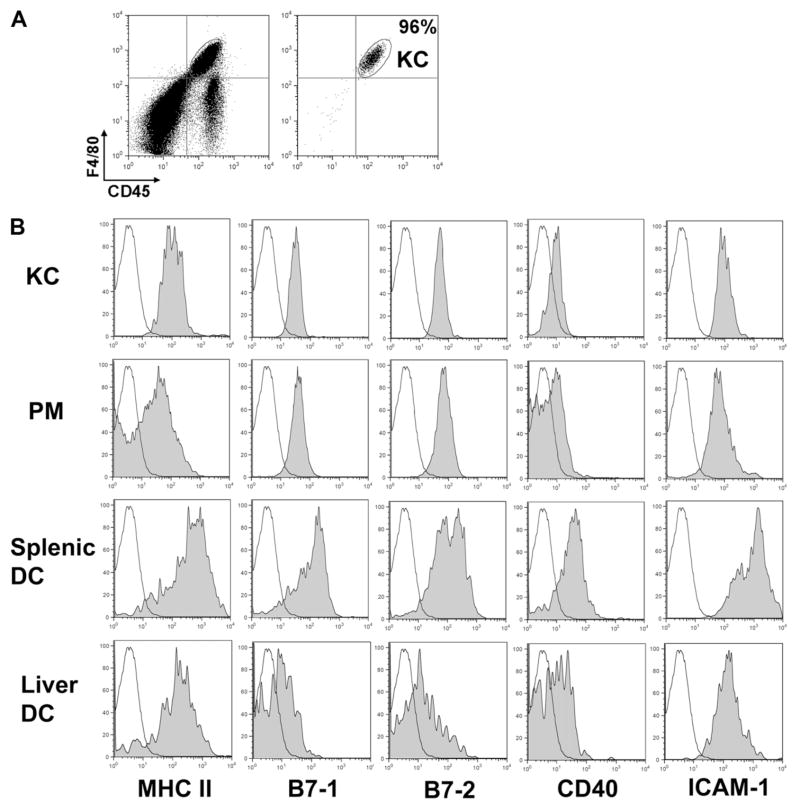
Even though KCs represent the largest APC population within the liver, and the in vivo KC depletion data suggested a possible immune tolerogenic role of these cells,19–22 studies investigating the underlying mechanism accounting for their tolerogenic function have been scarce. One reason may be that it is not straightforward to isolate mouse KCs with high purity and yield. Previous reports described isolation of KCs from rats or mice using differential plating or elutriation23–25; however, these methods are limited in achieving high cell purity. In the current study, we took an immunological approach to isolate mouse KCs using fluorescence-assisted cell sorting technique. After enrichment of liver NPCs as described in the literature, the cells were stained with anti-F4/80 and anti-CD45 antibodies, which recognize F4/80 (a tissue macrophage marker) and CD45 (a pan-leukocyte marker), respectively. KCs were identified as CD45- and F4/80-double positive cells. The purity of isolated KCs is greater than 95%, and the yield of the cells isolated from 6 mice is approximately 3 × 106 (Fig. 1A).
Fig. 1.
Phenotypical analysis of KCs in comparison with PMs and splenic DCs. (A) Liver NPCs were isolated and dual stained with anti-F4/80 and anti-CD45 antibodies. KCs were subsequently sorted by fluorescence-assisted cell sorting and used for T cell stimulation experiments. The purity of sorted KCs, indicated by the enclosed circle (CD45+F4/80+), is 96%. (B) Liver NPCs, splenocytes, and lavaged peritoneal cells were stained with various antibodies and analyzed by flow cytometry. The cells were gated on KCs (CD45+F4/80+), PMs (CD11b+F4/80+), liver DCs (CD45+CD11c+), or splenic DCs (CD45+CD11c+), and further analyzed for the expression of various surface molecules. The results are shown as histograms with fluoresce intensity on x-axis and cell number on y-axis. Solid black lines represent isotype control antibodies, and shaded histograms, specific staining. The data depicted represent three independent experiments producing similar results.
Although evidence suggests that KCs represent a primary population of hepatic APCs, their surface expression of molecules related to antigen presentation has not been examined in comparison with other APCs. Using flow cytometric analysis, we determined KC expression of MHC II, B7-1, B7-2, CD40, and ICAM-1. The expression levels on KCs were compared with those on naïve PMs, liver DCs, and splenic DCs (known as potent “professional” APCs). The data showed that KCs expressed similar levels of MHC II and costimulatory molecules, including B7-1, B7-2, and CD40, when compared with PM, but lower when compared with splenic DCs. Among all types of cells compared, liver DCs expressed the lowest levels of MHC II and costimulatory molecules (Fig. 1B).
KCs Are Not Potent in Induction of Antigen-Specific T Cell Activation
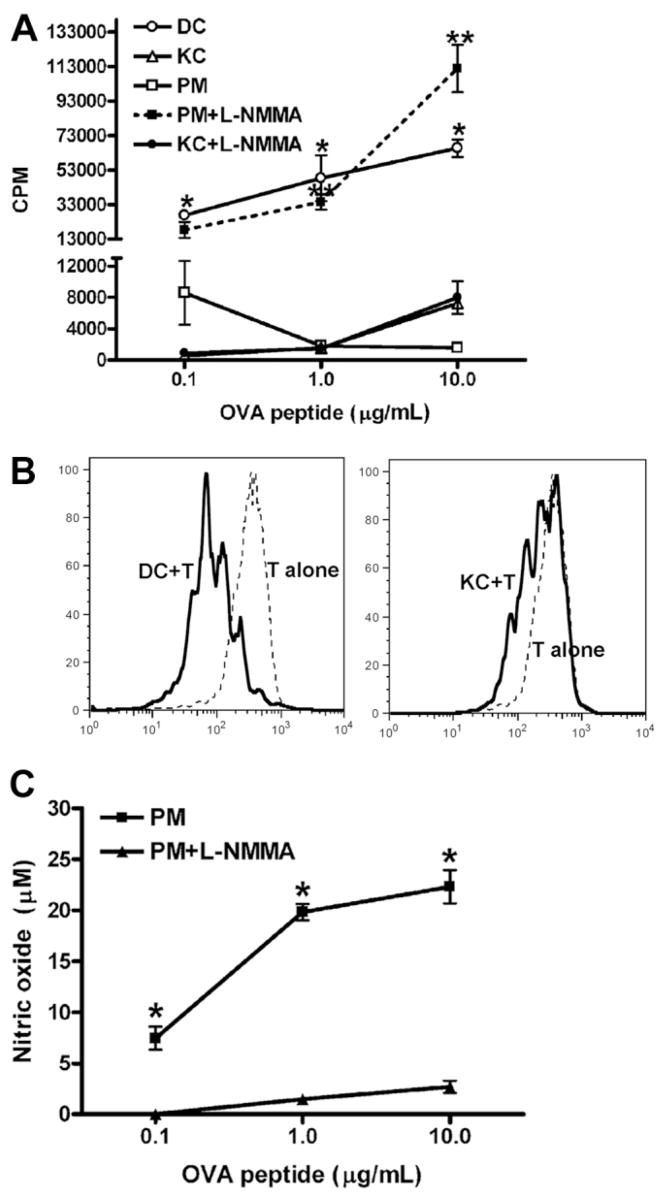
The ability of KCs to induce antigen-specific T cell activation was examined using CD4+ OVA-TCR (ovalbumin-T cell receptor) transgenic T cells isolated from OT II mice. KCs, PMs, and splenic DCs were isolated from syngeneic male C57BL/6J wild-type mice. The T cells were cocultured with APCs in the presence of OVA peptide 323–339 (OVA323-339). T cell activation and proliferation were determined by measuring 3H-thymidine uptake and CFSE fluorescence intensities. Consistent with reports in the literature,14,26 we found that splenic DCs elicited a strong T cell proliferative response as evidenced by high uptake of 3H-thymidine and the increased number of differentiated cells that express lower CFSE intensities. However, measurement of 3H-thymidine uptake and CFSE fluorescence intensities showed that T cells were nearly completely refractory to KC-induced activation (Fig. 2A, B). The data demonstrated that PMs could induce T cell activation in the presence of a low concentration of OVA323-339 (0.1 μg/mL), but T cell proliferation decreased when the amount of antigen was increased to 1 and 10 μg/mL (Fig. 2A). In these cultures, significant amounts of NO were detected (Fig. 2C). Because numerous studies have demonstrated that NO can inhibit T cell proliferation,27–34 the role of NO in the suppression of PM-mediated T cell activation was investigated by adding L-NMMA in the cultures to inhibit NO production (Fig. 2C). The results showed that inhibition of NO drastically increased PM-induced T cell proliferation, at a level similar to that induced by DCs (Fig. 2A). In contrast, NO production was not detectable in KC/T cocultures (data not shown), and the addition of L-NMMA did not affect T cell proliferation (Fig. 2A). These data suggested that the mechanisms for the lack of T cell proliferation induced by KCs and PMs are different.
