Abstract
Objective
To describe baseline night vision symptoms and their association with ≥3-lines loss in visual acuity (VA), choroidal neovascularization (CNV) and geographic atrophy (GA).
Design
Cohort study within a multicenter randomized clinical trial.
Participants
1052 participants with ≥10 large (>125 μ) drusen and VA ≥20/40 in each eye.
Methods
At baseline, participants self-administered a 10-item night vision questionnaire (NVQ-10). VA testing was performed by certified examiners at baseline, 6 months and annually. One eye of each participant was randomly assigned to laser treatment and the contralateral eye was assigned to observation. During follow-up, trained readers identified CNV based on fluorescein angiograms and endpoint GA, defined as >1 disc area of new GA, based on color photographs. Evaluation of NVQ-10 score as a risk factor for ≥3-lines loss in VA was performed by repeated-measures logistic regression, and for CNV and GA by survival analysis, with and without adjustment for participant and ocular characteristics. Evaluations were based on observed eyes and treated eyes, considered separately and combined.
Main Outcome Measures
≥3-lines loss in VA, development of CNV and of endpoint GA.
Results
At baseline, NVQ-10 scores ranged from 3 to 100 with a mean of 70 (100 corresponds to no night vision symptoms). Compared to participants with the best night vision (4th quartile of NVQ-10 scores), participants with the worst night vision (1st quartile of scores) were at increased risk of ≥3-lines loss in VA in both observed and treated eyes; odds ratios (95% confidence interval) were 2.85 (1.85 – 4.39) and 2.00 (1.27 – 3.14), respectively. The relative risk for the 1st quartile vs. the 4th quartile for development of GA was 4.18 (1.80 – 9.68) in observed eyes, and 2.59 (1.13 – 5.95) in treated eyes. The relative risk for CNV incidence was 1.99 (1.12 – 3.54) in observed eyes and 1.33 (0.81 – 2.19) in treated eyes. These relationships were maintained after adjustment for baseline participant and ocular characteristics.
Conclusions
Complications of AMD Prevention Trial (CAPT) participants who perceived the most problems in their night vision at baseline had an increased risk of ≥3-lines loss in VA, CNV and GA. These associations are independent of established risk factors.
INTRODUCTION
Age-related macular degeneration (AMD) is the leading cause of vision loss among older adults in the United States.1 AMD can be characterized as a progressive regionalized degeneration of the photoreceptors in the macula. The dysfunction and death of photoreceptors, through an atrophic process or a neovascular event, accounts for vision loss associated with the advanced stages of AMD.2 Patients with early and intermediate AMD can have unimpaired visual acuity, but may report difficulty with activities performed at night and under low illumination (e.g. driving, reading at night).3–10 Impairment of night vision may be due to the slowing of rod-mediated dark adaptation in AMD resulting from the degeneration and loss of rod photoreceptors.11–13
Histopathological studies of human donor retinas with AMD have shown a predilection for parafoveal loss of rods over cones in the nonadvanced AMD. Although both rods and cones in the parafovea degenerated in early AMD, rod loss preceded and was more severe than cone loss in most of the donor retinas evaluated.14–17 Psychophysical functional studies also have demonstrated preferential vulnerability of rods over cones in early AMD. Photoreceptor degeneration and loss occurs before disease in the retinal pigment epithelium (RPE)/Bruch’s membrane complex progresses to late AMD.2, 18–21
In vivo and in vitro studies of photoreceptors suggest that a significant inter-dependence exists between rod and cone photoreceptors.2 Death of rod photoreceptors may contribute to the later degeneration of cones, possibly induced by either excitotoxicity or changes in the structural and biochemical microenvironment.2 Furthermore, rods are necessary for continued cone survival because rods produce a diffusible substance essential for cone survival.2, 22, 23 Thus, dysfunction of rod photoreceptors may serve as an indicator for impending cone dysfunction.16
Because of the body of evidence that rod dysfunction and resulting problems with night vision may indicate more advanced age-related maculopathy and higher risk of vision loss from progression to the late stage of the disease, we administered a 10-item questionnaire on night vision to participants enrolling in the Complications of AMD Prevention Trial (CAPT).24 CAPT was a multicenter clinical trial sponsored by the National Eye Institute to evaluate the efficacy and safety of low-intensity laser treatment in preventing loss of vision in people with bilateral large drusen. Participants were followed longitudinally, visual acuity was measured annually, and development of choroidal neovascularization (CNV) and geographic atrophy (GA) were monitored closely for at least five years. The CAPT found that light-intensity laser treatment did not reduce the risk of the development of CNV, GA, or loss of visual acuity.25 This paper seeks to assess whether baseline night vision symptoms predict subsequent vision loss and development of CNV and GA in CAPT participants.