Fig. 2.
Comparing the abilities of KCs, PMs, and DCs to activate OVA TCR transgenic T cells. (A) CD4+ OVA-TCR transgenic T cells (5 × 104/well) were incubated with DCs, PMs, or KCs (2.5 × 104/well) for 3 days in the presence of various concentrations of OVA323-339. L-NMMA (0.1 mM) was included in some KC/T cell or PM/T cell cocultures. T cell proliferation was determined by [3H]-thymidine incorporation during the last 16 hours of incubation. The experiments were carried out in triplicate and the results represent mean ± standard error of the mean (SEM). *P < 0.05 compared with T cells stimulated by KCs. **P < 0.05 compared with T cells stimulated by PM. (B) CD4+ OVA-TCR transgenic T cells were labeled with CFSE, followed by incubation for 3 days with DCs or KCs in the presence of 10 μg/mL OVA323-339. Fluorescence intensities of CFSE on CD4+ T cells were measured by flow cytometry. (C) The levels of NO production in PM/T cell cocultures in the presence or absence of L-NMMA. The experiments were carried out in triplicate, and the results represent mean ± SEM. *P < 0.05 compared with cultures including L-NMMA. The data shown in panels A, B, and C represent four separate experiments producing similar results.
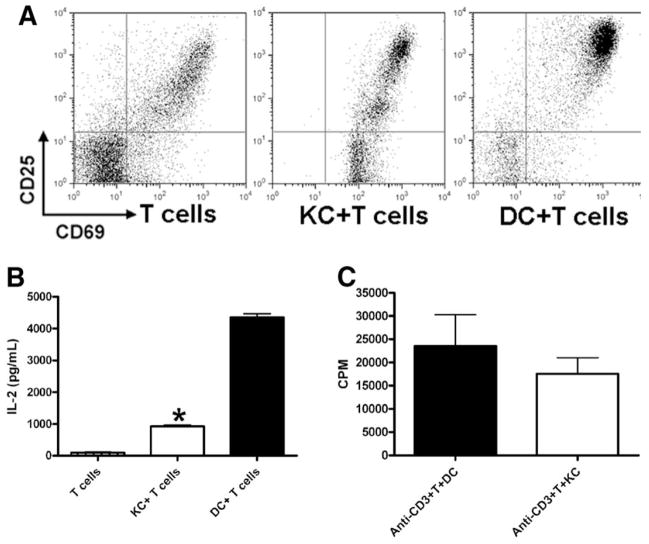
It is known that T cell activation results in the production of IL-2, up-regulation of IL-2 receptor γ chain (CD25), and the expression of an early activation marker CD69. Subsequently, the activated T cells undergo cell cycle progression and proliferation. To examine whether KCs are incompetent in initiating T cell activation or whether they negatively affect T cell proliferation, the indicators for T cell activation were examined by flow cytometry. We found that KC-stimulated T cells expressed similar levels of CD69 as those stimulated by DCs (Fig. 3A). However, instead of one major CD25high T cell population observed after DC stimulation, KC-activated T cells contained two additional subsets that were CD25dim and CD25-negative. Moreover, KC-stimulated T cells produced much lower levels of IL-2 compared with those activated by DCs (Fig. 3B). This suggested that KCs might be able to cause T cell anergy. Anergized T cells should be able to proliferate when exposed to exogenous IL-2. As well, once T cells are anergized, they should not proliferate even when they encounter potent APCs subsequently. We performed the rescue experiment using exogenous IL-2; however, KC-induced T cell proliferation was not increased (data not shown). Moreover, we examined the ability of OTII T cells to respond to DC stimulation after they were cocultured with KCs for 3 days and found that the degree of proliferation of these T cells (counts per minute [CPM] = 75,257 ± 8344) was comparable to that of naïve T cells (CPM = 69,196 ± 11,143), in response to DC elicitation. These results indicated that KCs did not induce T cell anergy.
Fig. 3.
The activation status of T cells in response to stimulation by KCs and DCs. (A and B) CD4+ OVA-TCR transgenic T cells were incubated with DCs or KCs as described. (A) After 12 hours, the cells were collected and stained with anti-CD4, anti-CD25, and anti-CD69 antibodies. Results shown are flow cytometric analyses of gated CD4+ T cells. (B) After 12 hours, the supernatants were collected and IL-2 levels determined. The experiments were carried out in triplicate and the results represent mean ± SEM. *P < 0.05 compared with DC/T cell cocultures. The data shown represent two separate experiments producing similar results. (C) CD4+ T cells (2 × 105/well) isolated from C57BL/6J mice were cocultured with DCs or KCs in the wells of 96-well plate pre-coated with anti-CD3 antibody (5 μg/mL). T cell proliferation was determined by [3H]-thymidine incorporation during the last 16 hours of incubation. The experiments were carried out in triplicate and the results represent mean ± SEM.
To determine whether the lack of T cell proliferation in response to KC stimulation was attributable to the insufficient expression of signal 1 or signal 2 by KCs, we conducted a series of experiments. We added anti-CD28 antibody in some KC/T cell cocultures, and observed a onefold increase in T cell proliferation (data not shown), suggesting that enhancing signal 2 could not restore T cell activation to the level that was induced by splenic DCs. However, when examining T cell activation induced by anti-CD3 antibody in the presence of DCs or KCs, we found that KCs were as potent as DCs in eliciting T cell proliferation (Fig. 3C). These data suggested that the lack of priming of OVA-specific T cells by KCs was predominantly attributable to KCs’ inability to present antigen and provide signal 1.
Poly I:C Enhanced the Ability of KCs To Induce T Cell Activation
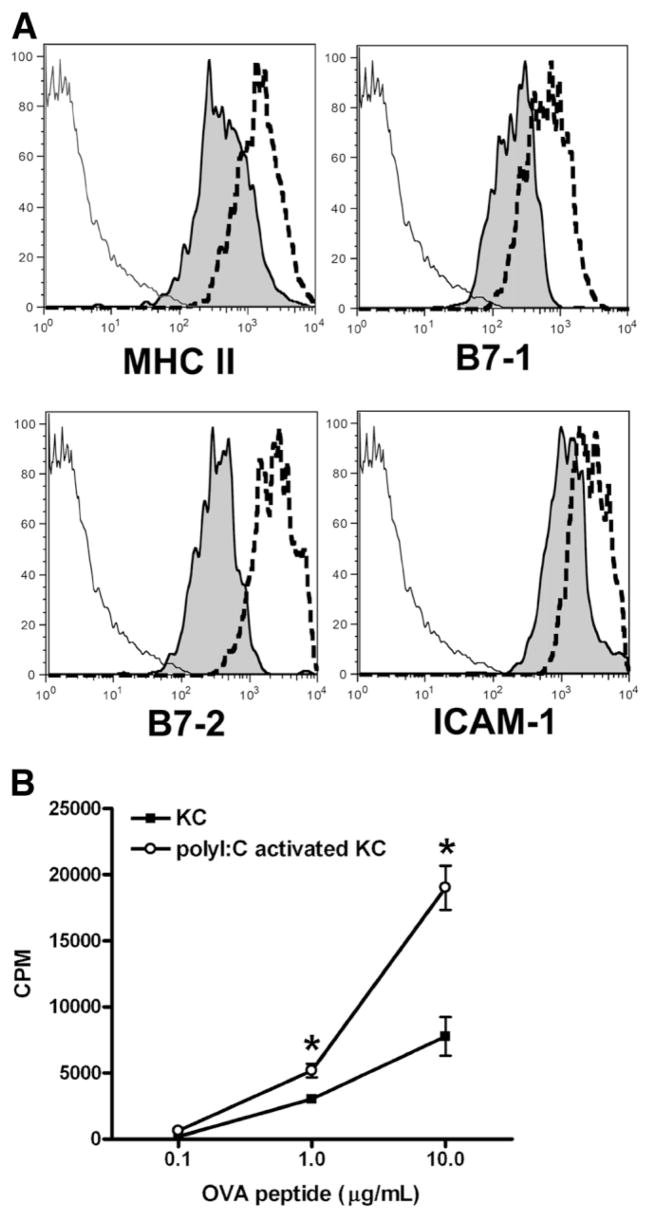
It is known that bacterial or viral infection causes activation of DC and increases their ability to initiate stronger immune responses.35–37 Similar immunostimulatory effects on KCs have not been explored. To examine whether KCs can be stimulated to increase their T cell–activating capacity, male C57BL/6J mice were treated with poly I:C, a viral RNA mimic. After 12 hours, KCs were isolated and their ability in T cell activation evaluated. The data showed that, compared with naïve KCs, poly I:C-stimulated KCs were much more potent in the induction of OVA-specific T cell responses (Fig. 4B). Furthermore, flow cytometric analysis indicated that poly I:C treatment significantly increased KC expression of MHC II, B7-1, B7-2, and ICAM-1, which could account for the increased ability of these cells to activate T cells (Fig. 4A).