METHODS
Details of the design and methods have been reported elsewhere,9, 24,25 only major features related to this paper are described here.
Participants were enrolled through 22 clinical centers. The institutional review board associated with each center approved the study protocol and written informed consent was obtained from each participant. Data management was compliant with Health Insurance Portability and Accountability Act guidelines. The conduct of the clinical trial adhered to the tenets of the Declaration of Helsinki. A total of 1,052 participants were enrolled between May 1999 and March 2001. Both eyes of the participants were enrolled in the CAPT; one eye of each participant was randomized to laser treatment with the contralateral eye assigned to observation. CAPT eligibility criteria specified that each eye have ≥ 10 large drusen (≥ 125 microns in diameter) and visual acuity ≥ 20/40. Neither eye was to have evidence of CNV, serous pigment epithelial detachment, GA within 500 microns of foveal center or total area >1 MPS disc area, or other ocular conditions that were likely to compromise visual acuity or contraindicate application of laser treatment.
During the initial visit, participants provided information on demographic characteristics, history of diabetes mellitus, history of cigarette smoking, current use of aspirin, and current use of anti-hypertensive medications. Blood pressure (BP) was measured one time while the participant was seated. During the initial visit and follow-up visits, visual acuity was measured following the procedures developed for the Early Treatment Diabetic Retinopathy Study (ETDRS) as adapted for the Age-Related Eye Disease Study (AREDS).26, 27 Modified ETDRS Charts 1 and 2 were used at a distance of 3.2 meters. Scoring of VA test was based on the number of letters read correctly. The range of possible scores was 0 to 95, corresponding to Snellen visual acuity equivalents of < 20/800 to 20/12.
At the initial visit and annually thereafter, certified photographers adhering to a standardized protocol for field definition and image sequencing took stereoscopic, color fundus photographs on film and a fluorescein angiogram on film, with frames from each eye. Color photographs were taken also at 6 months. All photographic images were graded independently by two trained readers in the CAPT Reading Center who later openly discussed their discrepancies to arrive at consensus. At baseline, the fundus features described in the grading included number of drusen, largest drusen size, percent of area covered by drusen, drusen confluence, focal hyperpigmentation and RPE depigmentation.
Readers in the CAPT Reading Center also evaluated the follow-up images for the presence of CNV and GA. Fluorescein angiograms were used to identify CNV, defined as expansion or persistent staining of an area of hyperfluorescence as the time from injection increased. GA was considered present when the color photographs showed an area of atrophy of the retinal pigment epithelium with a diameter of at least 250 μ with 2 of the following 3 features: visible choroidal vessels, sharp edges, and a more or less circular shape. “Endpoint GA” was defined as development of a total of >1 Macular Photocoagulation Study disc area of new, additional atrophy when all areas of GA within 3000 μ of the foveal center were combined. Evaluation of GA was not performed after an eye developed CNV because the neovascular complex and subsequent scarring often occupied or obscured the retinal area most likely to develop GA.
10-item Night Vision Questionnaire
CAPT participants completed the 25-item National Eye Institute Visual Functioning Questionnaire (NEI-VFQ-25) at the initial visit. Participants also completed 6 items concerning night vision based on a symptom list designed by Cynthia Owsley, Ph.D. and Samuel Jacobson, M.D, Ph.D. for patients with age-related macular degeneration. The 4 items concerning night vision from the NEI-VFQ-25 and the 6 items on night vision symptoms are referred to as the 10-item Night Vision Questionnaire (NVQ-10) (see Appendix 2, available at http://aaojournal.org). The first 4 items are on a 5-point scale from “None” to “Stopped doing because of my eyesight” and ask about the difficulty in seeing moving subjects, reading street signs when driving at night, difficulty in seeing street signs as a passenger in the car at night, and difficulty with the oncoming headlights or streetlights when driving at night. The next 6 items are on a 4-point scale from “Not at all” to “Very” and ask about how bothered the participant is by: poor vision at night, problem in reading in dim light, a dark spot in the middle of vision in dim light, poor vision in dim lighting, problems adjusting to the dark when entering a theater, and trouble seeing the stars in the sky at night. Each item is scored between 100 (none or not at all) and 0 (stop doing because of eyesight, or very bothered). An item cannot be scored if the participant answered with “not currently driving” or “Stopped doing this for other reasons or not interested in doing this”. An overall NVQ-10 score for each participant based on the average score of the items with a score (i.e., excluding items that cannot be scored) is expressed on a scale range from 0 to 100; lower score indicates worse night vision.