Fig. 4.
Poly I:C can stimulate KCs and increase their ability to activate T cells. Male C57BL/6J mice were treated by poly I:C (50 μg/mouse), or phosphate-buffered saline as control. After 12 hours, the animals were sacrificed, NPCs isolated, and KCs purified. (A) Liver NPCs were analyzed for the expression of various surface molecules. Results shown are flow cytometric analyses of gated CD45+F4/80+ KCs. The solid lines represent isotype controls, shaded histograms staining of naïve KCs, and the dotted lines staining of poly I:C-stimulated KCs. (B) CD4+ OVA-TCR transgenic T cells were incubated with either naïve or poly I:C-stimulated KCs for 3 days in the presence of various concentrations of OVA323-339. T cell proliferation was determined as described. The measurements were carried out in triplicate and the results represent mean ± SEM. *P < 0.05 compared with T cells stimulated by naïve KCs. The data shown are representative of three independent experiments producing similar results.
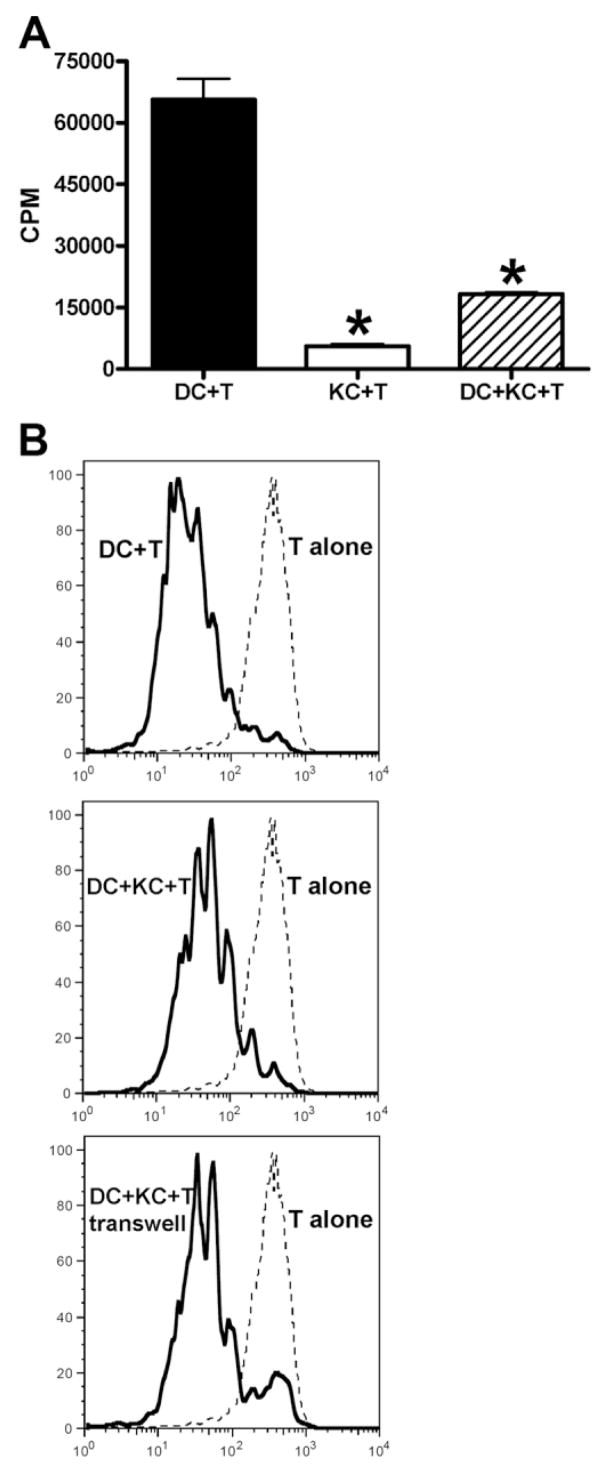
Suppression of DC-Induced T Cell Activation by KCs
It is known that the frequency of effector and memory T cells in the liver is higher than in the circulation38; however, these activated T cells appear to be quiescent within the liver. One possible explanation is that T cell activation may be suppressed and that KCs may be involved in the mechanism. To examine this hypothesis, KCs were included in the cocultures of DCs and CD4+OVA-TCR transgenic T cells. The data demonstrated that addition of KCs significantly inhibited DC-induced T cell activation, evidenced by both decreased [3H]-thymidine uptake and the decreased number of differentiated cells with lower CFSE intensity (Fig. 5). To determine whether KC-mediated T cell suppression was cell–cell contact dependent, the experiments were repeated using transwell plates to separate KCs from the DC/T cell cultures. The pattern of the CFSE staining was not affected by where KCs were placed, either above the membrane or mixed with DCs and T cells (Fig. 5B). This finding suggests that soluble mediator(s) are involved in KC-induced T cell suppression.
Fig. 5.
KCs inhibit splenic DC-induced expansion of OVA-TCR transgenic T cells. (A) CD4+ OVA-TCR transgenic T cells (5 × 104/well) were incubated with 2.5 × 104/well DCs, KCs, or DCs plus KCs (1:1 ratio) for 3 days in the presence of 10 μg/mL OVA323-339. T cell proliferation was determined by [3H]-thymidine incorporation during the last 16 hours of incubation. The experiments were carried out in triplicate and the results represent mean ± SEM. *P < 0.05 compared with T cells stimulated by DCs alone. (B) CD4+ OVA-TCR transgenic T cells were labeled with CFSE, followed by incubation with DCs alone, or in the presence of KCs with or without separation by a transwell membrane. OVA323-339 (10 μg/mL) were included in all cultures, and after 3 days the cells analyzed for CFSE staining on CD4+ T cells. The data shown in A and B are representative of four independent experiments producing similar results.
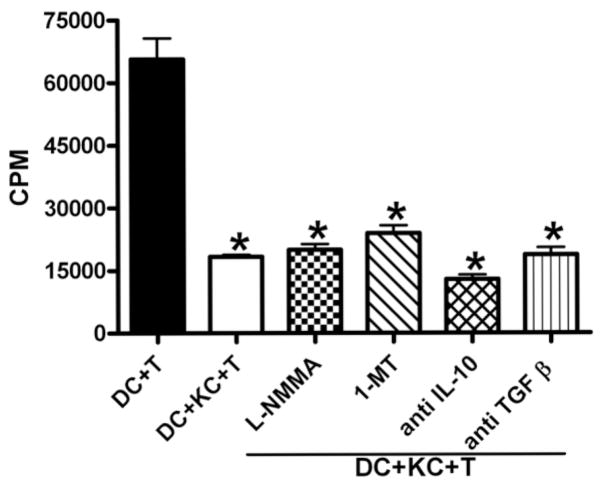
Evidence suggests that KCs represent a major hepatic source of IL-10 and TGF-β 39–41 two cytokines that have been widely shown to be potent suppressors of T cell activation. To examine whether the production of these two cytokines may contribute to KC-induced T cell suppression, neutralizing anti–IL-10 or anti–TGF-β antibody was included in some cultures. However, reversal of T cell inhibition was not observed, suggesting that IL-10 and TGF-β are not involved in KC-induced T cell suppression (Fig. 6).