The questionnaires were self-administered during the initial visit. The local clinic coordinator reviewed the instructions with the participant and answered any questions that arose for participants self-administering the questionnaires. Upon completion, the clinic coordinator immediately reviewed the form to ensure that all questions were answered and the responses were legible. If any problems were identified, the clinic coordinator requested that the participant complete or revise missing or illegible responses.
Statistical Analysis
Hypertension was classified according to the blood pressure measured at initial visit and the reported use of anti-hypertensive medications. Definite hypertension was defined as systolic BP ≥ 160 mmHg, diastolic BP ≥ 95 mmHg, or current use of anti-hypertensive medications.
The distribution of night vision scores was summarized by mean, standard deviation (SD), median and range. For the primary analysis, due to the skewed distribution of night vision score (skewed towards the ceiling of the score with 42 (4.0%) participants scoring 100), we grouped the CAPT participants into four groups based on 4 quartiles of NVQ-10 score, the participants with NVQ-10 scores in the 1st quartile (lowest) have the worst night vision, while the participants with NVQ-10 scores in the 4th quartile (highest) have the best night vision. The prevalence of vision loss ≥ 3-lines at each follow-up visit, and cumulative incidence of CNV and GA over follow-up time were calculated and compared among these 4 groups of participants. The cumulative incidence of CNV over follow-up time was calculated using the Kaplan-Meier method,28 and the cumulative incidence estimates of GA were calculated using a competing risk model to accommodate the fact that eyes that developed CNV were no longer considered at risk of developing GA.29
Eyes with CNV identified by the Reading Center from review of baseline photographs (N=20) were excluded from the analysis of development of CNV. Eyes with CNV (N=20), serous pigment epithelial detachment (N=2), or any geographic atrophy (N=66) identified by the Reading Center from review of baseline photographs or no photographs allowing assessment of GA during follow-up (N=28), were excluded from the analysis of development of endpoint GA.
The association of night vision symptoms with risk of 3-lines loss in VA was evaluated by odds ratios (OR) from repeated logistic regression models. The association of night vision symptoms with risk of CNV and GA was evaluated by the relative risks (RR) from proportional hazard models. The group with NVQ-10 score in 4th quartile (with the best night vision) was used as the reference group in calculating OR and RR. These evaluations were performed without and with the adjustment of significant participant and ocular characteristics as determined from CAPT study.30 The above analysis was performed for observed eyes and treated eyes, considered separately and combined. For the analysis of the combined data from observed and treated eyes, assigned treatment was included as a covariate, and the correlation between paired eyes of participants was accommodated by using a robust estimator of variance.31 All the data analysis was performed in SAS 9.1. (SAS Institute, Inc., Cary, NC).
RESULTS
NVQ-10 score at baseline
At baseline, 1,051 of 1,052 CAPT participants completed NVQ-10. The distribution of NVQ-10 scores shows that many CAPT participants reported problems with their night vision (Figure 1). The mean (±SD) NVQ-10 score was 70 (±20), and the median was 73 (range: 3 –100). Forty-two (4.0%) participants reported no problems with night vision and attained the maximum NVQ-10 score of 100. The NVQ-10 score ranged from 3 to 57 (mean: 42.1) in the 1st quartile, 58 to 73 (mean: 66.8) in the 2nd quartile, 74 to 85 (mean: 79.8) in the 3rd quartile, and 86 to 100 in the 4th quartile (mean: 93.1) (Figure 1). The NVQ-10 items showed strong internal consistency and reliability with Cronbach’s α = 0.90.
Figure 1.
The distribution of night vision scores calculated from the 10-item night vision questionnaire (NVQ-10) administered at baseline. The scores were scaled from 0 to 100 with 100 indicating no night vision symptoms. The ranges of the four quartiles (Q1, Q2, Q3, and Q4) are indicated in the figure.
Association with VA
When participants were compared based on the quartiles of NVQ-10, the participants with the best night vision (in the 4th quartile of NVQ-10) had the lowest proportions of observed eyes with ≥ 3-lines loss in VA at every visit when visual acuity was measured (Figure 2). Participants with the worst night vision (in the 1st quartile) generally had the highest proportion of observed eyes with ≥ 3-lines loss, although the differences among the first three quartiles were not large (Figure 2). The association between loss in visual acuity and quartiles of night vision scores followed a similar pattern in treated eyes (data not shown). As compared with participants with the best night vision (in the 4th quartile), participants with worse night vision at baseline (either in the 1st, 2nd or 3rd quartile) had at least a two-fold increased risk of vision loss ≥ 3-lines in observed eyes. This significant association was maintained after adjustment by the other factors significantly associated with loss of visual acuity (age, current smoking status, hypertension, and focal hyperpigmentation) (Table 1). Weaker associations were seen in treated eyes, and in the combined set of observed and treated eyes (Table 1). Interaction between treatment assignment and quartiles of night vision score was not found (p=0.63)
Figure 2.