Fig. 6.
KC-induced T cell suppression was not mediated by NO, IDO, IL-10, or TGF-β. CD4+ OVA-TCR transgenic T cells were incubated with DCs or the combination of DCs and KCs as described. L-NMMA (0.1 mM), 1-MT (1 mM), anti–IL-10 (10 μg/mL), or anti–TGF-β (10 μg/mL) neutralizing antibody was included in some DC/KC/T cell cocultures. T cell proliferation was determined after 3 days as described. The experiments were carried out in triplicate, and the results represent mean ± SEM. *P < 0.05 compared with T cells stimulated by DCs alone. Similar results were obtained in four separate experiments conducted in the same manner.
Many studies have demonstrated that indoleamine 2,3-dioxygenase (IDO) expressed by APCs plays an important role in mediating T cell suppression through depletion of tryptophan, thereby inhibiting T cell proliferation.42,43 To determine whether IDO could contribute to KC-induced T cell suppression, 1-MT was included in some cultures. The data showed that T cell activation was still suppressed even when IDO activity was inhibited (Fig. 6), negating a possible role for IDO in KC-mediated T cell suppression.
It has been demonstrated that macrophages can be activated by lipopolysaccharides, IFN-γ, or other stimuli to produce NO, which can inhibit T cell activation.28,29 Therefore, it is possible that IFN-γ production by DC-activated T cells could stimulate KCs to produce NO, which in turn, suppresses T cell proliferation. To examine this hypothesis, L-NMMA was added in some cultures. However, the data demonstrated that NO inhibition did not affect KC-induced T cell suppression (Fig. 6).
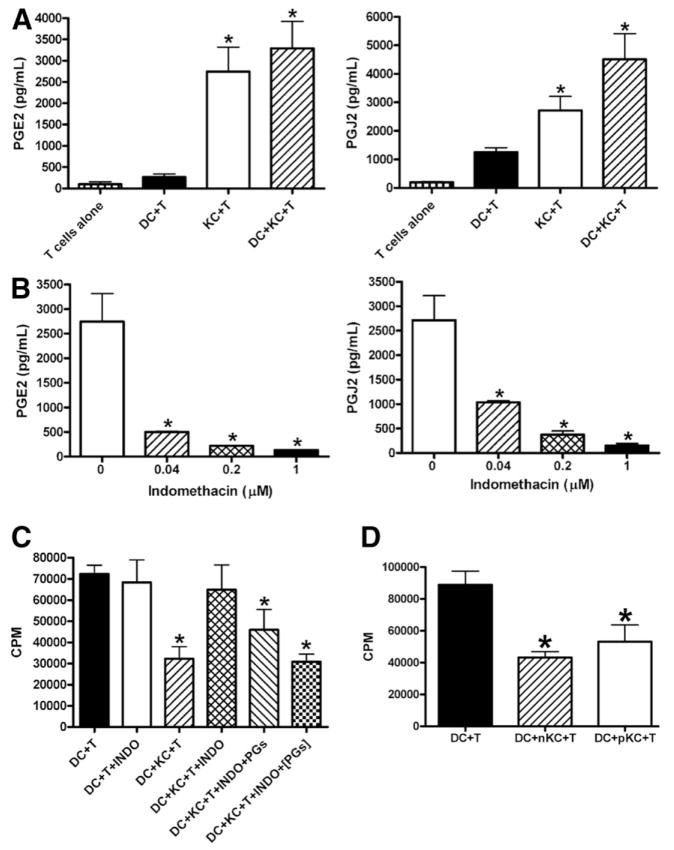
Lastly, macrophages are known to be a major source of prostaglandins (PGs), such as PGE2 and 15d-PGJ2; both have been shown to suppress T cell proliferation.44 The ability of KCs to produce PGE2 and 15d-PGJ2 was evaluated by measuring the release of these prostaglandins into the culture supernatants. The data showed that isolated naïve KCs produce significant amounts of PGE2 and 15d-PGJ2, much higher than the levels detected in the culture supernatants of T cell alone or DC/T cell cocultures (Fig. 7A). To examine whether PGE2 or 15d-PGJ2 release by KCs may contribute to their tolerogenic function, 1 μM indomethacin was added to DC/KC cocultures overnight before the addition of T cells. The data demonstrated that indomethacin effectively inhibited PGE2 and 15d-PGJ2 production in a dose-dependent manner (Fig. 7B) and inhibited KC-mediated T cell suppression (Fig. 7C). However, this inhibitory effect on T cell suppression was reversed by the addition of exogenous PGE2 and 15d-PGJ2 (Fig. 7C). Interestingly, this effect was greater if exogenous PGs were added together with T cells than if they were added before the T cell addition (Fig. 7C), suggesting PGs acting directly on T cells. Furthermore, we found that, compared with naïve KCs, poly I:C-stimulated KCs produce similar levels of PGE2 (2768 ± 271 pg/mL versus 2744 ± 575 pg/mL in naïve KCs) and 15d-PGJ2 (2995 ± 740 pg/mL versus 2718 ± 499 pg/mL in naïve KCs), and were equally potent in suppressing DC-induced T cell activation (Fig. 7D). Collectively, these results suggested that PGE2 or 15d-PGJ2 produced by KCs play an essential role in their suppression of antigen-specific T cell activation induced by DCs.
Fig. 7.
KC-induced T cell suppression was mediated by PGs. (A) CD4+ OVA-TCR transgenic T cells were cultured alone, or in the presence of DCs, KCs, or DCs and KCs. After 48 hours, the supernatants were collected and the levels of PGE2 and 15d-PGJ2 were determined. The measurements were carried out in triplicate and the results represent mean ± SEM. *P < 0.05 compared with T cells alone. (B) KCs (2.5 × 104/well) were cultured in the absence and presence of various concentrations of indomethacin. After 48 hours, the supernatants were collected and the levels of PGE2 and PGJ2 measured. The measurements were carried out in triplicate and the results represent mean ± SEM. *P < 0.05 compared with KCs cultured in the absence of indomethacin. (C) DCs (2.5 × 104/well) and KCs (2.5 × 104/well) were cultured overnight before the addition of CD4+ OVA-TCR transgenic T cells (5 × 104/well). Indomethacin (INDO, 1 μM) was included in some DC/KC overnight cocultures. Furthermore, a combination of exogenous PGE2 (3 ng/mL) and PGJ2 (3 ng/mL) wad added either in DC/KC overnight cultures, or in DC/KC/T cell cocultures at the same time as the addition of T cells (PGs). T cell proliferation was determined after 3 days as described. (D) CD4+ OVA-TCR transgenic T cells were incubated with DCs alone, or in the presence of naïve KCs (nKC) or KCs isolated from poly I:C-treated mice (pKC). T cell proliferation was determined after 3 days as described. (C and D) The measurements were carried out in triplicate and the results represent mean ± SEM. *P < 0.05 compared with T cells stimulated by DCs. The data shown in panels A, B, C, and D represent four independent experiments producing similar results.
Discussion
The tolerogenic properties of the liver may be explained by its composition of “tolerogenic” APCs, including LSECs, hepatic DCs, and KCs. Although the immunosuppressive functions of LSECs and hepatic DCs have been investigated extensively,10–16 very little was known about the immunogenicity or tolerogenicity of KCs as APCs toward T cell activation. Our previous studies revealed an immunosuppressive function of KCs by using a murine model of T cell–mediated delayed-type hypersensitivity reaction induced by a hapten, 2,4-dinitrochlorobenzene.22 The data demonstrated that pretreatment of mice with a bovine serum albumin adduct of 2,4-dinitrochlorobenzene led to tolerance against the 2,4-dinitrochlorobenzene–induced delayed-type hypersensitivity reaction response. We also found that adoptive transfer of KC-enriched, but not KC-depleted, NPCs, from tolerized mice, could induce tolerance in naïve recipient mice. These results suggested that KCs are important in down-regulating T cell reactions to soluble antigens. Other evidence also indicated a regulatory role for KCs in allograft immune reactions.19–21 However, the underlying mechanism(s) for the immunotolerogenic role of KCs has not been well studied.