The proportion of observed eyes with ≥ 3-lines loss in visual acuity (VA) across follow-up time by quartiles of the night vision score from the 10-item night vision questionnaire (NVQ-10). The proportion of observed eyes with ≥ 3-lines loss in VA is significantly different among the 4 quartiles of night vision score (p<0.0001).
Table 1.
The Association of NVQ-10 Score at Baseline with Risk of ≥ 3-lines Loss in VA in Follow-up
| Observed Eyes
|
Treated Eyes
|
Combined § |
|
|---|---|---|---|
| NVQ-10 Quartile | OR† (95% C.I.) | OR† (95% C.I.) | OR† (95% C.I.) |
| Univariate Analysis | |||
| 1st (lowest) | 2.85 (1.85, 4.39) | 2.00 (1.27, 3.14) | 2.39 (1.69, 3.40) |
| 2nd | 2.54 (1.62, 3.97) | 2.04 (1.31, 3.17) | 2.27 (1.39, 3.24) |
| 3rd | 2.14 (1.39, 3.32) | 1.78 (1.13, 2.81) | 1.95 (1.36, 2.79) |
| 4th (highest) | 1.00 | 1.00 | 1.00 |
| Overall P-value | <0.0001 | 0.0002 | <0.0001 |
| Adjusted Analysis‡ | |||
| 1st (lowest) | 2.67 (1.69, 4.22) | 1.50 (0.94, 2.39) | 2.02 (1.41, 2.89) |
| 2nd | 2.48 (1.55, 3.95) | 1.75 (1.12, 2.74) | 2.08 (1.46, 2.97) |
| 3rd | 2.14 (1.36, 3.36) | 1.69 (1.08, 2.65) | 1.90 (1.33, 2.71) |
| 4th (highest) | 1.00 | 1.00 | 1.00 |
| Overall P-value | <0.0001 | 0.04 | <0.0001 |
NVQ-10: 10-item night vision questionnaire; VA: Visual Acuity; OR: Odds Ratio; 95% C.I.: 95% Confidence Interval.
Repeated measures logistic regression.
Adjusted by age, current smoking status, hypertension and focal hyperpigmentation.
Also adjusted by the assigned treatment.
Association with CNV
The proportion of participants developing CNV in their observed eye, regardless of length of follow-up, was lowest for the participants in the 4th quartile of night vision scores (least reported night vision problems; Table 2). These crude proportions and the Kaplan-Meier estimates of the cumulative proportion of developing CNV (Figure 3) for the other three quartiles did not differ consistently over time and did not exhibit a clear dose-response pattern. The relative risk for each of the three groups was approximately 2 and adjustment for the other risk factors for CNV in the CAPT participants (age, current smoking status, hypertension, and focal hyperpigmentation) resulted in only minor changes in the estimated relative risks (Table 2). In treated eyes, worse night vision (lower quartile number) was associated with slightly increased risk of CNV (Table 2). Interaction between treatment assignment and night vision score (4 categorical levels) was not found (p=0.34).
Table 2.
The Association of NVQ-10 Score at Baseline with Risk of CNV in Follow-up
| NVQ-10 Quartile | Observed eyes
|
Treated eyes
|
Combined§ |
|||
|---|---|---|---|---|---|---|
| n | CNV (%) | n | CNV (%) | n | CNV (%) | |
| 1st (lowest) | 267 | 35 (13.1) | 266 | 37 (13.9) | 533 | 72 (13.5) |
| 2nd | 267 | 45 (16.9) | 266 | 38 (14.3) | 533 | 83 (15.6) |
| 3rd | 261 | 43 (16.5) | 259 | 37 (14.3) | 520 | 80 (15.4) |
| 4th (highest) | 248 | 18 (7.26) | 248 | 28 (11.3) | 496 | 46 (9.27) |
|
| ||||||
| RR† (95% C.I.) | RR† (95% C.I.) | RR† (95% C.I.) | ||||
|
| ||||||
| Univariate Analysis | ||||||
| 1st (lowest) | 1.99 (1.12, 3.54) | 1.33 (0.81, 2.19) | 1.59 (1.05, 2.41) | |||
| 2nd | 2.50 (1.44, 4.34) | 1.34 (0.81, 2.19) | 1.79 (1.18, 2.71) | |||
| 3rd | 2.36 (1.36, 4.12) | 1.27 (0.77, 2.09) | 1.70 (1.13, 2.56) | |||
| 4th (highest) | 1.00 | 1.00 | 1.00 | |||
| Overall P-value | 0.008 | 0.64 | 0.03 | |||
| Adjusted Analysis‡ | ||||||
| 1st (lowest) | 1.92 (1.08, 3.44) | 1.07 (0.64, 1.78) | 1.41 (0.92, 2.16) | |||
| 2nd | 2.38 (1.36, 4.14) | 1.15 (0.69, 1.91) | 1.63 (1.06, 2.48) | |||
| 3rd | 2.29 (1.31, 4.00) | 1.22 (0.74, 2.01) | 1.64 (1.08, 2.49) | |||
| 4th (highest) | 1.00 | 1.00 | 1.00 | |||
| Overall P-value | 0.01 | 0.87 | 0.09 | |||
NVQ-10: 10-item night vision questionnaire; CNV: Choroidal Neovascularization; RR: Risk Ratio; 95% C.I.: 95% Confidence Interval.