The current study characterized KCs’ expression of APC-related molecules and compared the levels with those on other APCs. The data demonstrated that KCs expressed significant levels of MHC II and costimulatory molecules, such as B7-1 and B7-2, even though the levels were lower than those expressed by splenic DCs. Moreover, KCs expressed high levels of ICAM-1, suggesting that these cells, ideally situated to encounter circulating lymphocytes in the sinusoidal lumen, are capable of interacting and immobilizing T cells in the liver through binding to leukocyte functional antigen expressed by T cells. The immunogenicity of KCs was evaluated by in vitro T cell proliferation assay using OVA-specific TCR transgenic CD4+ T cells. The data showed that splenic DCs could induce a strong activation of T cells; however, T cell proliferative response was low when KCs were used as APCs (Fig. 2). Moreover, the level of IL-2 production by KC-stimulated T cells was markedly lower than that by DC-activated T cells (Fig. 3B). There are three possible causes for the observed phenomenon: (1) induction of T cell apoptosis, (2) activation of regulatory T cells (Tregs), and (3) inadequate stimulation by KCs.
It is known that a large proportion of T cells activated in the lymph nodes and the spleen flow into the liver.45 However, evidence suggests that a large percentage of these T cells undergo apoptosis within the liver.46–48 Recent studies demonstrated that KCs may be involved in the induction of T cell apoptosis through the expression of programmed death L1, a ligand for programmed death 1, that is expressed on activated T cells.45,49 Our flow cytometric analyses of T cell apoptosis demonstrated a onefold increase in the percentage of apoptotic T cells in the presence of KCs (21.6% ± 3.4%), compared with T cells alone (4% ± 0.6%) or DC/T cell cocultures (10.1% ± 1.7%). However, we did not observe a significant increase in T cell proliferation when a caspase inhibitor (Z-VAD) was added in the KC/T cell cocultures to prevent apoptosis (data not shown). These data suggest that the slight increase in T cell apoptosis induced by KCs could not explain the drastically impaired T cell proliferative response.
Another potential cause for the defective T cell priming is that KCs may activate and expand Tregs, which can deplete IL-2 and suppress effector T cell proliferation.50,51 A previous study using CD4+CD25+ cells isolated from the mouse spleen demonstrated that KCs could induce proliferation of these cells in the presence of anti-CD3 antibody.52 However, using Foxp3 intracellular staining approach to identify Tregs, we did not observe a significant increase in the percentage of Foxp3+CD4+T cells in the KC/T cocultures compared with T cell alone or DC/T cocultures (data not shown). Aside from the fact that two different in vitro T cell activation systems (anti-CD3 antibody versus OVA peptide) were used, this discrepancy is likely due to the different methods used for the identification of Tregs. Foxp3+CD4+cells were defined as Tregs in our studies; however, the published study used CD25 as the marker for Treg, most likely because the Foxp3 method was not available at the time the work was conducted. It is well established now that Foxp3 is the most accurate marker for Treg, and that CD25 is not a specific marker. Not all CD25+ cells are Tregs and Tregs are not all CD25-positive.53
It is known that T cell activation requires two signals. T cell receptor recognition of a specific MHC/antigenic peptide complex produces signal 1.54 Signal 2 is provided by the binding of costimulatory molecules expressed on APCs to their ligands on T cells.55 For example, CD40 and B7 molecules (B7-1 and B7-2) on APCs interact with CD40 ligand and CD28 on T cells, respectively.56,57 Our data showed that, compared with DCs, KCs expressed significantly lower levels of costimulatory molecules, including B7-1, B7-2, and CD40 (Fig. 1B), which may contribute to the lack of T cell activation. To examine this hypothesis, in some KC/T cell cocultures, anti-CD28 antibody was added to provide sufficient costimulation. Although a onefold increase in T cell proliferation was observed, the enhanced signal 2 could not restore T cell activation to the level that is induced by splenic DCs (data not shown). Our data showed that, compared with DCs, KCs also expressed lower levels of MHC II, suggesting that these cells may be incompetent in presenting antigens and providing signal 1. Moreover, we cocultured T cells with DCs or KCs in the presence of anti-CD3 antibody, and found that when signal 1 levels were the same, KCs and DCs were equally potent to induce T cell activation (Fig. 3C). The data further supported that the lack of priming of OVA-specific T cells by KCs was predominantly attributable to KCs’ inability to present antigen (signal 1). However, when KCs were stimulated by poly I:C, their expression of signal 1 and signal 2 were increased, and they became much more potent in induction of OVA-specific T cell activation (Fig. 4). To investigate the role of KC-derived PGs in regulating T cell priming, we conducted experiments using indomethacin to inhibit PGs in naïve KCs, and found a onefold increase of T cell priming. Similarly, using poly I:C-stimulated KCs, we observed that adding indomethacin caused an increase in T cell priming, whereas adding exogenous PGs caused a decrease (data not shown). Collectively, these findings suggest that the inability of KCs to activate antigen-specific T cell responses is primarily attributable to their inability to present antigens, and also attributable to their inadequate expression of costimulatory molecules and production of PGs.
Interestingly, our data demonstrated that, although KCs were incompetent in eliciting T cell activation, they could actively suppress T cell proliferation induced by splenic DCs. KC-mediated T cell suppression was evidenced by both decreased [3H]-thymidine uptake and increased CFSE intensity (Fig. 5). Moreover, the trans-well experiments showed the involvement of soluble mediators in such suppression. Numerous studies have demonstrated that KCs represent a major source of IL-10 and TGF-β, two most potent immunosuppressive cytokines.39–41 The possibility that IL-10 or TGF-β are involved in KC-induced T cell inhibition was examined by including neutralizing anti–IL-10 or anti–TGF-β antibody in the KC/DC/T cell cocultures. However, reversal of T cell suppression was not observed (Fig. 6), suggesting that these two cytokines did not play a role. Furthermore, no significant levels of IL-10 and TGF-β could be detected in cultures containing KCs. This is not contradictory to the reports describing production of these cytokines by KCs, because the KCs used in our studies were naïve rather than activated. In fact, this result confirmed that the KCs were not activated during our isolation of the cells, as macrophages are sensitive to lipopolysaccharides that might be present during cell isolation and culturing systems.
It has been demonstrated that macrophages produce NO in response to IFN-γ released by activated T cells, and that NO in turn inhibits T cell proliferation.27,28,58 Consistent with these findings, our data demonstrated that significant amounts of NO were produced in PM/T cell cocultures, and that inhibition of NO drastically increased PM-induced T cell proliferation (Fig. 2). However, NO was not detected in cultures containing KCs (data not shown), and the addition of L-NMMA did not reverse KC-induced inhibition of T cell activation (Fig. 6). These data suggest that NO production cannot account for the mechanism of KC-mediated T cell suppression.
Evidence suggests that amino acid metabolism in myeloid cells is a mechanism for effectively limiting T cell activation and proliferation. L-tryptophan and L-arginine are central to the control of T cell responses. Myeloid cells regulate the levels of these two amino acids by the expression of arginase and IDO, which degrades arginine and tryptophan, respectively. A considerable body of evidence supports that IDO-expressing cells negatively regulate T cell–mediated immune responses in inflammatory disease, transplant rejection, pregnancy, and cancer.43 The initial studies demonstrated that IDO expression by macrophages suppressed T cell proliferation.59,60 Further studies showed that certain subsets of DCs can express IDO and induce T cell suppression.42 To examine the potential involvement of IDO in the mechanism of KC-mediated T cell suppression, 1-MT was added in some cultures to inhibit IDO activity. However, no effect on T cell activation was observed, indicating that IDO did not contribute to the mechanism of KC-induced T cell suppression (Fig. 6). The role of L-arginine in regulating immune response has been suggested by the markedly decreased T cell response found in patients with liver transplantation, trauma, and certain cancers in which the L-arginine level is reduced.61–63 A recent study showed that the mechanism by which L-arginine starvation blocks T-cell proliferation is due to impaired expression of cell cycle regulating proteins, thereby resulting in cell cycle arrest.64 In the current study, L-arginine was added to some cultures containing KCs, DCs, and T cells. However, KC-induced inhibition of DC-mediated T cell activation was not affected (data not shown), suggesting that regulation of L-arginine levels does not play a role in our model.