Cox proportional hazards model.
Adjusted by age, current smoking status, hypertension, and focal hyperpigmentation.
Also adjusted by the assigned treatment.
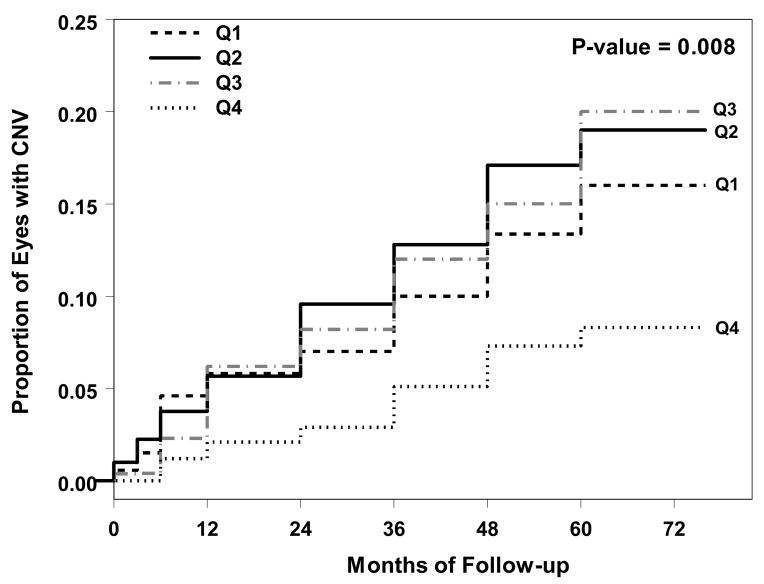
Figure 3.
Kaplan-Meier curves for the risk of Choroidal Neovascularization (CNV) in observed eyes by quartiles of night vision score from the 10-item night vision questionnaire (NVQ-10). The incidence of CNV is significantly different among 4 quartiles of night vision score (p=0.008).
Association with GA
The proportion of participants developing GA in their observed eye, regardless of length of follow-up, was lower for the participants in the 3rd and 4th quartile of night vision scores (least reported problems) than participants in the 1st and 2nd quartile (Table 3). The cumulative incidence estimate of GA from the competing risk model (Figure 4) also showed a large difference between quartiles 1 and 2 vs. quartiles 3 and 4. The unadjusted relative risk for each of the 1st and 2nd quartiles was 4.2 and 3.1, respectively. With adjustment for the other risk factors for GA in the CAPT participants (age, hypertension, larger area of drusen, focal hyperpigmentation, and RPE depigmentation), the estimated relative risks increased to 4.6 and 3.2, respectively. In treated eyes, there was a similar trend for incidence of GA in quartiles 1 and 2 and within quartiles 3 and 4 (Table 3). Interaction between treatment assignment and quartiles of night vision score was not found (p=0.52).
Table 3.