Macrophages are known to be a major source of PGs, such as PGE2 and 15d-PGJ2, which have been shown by numerous studies to inhibit T cell proliferation.44 Our data demonstrated that isolated naïve KCs produced significant amounts of PGE2 and 15d-PGJ2, much higher than the levels detected in the culture supernatants of T cell alone or DC plus T cells (Fig. 7A). When KCs were cultured in the presence of various concentrations of indomethacin, the production of PGE2 and 15d-PGJ2 was effectively reduced (Fig. 7B), and more importantly, KC-induced T cell suppression was inhibited (Fig. 7C). However, addition of exogenous PGE2 and PGJ2 at the levels detected in cultures containing KCs (as shown in Fig. 7A) did reverse the effect of indomethacin (Fig. 7C). We also found that polyI:C-stimulated KCs produced similar levels of PGE2 and 15d-PGJ2, and were as potent in T cell suppression (Fig. 7D). Collectively, these results suggest that PGE2 or 15d-PGJ2 produced by KCs play an essential role in their suppression of antigen-specific T cell activation induced by DCs.
We further examined which cells, KCs, DCs, or T cells, were targeted by KC-derived PGE2 and 15d-PGJ2 to exert their tolerogenic effect. We found no autocrine effect of PGs on KCs, because the addition of indomethacin did not alter MHC II, B7-1, and B7-2 expression by KCs (data not shown). To investigate whether KCs would affect the phenotype of DCs, DCs were cultured alone or with KCs overnight, and the surface expression of MHC II, B7.1, and B7.2 by DCs was determined. We found that coculturing with KCs did not alter DC’s phenotype related to antigen presentation (data not shown). These results suggested that the tolerogenic effect of KCs, via their production of PGE2 and 15d-PGJ2, is mediated through a direct action on T cells, rather than on KCs or DCs. This was further supported by our findings from the following experiment: KCs and DCs were cocultured for 16 hours before the addition of T cells; if PGs were added simultaneously with T cells, a greater suppression of T cell proliferation was observed than if PGs were added earlier into the KC/DC cocultures (Fig. 7C). Although the mechanism of T cell inhibition induced by 15d-PGJ2 is currently unclear,65–67 ample evidence suggests that the mechanisms by which PGE2 inhibits T cell proliferation include suppression of intracellular calcium elevation,68 reduction of IL-2 secretion,69 and inhibition of the activity of tyrosine kinases, such as JAK3 and p59.70,71 More recent studies also demonstrated that PGE2 can induce Foxp3+ regulatory T cells, which are pivotal in suppressing immune responses and maintaining tolerance.72,73 However, in our experimental system, the molecular mechanism for the involvement of PGE2 or 15d-PGJ2 in mediating KC-induced T cell suppression warrants further investigation.
In summary, this study fully characterizes the immunogenicity/tolerogenicity of KCs. The data demonstrated that KCs cannot elicit T cell activation, and more importantly, they can inhibit antigen-specific T cell responses induced by other APCs. Further investigation of the underlying mechanism revealed the role of PGE2 and 15d-PGJ2 in mediating the suppressive function of KCs, and the studies ruled out the involvement of IL-10, TGF-β, NO, and amino acid starvation in the mechanism. Our findings suggest that KCs are a tolerogenic population of APC in the liver and that they may play an important role in the mechanisms of liver-mediated systemic immunological tolerance.
Acknowledgments
Supported by U.S. National Institutes of Health grant RO1 ES012914 (to C.J.).
The authors thank Dr. Philippa Marrack (National Jewish Medical and Research Center, Denver, CO) for the generous gift of the OTII OVA-TCR transgenic mice.
Abbreviations
- 1-MT
1-methyl-d,l-trytophan
- 15d-PGJ2
15-deoxy-delta12,14-PGJ2
- APC
antigen-presenting cell
- CFSE
carboxy-fluorescein diacetate succinimidyl ester
- CPM
counts per minute
- DC
dendritic cell
- HBSS
Hanks’ balanced-salt solution
- ICAM
intercellular adhesion molecule
- IDO
indoleamine 2,3-dioxygenase
- IFN
interferon
- IL
interleukin
- KC
Kupffer cell
- L-NMMA
NG-mono-methyl-L-arginine
- LSEC
liver sinusoidal endothelial cells
- MHC
major histocompatibility complex
- NO
nitric oxide
- NPC
nonparenchymal cell
- PG
prostaglandin
- PM
peritoneal macrophages
- poly I
C, polyinosinic-polycytidylic acid
- SEM
standard error of the mean
- TCR
T cell receptor
- TGF
transforming growth factor
- Th
T helper
- Treg
regulatory T cell
Footnotes
Potential conflict of interest: Nothing to report.
References
- 1.Calne RY, Sells RA, Pena JR, et al. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;223:472–476. doi: 10.1038/223472a0. [DOI] [PubMed] [Google Scholar]
- 2.Gorczynski RM, Chan Z, Chung S, Cohen Z, Levy G, Sullivan B, et al. Prolongation of rat small bowel or renal allograft survival by pretransplant transfusion and/or by varying the route of allograft venous drainage. Transplantation. 1994;58:816–820. [PubMed] [Google Scholar]
- 3.Rao VK, Burris DE, Gruel SM, Sollinger HW, Burlingham WJ. Evidence that donor spleen cells administered through the portal vein prolong the survival of cardiac allografts in rats. Transplantation. 1988;45:1145–1146. doi: 10.1097/00007890-198806000-00032. [DOI] [PubMed] [Google Scholar]
- 4.Cantor HM, Dumont AE. Hepatic suppression of sensitization to antigen absorbed into the portal system. Nature. 1967;215:744–745. doi: 10.1038/215744a0. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Ong CR, McKenna GJ, Mui AL, Smith RM, Chung SW. Induction of immune hyporesponsiveness after portal vein immunization with ovalbumin. Surgery. 2001;129:66–75. doi: 10.1067/msy.2001.109059. [DOI] [PubMed] [Google Scholar]
- 6.Bertolino P, Heath WR, Hardy CL, Morahan G, Miller JF. Peripheral deletion of autoreactive CD8+ T cells in transgenic mice expressing H-2Kb in the liver. Eur J Immunol. 1995;25:1932–1942. doi: 10.1002/eji.1830250721. [DOI] [PubMed] [Google Scholar]
- 7.Huang L, Soldevila G, Leeker M, Flavell R, Crispe IN. The liver eliminates T cells undergoing antigen-triggered apoptosis in vivo. Immunity. 1994;1:741–749. doi: 10.1016/s1074-7613(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 8.Keating R, Yue W, Rutigliano JA, So J, Olivas E, Thomas PG, et al. Virus-specific CD8+ T cells in the liver: armed and ready to kill. J Immunol. 2007;178:2737–2745. doi: 10.4049/jimmunol.178.5.2737. [DOI] [PubMed] [Google Scholar]
- 9.Polakos NK, Klein I, Richter MV, Zaiss DM, Giannandrea M, Crispe IN, et al. Early intrahepatic accumulation of CD8+ T cells provides a source of effectors for nonhepatic immune responses. J Immunol. 2007;179:201–210. doi: 10.4049/jimmunol.179.1.201. [DOI] [PubMed] [Google Scholar]
- 10.Klugewitz K, Blumenthal-Barby F, Schrage A, Knolle PA, Hamann A, Crispe IN. Immunomodulatory effects of the liver: deletion of activated CD4+ effector cells and suppression of IFN-gamma-producing cells after intravenous protein immunization. J Immunol. 2002;169:2407–2413. doi: 10.4049/jimmunol.169.5.2407. [DOI] [PubMed] [Google Scholar]
- 11.Knolle PA, Schmitt E, Jin S, Germann T, Duchmann R, Hegenbarth S, et al. Induction of cytokine production in naive CD4+) T cells by antigen-presenting murine liver sinusoidal endothelial cells but failure to induce differentiation toward Th1 cells. Gastroenterology. 1999;116:1428–1440. doi: 10.1016/s0016-5085(99)70508-1. [DOI] [PubMed] [Google Scholar]
- 12.Limmer A, Ohl J, Kurts C, Ljunggren HG, Reiss Y, Groettrup M, et al. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nat Med. 2000;6:1348–1354. doi: 10.1038/82161. [DOI] [PubMed] [Google Scholar]
- 13.Katz SC, Pillarisetty VG, Bleier JI, Shah AB, DeMatteo RP. Liver sinusoidal endothelial cells are insufficient to activate T cells. J Immunol. 2004;173:230–235. doi: 10.4049/jimmunol.173.1.230. [DOI] [PubMed] [Google Scholar]
- 14.Pillarisetty VG, Shah AB, Miller G, Bleier JI, DeMatteo RP. Liver dendritic cells are less immunogenic than spleen dendritic cells because of differences in subtype composition. J Immunol. 2004;172:1009–1017. doi: 10.4049/jimmunol.172.2.1009. [DOI] [PubMed] [Google Scholar]
- 15.Lian ZX, Okada T, He XS, Kita H, Liu YJ, Ansari AA, et al. Heterogeneity of dendritic cells in the mouse liver: identification and characterization of four distinct populations. J Immunol. 2003;170:2323–2330. doi: 10.4049/jimmunol.170.5.2323. [DOI] [PubMed] [Google Scholar]
- 16.Jomantaite I, Dikopoulos N, Kroger A, Leithäuser F, Hauser H, Schirmbeck R, et al. Hepatic dendritic cell subsets in the mouse. Eur J Immunol. 2004;34:355–365. doi: 10.1002/eji.200324336. [DOI] [PubMed] [Google Scholar]
- 17.MacPhee PJ, Schmidt EE, Groom AC. Intermittence of blood flow in liver sinusoids, studied by high-resolution in vivo microscopy. Am J Physiol. 1995;269:G692–G698. doi: 10.1152/ajpgi.1995.269.5.G692. [DOI] [PubMed] [Google Scholar]
- 18.McCuskey RS, Reilly FD. Hepatic microvasculature: dynamic structure and its regulation. Semin Liver Dis. 1993;13:1–12. doi: 10.1055/s-2007-1007333. [DOI] [PubMed] [Google Scholar]
- 19.Callery MP, Kamei T, Flye MW. Kupffer cell blockade inhibits induction of tolerance by the portal venous route. Transplantation. 1989;47:1092–1094. [PubMed] [Google Scholar]
- 20.Kamei T, Callery MP, Flye MW. Kupffer cell blockade prevents induction of portal venous tolerance in rat cardiac allograft transplantation. J Surg Res. 1990;48:393–396. doi: 10.1016/0022-4804(90)90001-i. [DOI] [PubMed] [Google Scholar]
- 21.Sato K, Yabuki K, Haba T, Maekawa T. Role of Kupffer cells in the induction of tolerance after liver transplantation. J Surg Res. 1996;63:433–438. doi: 10.1006/jsre.1996.0288. [DOI] [PubMed] [Google Scholar]
- 22.Ju C, McCoy JP, Chung CJ, Graf ML, Pohl LR. Tolerogenic role of Kupffer cells in allergic reactions. Chem Res Toxicol. 2003;16:1514–1519. doi: 10.1021/tx0341761. [DOI] [PubMed] [Google Scholar]
- 23.Perez RV, Swanson C, Morgan M, Erickson K, Hubbard NE, German JB. Portal venous transfusion up-regulates Kupffer cell cyclooxygenase activity: a mechanism of immunosuppression in organ transplantation. Transplantation. 1997;64:135–139. doi: 10.1097/00007890-199707150-00023. [DOI] [PubMed] [Google Scholar]
- 24.Roland CR, Mangino MJ, Duffy BF, Flye MW. Lymphocyte suppression by Kupffer cells prevents portal venous tolerance induction: a study of macrophage function after intravenous gadolinium. Transplantation. 1993;55:1151–1158. doi: 10.1097/00007890-199305000-00041. [DOI] [PubMed] [Google Scholar]
- 25.Squiers EC, Brunson ME, Salomon DR. Kupffer cells can present alloantigen in vitro: an effect abrogated by gadolinium. J Surg Res. 1993;55:571–574. doi: 10.1006/jsre.1993.1186. [DOI] [PubMed] [Google Scholar]
- 26.Iwasaki A, Kelsall BL. Freshly isolated Peyer’s patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J Exp Med. 1999;190:229–239. doi: 10.1084/jem.190.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mazzoni A, Bronte V, Visintin A, Spitzer JH, Apolloni E, Serafini P, et al. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J Immunol. 2002;168:689–695. doi: 10.4049/jimmunol.168.2.689. [DOI] [PubMed] [Google Scholar]
- 28.Bingisser RM, Tilbrook PA, Holt PG, Kees UR. Macrophage-derived nitric oxide regulates T cell activation via reversible disruption of the Jak3/STAT5 signaling pathway. J Immunol. 1998;160:5729–5734. [PubMed] [Google Scholar]
- 29.Albina JE, Abate JA, Henry WL., Jr Nitric oxide production is required for murine resident peritoneal macrophages to suppress mitogen-stimulated T cell proliferation: role of IFN-gamma in the induction of the nitric oxide-synthesizing pathway. J Immunol. 1991;147:144–148. [PubMed] [Google Scholar]
- 30.Young MR, Wright MA, Matthews JP, Malik I, Prechel M. Suppression of T cell proliferation by tumor-induced granulocyte-macrophage progenitor cells producing transforming growth factor-beta and nitric oxide. J Immunol. 1996;156:1916–1922. [PubMed] [Google Scholar]
- 31.Medot-Pirenne M, Heilman MJ, Saxena M, McDermott PE, Mills CD. Augmentation of an antitumor CTL response In vivo by inhibition of suppressor macrophage nitric oxide. J Immunol. 1999;163:5877–5882. [PubMed] [Google Scholar]
- 32.Lejeune P, Lagadec P, Onier N, Pinard D, Ohshima H, Jeannin JF. Nitric oxide involvement in tumor-induced immunosuppression. J Immunol. 1994;152:5077–5083. [PubMed] [Google Scholar]
- 33.Angulo I, las Heras FG, Garcia-Bustos JF, Gargallo D, Muñoz-Fernández MA, Fresno M. Nitric oxide-producing CD11b(+)Ly-6G(Gr-1)(+)CD31(ER-MP12)(+) cells in the spleen of cyclophosphamide-treated mice: implications for T-cell responses in immunosuppressed mice. Blood. 2000;95:212–220. [PubMed] [Google Scholar]
- 34.Bobe P, Benihoud K, Grandjon D, Opolon P, Pritchard LL, Huchet R. Nitric oxide mediation of active immunosuppression associated with graft-versus-host reaction. Blood. 1999;94:1028–1037. [PubMed] [Google Scholar]
- 35.Amati L, Pepe M, Passeri ME, Mastronardi ML, Jirillo E, Covelli V. Toll-like receptor signaling mechanisms involved in dendritic cell activation: potential therapeutic control of T cell polarization. Curr Pharm Des. 2006;12:4247–4254. doi: 10.2174/138161206778743583. [DOI] [PubMed] [Google Scholar]
- 36.Pearce EJ, Kane CM, Sun J. Regulation of dendritic cell function by pathogen-derived molecules plays a key role in dictating the outcome of the adaptive immune response. Chem Immunol Allergy. 2006;90:82–90. doi: 10.1159/000088882. [DOI] [PubMed] [Google Scholar]
- 37.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 38.Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43:S54–S62. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- 39.Bissell DM, Wang SS, Jarnagin WR, Roll FJ. Cell-specific expression of transforming growth factor-beta in rat liver: evidence for autocrine regulation of hepatocyte proliferation. J Clin Invest. 1995;96:447–455. doi: 10.1172/JCI118055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knolle PA, Uhrig A, Protzer U, Trippler M, Duchmann R, Meyer zum Büschenfelde KH, et al. Interleukin-10 expression is autoregulated at the transcriptional level in human and murine Kupffer cells. Hepatology. 1998;27:93–99. doi: 10.1002/hep.510270116. [DOI] [PubMed] [Google Scholar]