The Association of NVQ-10 Score at baseline with Risk of GA in Follow-up
| Observed eyes
|
Treated eyes
|
Combined§ |
||||
|---|---|---|---|---|---|---|
| NVQ-10 Quartile | n | GA (%) | n | GA (%) | n | GA (%) |
| 1st (lowest) | 247 | 26 (10.5) | 250 | 19 (7.60) | 497 | 45 (9.05) |
| 2nd | 250 | 20 (8.00) | 254 | 21 (8.27) | 504 | 41 (8.13) |
| 3rd | 251 | 8 (3.19) | 250 | 10 (4.00) | 501 | 18 (3.59) |
| 4th (highest) | 240 | 7 (2.92) | 244 | 8 (3.28) | 484 | 15 (3.10) |
|
| ||||||
| RR† (95% C.I.) | RR† (95% C.I.) | RR† (95% C.I.) | ||||
|
| ||||||
| Univariate Analysis | ||||||
| 1st (lowest) | 4.18 (1.80, 9.68) | 2.59 (1.13, 5.95) | 3.32 (1.69, 6.53) | |||
| 2nd | 3.10 (1.30, 7.37) | 2.72 (1.20, 6.18) | 2.90 (1.46, 5.76) | |||
| 3rd | 1.16 (0.42, 3.22) | 1.22 (0.48, 3.10) | 1.20 (0.55, 2.61) | |||
| 4th (highest) | 1.00 | 1.00 | 1.00 | |||
| Overall P-value | 0.0005 | 0.02 | 0.0002 | |||
| Adjusted Analysis‡ | ||||||
| 1st (lowest) | 4.60 (1.81, 11.6) | 2.44 (1.03, 5.77) | 3.42 (1.69, 6.96) | |||
| 2nd | 3.17 (1.23, 8.18) | 2.97 (1.27, 6.93) | 3.10 (1.50, 6.40) | |||
| 3rd | 1.16 (0.38, 3.53) | 1.33 (0.51, 3.45) | 1.22 (0.54, 2.79) | |||
| 4th (highest) | 1.00 | 1.00 | 1.00 | |||
| Overall P-value | 0.001 | 0.03 | 0.0008 | |||
NVQ-10: 10-item night vision questionnaire; GA: Geographic Atrophy; RR: Risk Ratio; 95% C.I.: 95% Confidence Interval.
Cox proportional hazards model.
Adjusted by age, hypertension, global area covered by drusen, focal hyperpigmentation, and retinal pigment epithelium depigmentation.
Also adjusted by the assigned treatment.
Figure 4.
Kaplan-Meier curves for the risk of Geographic Atrophy (GA) in observed eyes by quartiles of night vision score from the 10-item night vision questionnaire (NVQ-10). The incidence of GA is significantly different among 4 quartiles of night vision score (p=0.0005).
DISCUSSION
The data from CAPT show that many patients with multiple large drusen bilaterally and good visual acuity (≥ 20/40) have reported night vision symptoms, and more night vision symptoms are associated with increased risk of developing loss in visual acuity, CNV, and GA. Furthermore, the associations are independent of other risk factors including participant and ocular characteristics. These findings are consistent with the biological and psychophysical findings that rod photoreceptor degeneration precedes cone degeneration in early AMD,11, 15, 18, 19, 21, 32–34 and rod dysfunction may contribute to the later degeneration of cones because of their inter-dependence.2, 22, 23 The predictive value of night vision symptoms on late AMD development is in agreement with the findings from a study by Sunness et al on a small group of patients with drusen, in which the degree of loss of foveal dark-adapted sensitivity at baseline predicted the development of advanced AMD with 100% sensitivity and 92% specificity.35
Results from previous studies have established several risk factors for progression to CNV and GA.1 The risk factors identified within the CAPT data were consistent with previous findings for increased risk with the personal characteristics of advanced age, current cigarette smoking, hypertension and the ocular characteristics of drusen area, focal hyperpigmentation and RPE depigmentation.30 The results of the analyses presented in this paper support night vision symptoms as a novel risk factor of vision loss and development of CNV and GA. It is interesting to note that the association of CNV and GA with night vision symptoms seems different. As shown in Figure 3, the risk of CNV in the 4th quartile is much lower than that from the first 3 quartiles, and the risk of CNV in the first three quartiles does not show dose-response pattern; while the risk of GA in the 3rd and 4th quartile is similar, which is much lower than that in the 1st and 2nd quartiles (Figure 4). These results imply that the CNV and GA may arise from two different disease physiological processes.
The assessment of night vision symptoms provides additional valuable predictive information, as it is independent of the effects of established ocular and other participant risk factors. During the period that CAPT was being carried out, Owsley et al developed the 32-item Low-Luminance Questionnaire (LLQ) to characterize the vision problems in low luminance and found that the LLQ scores were related to rod-mediated dark adaptation parameters but not to cone-mediated parameters. 10 Because of the ease of ascertainment compared to testing dark adaptation or rod sensitivity, assessing night vision symptoms may be useful in identifying patients with early or intermediate AMD who are at relatively high risk of progression. Several agents are currently under evaluation in clinical trials as treatments to prevent the development or progression of GA. Including only patients with night vision symptoms, and therefore higher risk of progression and loss of vision, would be one way to decrease the risk-benefit ratio in these clinical trials as well as to decrease the total sample size or follow-up period required to attain a specific amount of statistical power.
Supplementary Material
This article contains online-only material. The following should appear online-only: Appendix 1 and Appendix 2.
Acknowledgments
Supported by grants EY012211, EY012261, and EY012279, from the National Eye Institute, National Institutes of Health, Department of Health and Human Services.
Footnotes
The Writing Committee has no conflict of interest with regard to the material presented in the article.
Presented in part at the meetings of the Association for Research and Vision in Ophthalmology in Fort Lauderdale, Florida in May 1, 2005; the 4th US Symposium on Ocular Epidemiology, January 31, 2007.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age-related macular degeneration. Am J Ophthalmol. 2004;137:486–95. doi: 10.1016/j.ajo.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 2.Mohand-Said S, Hicks D, Leveillard T, et al. Rod-cone interactions: developmental and clinical significance. Prog Retin Eye Res. 2001;20:451–67. doi: 10.1016/s1350-9462(01)00006-4. [DOI] [PubMed] [Google Scholar]
- 3.Kosnik W, Winslow L, Kline D, et al. Visual changes in daily life throughout adulthood. J Gerontol. 1988;43:P63–70. doi: 10.1093/geronj/43.3.p63. [DOI] [PubMed] [Google Scholar]
- 4.Kuyk T, Elliott JL. Visual factors and mobility in persons with age-related macular degeneration. J Rehabil Res Dev. 1999;36:303–12. [PubMed] [Google Scholar]
- 5.Mangione CM, Gutierrez PR, Lowe G, et al. Influence of age-related maculopathy on visual functioning and health-related quality of life. Am J Ophthalmol. 1999;128:45–53. doi: 10.1016/s0002-9394(99)00169-5. [DOI] [PubMed] [Google Scholar]
- 6.Mangione CM, Lee PP, Gutierrez PR, et al. National Eye Institute Visual Function Questionnaire Field Test Investigators. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119:1050–8. doi: 10.1001/archopht.119.7.1050. [DOI] [PubMed] [Google Scholar]
- 7.Scilley K, Jackson GR, Cideciyan AV, et al. Early age-related maculopathy and self-reported visual difficulty in daily life. Ophthalmology. 2002;109:1235–42. doi: 10.1016/s0161-6420(02)01060-6. [DOI] [PubMed] [Google Scholar]
- 8.Clemons TE, Chew EY, Bressler SB, McBee W AREDS Research Group. National Eye Institute Visual Function Questionnaire in the Age-Related Eye Disease Study (AREDS): AREDS report no. 10. Arch Ophthalmol. 2003;121:211–7. doi: 10.1001/archopht.121.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Complications of Age-Related Macular Degeneration Prevention Trial Research Group. Baseline characteristics, the 25-item National Eye Institute Visual Functioning Questionnaire, and their associations in the Complications of Age-Related Macular Degeneration Prevention Trial (CAPT) Ophthalmology. 2004;111:1307–16. doi: 10.1016/j.ophtha.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 10.Owsley C, McGwin G, Jr, Scilley K, Kallies K. Development of a questionnaire to assess vision problems under low luminance in age-related maculopathy. Invest Ophthalmol Vis Sci. 2006;47:528–35. doi: 10.1167/iovs.05-1222. [DOI] [PubMed] [Google Scholar]
- 11.Owsley C, Jackson GR, White MF, et al. Delays in rod-mediated dark adaptation in early age-related maculopathy. Ophthalmology. 2001;108:1196–202. doi: 10.1016/s0161-6420(01)00580-2. [DOI] [PubMed] [Google Scholar]
- 12.Feigl B, Brown B, Lovie-Kitchin J, Swann P. Cone- and rod-mediated multifocal electroretinogram in early age-related maculopathy. Eye. 2005;19:431–41. doi: 10.1038/sj.eye.6701503. [DOI] [PubMed] [Google Scholar]
- 13.Dimitrov PN, Guymer RH, Zele AJ, et al. Measuring rod and cone dynamics in age-related maculopathy. Invest Ophthalmol Vis Sci. 2008;49:55–65. doi: 10.1167/iovs.06-1048. [DOI] [PubMed] [Google Scholar]
- 14.Curcio CA, Millican CL, Allen KA, Kalina RE. Aging of the human photoreceptor mosaic: evidence for selective vulnerability of rods in central retina. Invest Ophthalmol Vis Sci. 1993;34:3278–96. [PubMed] [Google Scholar]
- 15.Curcio CA, Medeiros NE, Millican CL. Photoreceptor loss in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1996;37:1236–49. [PubMed] [Google Scholar]
- 16.Curcio CA, Owsley C, Jackson GR. Spare the rods, save the cones in aging and age-related maculopathy. Invest Ophthalmol Vis Sci. 2000;41:2015–8. [PubMed] [Google Scholar]