- 41.Knolle PA, Gerken G. Local control of the immune response in the liver. Immunol Rev. 2000;174:21–34. doi: 10.1034/j.1600-0528.2002.017408.x. [DOI] [PubMed] [Google Scholar]
- 42.Grohmann U, Fallarino F, Bianchi R, Belladonna ML, Vacca C, Orabona C, et al. IL-6 inhibits the tolerogenic function of CD8 alpha+ dendritic cells expressing indoleamine 2,3-dioxygenase. J Immunol. 2001;167:708–714. doi: 10.4049/jimmunol.167.2.708. [DOI] [PubMed] [Google Scholar]
- 43.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 44.Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144–150. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- 45.Kuniyasu Y, Marfani SM, Inayat IB, Sheikh SZ, Mehal WZ. Kupffer cells required for high affinity peptide-induced deletion, not retention, of activated CD8+ T cells by mouse liver. Hepatology. 2004;39:1017–1027. doi: 10.1002/hep.20153. [DOI] [PubMed] [Google Scholar]
- 46.Qian S, Lu L, Fu F, Li Y, Li W, Starzl TE, et al. Apoptosis within spontaneously accepted mouse liver allografts: evidence for deletion of cytotoxic T cells and implications for tolerance induction. J Immunol. 1997;158:4654–4661. [PMC free article] [PubMed] [Google Scholar]
- 47.Iwakoshi NN, Goldschneider I, Tausche F, Mordes JP, Rossini AA, Greiner DL. High frequency apoptosis of recent thymic emigrants in the liver of lymphopenic diabetes-prone BioBreeding rats. J Immunol. 1998;160:5838–5850. [PubMed] [Google Scholar]
- 48.Mehal WZ, Azzaroli F, Crispe IN. Antigen presentation by liver cells controls intrahepatic T cell trapping, whereas bone marrow-derived cells preferentially promote intrahepatic T cell apoptosis. J Immunol. 2001;167:667–673. doi: 10.4049/jimmunol.167.2.667. [DOI] [PubMed] [Google Scholar]
- 49.Iwai Y, Terawaki S, Ikegawa M, Okazaki T, Honjo T. PD-1 inhibits antiviral immunity at the effector phase in the liver. J Exp Med. 2003;198:39–50. doi: 10.1084/jem.20022235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 51.de la RM, Rutz S, Dorninger H, Scheffold A. Interleukin-2 is essential for CD4+ CD25+ regulatory T cell function. Eur J Immunol. 2004;34:2480–2488. doi: 10.1002/eji.200425274. [DOI] [PubMed] [Google Scholar]
- 52.Wiegard C, Frenzel C, Herkel J, Kallen KJ, Schmitt E, Lohse AW. Murine liver antigen presenting cells control suppressor activity of CD4+ CD25+ regulatory T cells. Hepatology. 2005;42:193–199. doi: 10.1002/hep.20756. [DOI] [PubMed] [Google Scholar]
- 53.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 54.Paul WE. Fundamental immunology. 4. Philadelphia: Lippincott-Raven; 1999. [Google Scholar]
- 55.Germain RN. Accessory cell stimulation of T cell proliferation requires active antigen processing, Ia-restricted antigen presentation, and a separate nonspecific 2nd signal. J Immunol. 1981;127:1964–1966. [PubMed] [Google Scholar]
- 56.Greenfield EA, Nguyen KA, Kuchroo VK. CD28/B7 costimulation: a review. Crit Rev Immunol. 1998;18:389–418. doi: 10.1615/critrevimmunol.v18.i5.10. [DOI] [PubMed] [Google Scholar]
- 57.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 58.Apolloni E, Bronte V, Mazzoni A, Serafini P, Cabrelle A, Segal DM, et al. Immortalized myeloid suppressor cells trigger apoptosis in antigen-activated T lymphocytes. J Immunol. 2000;165:6723–6730. doi: 10.4049/jimmunol.165.12.6723. [DOI] [PubMed] [Google Scholar]
- 59.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 60.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189:1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Angele MK, Smail N, Ayala A, Cioffi WG, Bland KI, Chaudry IH. L-arginine: a unique amino acid for restoring the depressed macrophage functions after trauma-hemorrhage. J Trauma. 1999;46:34–41. doi: 10.1097/00005373-199901000-00006. [DOI] [PubMed] [Google Scholar]
- 62.Roth E, Steininger R, Winkler S, Längle F, Grünberger T, Függer R, et al. L-Arginine deficiency after liver transplantation as an effect of arginase efflux from the graft. Influence on nitric oxide metabolism. Transplantation. 1994;57:665–669. doi: 10.1097/00007890-199403150-00006. [DOI] [PubMed] [Google Scholar]
- 63.Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65:3044–3048. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- 64.Rodriguez PC, Quiceno DG, Ochoa AC. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood. 2007;109:1568–1573. doi: 10.1182/blood-2006-06-031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Clark RB, Bishop-Bailey D, Estrada-Hernandez T, Hla T, Puddington L, Padula SJ. The nuclear receptor PPAR gamma and immunoregulation: PPAR gamma mediates inhibition of helper T cell responses. J Immunol. 2000;164:1364–1371. doi: 10.4049/jimmunol.164.3.1364. [DOI] [PubMed] [Google Scholar]
- 66.Diab A, Deng C, Smith JD, Hussain RZ, Phanavanh B, Lovett-Racke AE, et al. Peroxisome proliferator-activated receptor-gamma agonist 15-deoxy-Delta12,14)-prostaglandin J(2) ameliorates experimental autoimmune encephalomyelitis. J Immunol. 2002;168:2508–2515. doi: 10.4049/jimmunol.168.5.2508. [DOI] [PubMed] [Google Scholar]
- 67.Harris SG, Phipps RP. The nuclear receptor PPAR gamma is expressed by mouse T lymphocytes and PPAR gamma agonists induce apoptosis. Eur J Immunol. 2001;31:1098–1105. doi: 10.1002/1521-4141(200104)31:4<1098::aid-immu1098>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 68.Choudhry MA, Hockberger PE, Sayeed MM. PGE2 suppresses mitogen-induced Ca2+ mobilization in T cells. Am J Physiol. 1999;277:R1741–R1748. doi: 10.1152/ajpregu.1999.277.6.R1741. [DOI] [PubMed] [Google Scholar]
- 69.Cosme R, Lublin D, Takafuji V, Lynch K, Roche JK. Prostanoids in human colonic mucosa: effects of inflammation on PGE(2) receptor expression. Hum Immunol. 2000;61:684–696. doi: 10.1016/s0198-8859(00)00131-2. [DOI] [PubMed] [Google Scholar]
- 70.Choudhry MA, Ahmed Z, Sayeed MM. PGE(2)-mediated inhibition of T cell p59(fyn) is independent of cAMP. Am J Physiol. 1999;277:C302–C309. doi: 10.1152/ajpcell.1999.277.2.C302. [DOI] [PubMed] [Google Scholar]
- 71.Kolenko V, Rayman P, Roy B, Cathcart MK, O’Shea J, Tubbs R, et al. Downregulation of JAK3 protein levels in T lymphocytes by prostaglandin E2 and other cyclic adenosine monophosphate-elevating agents: impact on interleukin-2 receptor signaling pathway. Blood. 1999;93:2308–2318. [PubMed] [Google Scholar]
- 72.Baratelli F, Lin Y, Zhu L, Yang SC, Heuzé-Vourc’h N, Zeng G, et al. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J Immunol. 2005;175:1483–1490. doi: 10.4049/jimmunol.175.3.1483. [DOI] [PubMed] [Google Scholar]
- 73.Sharma S, Yang SC, Zhu L, Reckamp K, Gardner B, Baratelli F, et al. Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res. 2005;65:5211–5220. doi: 10.1158/0008-5472.CAN-05-0141. [DOI] [PubMed] [Google Scholar]