- 17.Curcio CA. Photoreceptor topography in ageing and age-related maculopathy. Eye. 2001;15:376–83. doi: 10.1038/eye.2001.140. [DOI] [PubMed] [Google Scholar]
- 18.Steinmetz RL, Haimovici R, Jubb C, et al. Symptomatic abnormalities of dark adaptation in patients with age-related Bruch’s membrane change. Br J Ophthalmol. 1993;77:549–54. doi: 10.1136/bjo.77.9.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Owsley C, Jackson GR, Cideciyan AV, et al. Psychophysical evidence for rod vulnerability in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2000;41:267–73. [PubMed] [Google Scholar]
- 20.Jackson GR, Owsley C, Curcio CA. Photoreceptor degeneration and dysfunction in aging and age-related maculopathy. Ageing Res Rev. 2002;1:381–96. doi: 10.1016/s1568-1637(02)00007-7. [DOI] [PubMed] [Google Scholar]
- 21.Chen C, Wu L, Wu D, et al. The local cone and rod system function in early age-related macular degeneration. Doc Ophthalmol. 2004;109:1–8. doi: 10.1007/s10633-004-1041-0. [DOI] [PubMed] [Google Scholar]
- 22.Mohand-Said S, Deudon-Combe A, Hicks D, et al. Normal retina releases a diffusible factor stimulating cone survival in the retinal degeneration mouse. Proc Natl Acad Sci U S A. 1998;95:8357–62. doi: 10.1073/pnas.95.14.8357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hicks D, Sahel J. The implications of rod-dependent cone survival for basic and clinical research. Invest Ophthalmol Vis Sci. 1999;40:3071–4. [PubMed] [Google Scholar]
- 24.Complications of Age-Related Macular Degeneration Prevention Trial Research Group. The Complications of Age-Related Macular Degeneration Prevention Trial (CAPT): rationale, design and methodology. Clin Trials. 2004;1:91–107. doi: 10.1191/1740774504cn007xx. [DOI] [PubMed] [Google Scholar]
- 25.Complications of Age-Related Macular Degeneration Prevention Trial Research Group. Laser treatment in patients with bilateral large drusen: the Complications of Age-Related Macular Degeneration Prevention Trial. Ophthalmology. 2006;113:1974–86. doi: 10.1016/j.ophtha.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 26.Early Treatment Diabetic Retinopathy Study design and baseline patient characteristics: ETDRS report number 7. Ophthalmology. 1991;98(suppl):741–56. doi: 10.1016/s0161-6420(13)38009-9. [DOI] [PubMed] [Google Scholar]
- 27.Age-Related Eye Disease Study. Manual of Operations (MOP) [Accessed October 29, 2007];Examination procedures. Available at https://web.emmes.com/study/areds/mopfiles/chp7_mop.pdf.
- 28.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 29.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 30.Complications of Age-related Macular Degeneration Prevention Trial (CAPT) Research Group. Risk factors for choroidal neovascularization and geographic atrophy: Complications of Age-related Macular Degeneration Prevention Trial. Ophthalmology. doi: 10.1016/j.ophtha.2008.03.008. In press. [DOI] [PubMed] [Google Scholar]
- 31.Wei LJ, Lin DY, Weissfeld L. Regression analysis of multivariate incomplete failure time data by modeling marginal distributions. J Am Stat Assoc. 1989;84:1065–73. [Google Scholar]
- 32.Medeiros NE, Curcio CA. Preservation of ganglion cell layer neurons in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2001;42:795–803. [PubMed] [Google Scholar]
- 33.Jackson GR, Curcio CA, Sloan KR, Owsley C. Photoreceptor degeneration in aging and age-related maculopathy. In: Penfold PL, Provis JM, editors. Macular Degeneration. New York: Springer; 2004. pp. 45–62. [Google Scholar]
- 34.Haimovici R, Owens SL, Fitzke FW, Bird AC. Dark adaptation in age-related macular degeneration: relationship to the fellow eye. Graefes Arch Clin Exp Ophthalmol. 2002;240:90–5. doi: 10.1007/s00417-001-0417-z. [DOI] [PubMed] [Google Scholar]
- 35.Sunness JS, Massof RW, Johnson MA, et al. Diminished foveal sensitivity may predict the development of advanced age-related macular degeneration. Ophthalmology. 1989;96:375–81. doi: 10.1016/s0161-6420(89)32883-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article contains online-only material. The following should appear online-only: Appendix 1 and Appendix 2.