Abstract
Previous brain imaging studies have reported that Major Depressive Disorder (MDD) is characterized by decreased volumes of several cortical and subcortical structures, including the hippocampus, amygdala, anterior cingulate cortex, and caudate nucleus. The purpose of the present study was to identify structural volumetric differences between MDD and healthy participants using a method that allows a comparison of gray and white matter volume across the whole brain. In addition, we explored the relation between symptom severity and brain regions with decreased volumes in MDD participants. Twenty-two women diagnosed with MDD and 25 healthy women with no history of major psychiatric disorders participated in this study. Magnetic resonance brain images were analyzed using optimized voxel-based morphometry to examine group differences in regional gray and white matter volume. Compared with healthy controls, MDD participants were found to have decreased gray matter volume in the bilateral caudate nucleus and the thalamus. No group differences were found for white matter volume, nor were there significant correlations between gray matter volumes and symptom severity within the MDD group. The present results suggest that smaller volume of caudate nucleus may be related to the pathophysiology of MDD and may account for abnormalities of the cortico-striatal-pallido-thalamic loop in MDD.
Keywords: Major Depressive Disorder (MDD), magnetic resonance imaging (MRI), voxel-based morphometry (VBM), caudate, depression, brain
1. Introduction
Major Depressive Disorder (MDD) is among the most prevalent of all psychiatric disorders. It is estimated that between 8 and 18 percent of the general population will experience at least one clinically significant episode of depression during their lifetime (Kessler et al., 2003). Major depression can be both chronic and recurrent. Kessler (2002), for example, has estimated that between one-half and two-thirds of people who have ever been clinically depressed will be in an episode in any given year over the remainder of their lives (Kessler, 2002). This high chronicity and recurrence of depression, combined with its significant prevalence, personal loss, and societal costs, makes it imperative that we identify and elucidate factors that are involved in the onset and maintenance of, and recovery from, MDD.
Over the past decade investigators working to specify neurobiological aspects of depression have assessed differences between depressed and nondepressed individuals in the volume of specific neural structures. Recently, researchers have examined depression-associated anomalies in the volume of several structures that comprise the affective division of the cortico-striatal-pallidal-thalamic (CSPT) loop (Alexander et al., 1986; Botteron et al., 2002; Bremner et al., 2000; Pillay et al., 1998; Sheline et al., 1998). This circuit is thought to mediate reward-seeking behaviors, as well as anticipation and evaluation of rewarding stimuli (e.g., Gold, 2003; Kawagoe et al., 1998). Given that anhedonia is a cardinal symptom in depression, it is not surprising that the affective division of the CSPT loop has been the focus of considerable research. In this context, investigators have found that, compared with their nondepressed counterparts, depressed individuals have smaller volumes in the hippocampus (e.g., Sheline, 2000; Videbech and Ravnkilde, 2004), amygdala (e.g., Sheline et al., 1998), and subgenual anterior cingulate cotrex (ACC; e.g., Caetano et al., 2006), all of which provide input to the CSPT circuit. The striatum (composed of the caudate and putamen), which receives input from these cortical and allocortical inputs, has also been found to have decreased volume in depression (e.g., Krishnan et al., 1992; Parashos et al., 1998). The globus pallidus and thalamus, which complete the CSPT circuit, have not been the focus of as much empirical work, and there are not reported volumetric decreases in these structures associated with depression.
It is important to note that the magnetic resonance imaging (MRI) volumetric studies described above have used a region-of-interest (ROI) technique, in which investigators trace the brain region under study and then calculate its volume in depressed and nondepressed participants. Given the effort involved in manual tracing techniques, assessing the volume of more than one or two structures is prohibitive; indeed, very few volumetric studies have examined more than two structures in depressed participants. Further, no study has assessed whether all of the structures comprising the CSPT loop show volumetric decreases within a given sample of depressed individuals. In order to conduct a thorough assessment of the integrity of the CSPT loop in depression, in the present study we utilized voxel-based morphometry (VBM), a systematic imaging analysis tool for evaluating gray and white matter density and volume across multiple ROIs (Ashburner and Friston, 2000). Unlike conventional ROI approaches, VBM can detect tissue density changes in small or anatomically ill-defined brain regions (Ashburner and Friston, 2000). In fact, other investigators using VBM have now reported volumetric decreases associated with depression in the hippocampus (Bell-McGinty et al., 2002; Shah et al., 1998), superior frontal gyrus (Taki et al., 2005), middle frontal gyrus (Bell-McGinty et al., 2002), and superior and inferior temporal gyri (Shah et al., 1998).
One way to further improve the use of VBM to examine brain abnormalities in depression is to take into account differences between healthy and psychiatric samples in structural morphology during the brain image registration process, which would decrease the risk of biased estimates (Ashburner and Friston, 2001; Bookstein, 2001). Indeed, imperfect registration of the brain images by affine and low-order registration algorithms used in conventional VBM might be falsely aliased as volumetric group differences in gray or white matter (Ashburner and Friston, 2001; Bookstein, 2001; Mechelli et al., 2005). To overcome this and other issues with the use of conventional VBM, an optimized version of VBM has recently been developed (Good et al., 2001). Optimized VBM registers gray and white matter separately to a study-specific template rather than registering the whole brain to a common template during the normalization process, thereby helping to ensure the quality of image registration.
The purpose of the present study was to examine volumetric differences between currently depressed and never-depressed women in the structures comprising the affective division of the CSPT loop using an unbiased estimation technique that provides adequate control of family-wise Type-I error. We used optimized VBM to compare gray matter volume of depressed and never-depressed women within the hippocampus, amygdala, subgenual ACC, caudate, putamen, globus pallidus, and thalamus. We predicted that depressed women would have significantly lower gray matter volume in these structures than would their never-depressed counterparts. We hypothesized further that, within the group of MDD participants, symptom severity would be inversely correlated with gray matter volume in these structures.
2. Methods
2.1. Participants
Participants were recruited from two outpatient psychiatry clinics in a university teaching hospital and through advertisements posted in numerous locations within the local community. A phone screen established that participants were fluent in English and were between 18 and 60 years of age. Participants were excluded for severe head trauma, learning disabilities, current panic disorder, current social phobia, psychotic symptoms, bipolar disorder, and alcohol or substance abuse within the past six months. Eligible individuals were invited to come to the laboratory, where trained interviewers administered the Structured Clinical Interview for the DSM-IV (SCID; First et al., 1997). This interview schedule assesses Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV; American Psychiatric Association, 1994) current and lifetime diagnoses for anxiety, mood, psychotic, alcohol and substance use, somatoform, and eating disorders. The SCID has demonstrated good reliability for the majority of the disorders covered in the interview (Skre et al., 1991). The SCID interviewers achieved excellent inter-rater reliability for a diagnosis of MDD (κ=0.93) and for the “nonpsychiatric control” diagnosis (i.e., the absence of current or lifetime psychiatric diagnoses, according to the DSM-IV criteria; (Gotlib et al., 2004). Participants also completed the Beck Depression Inventory-II (BDI; (Beck et al., 1996), a 21-item, self-report measure of the severity of depressive symptoms. The high reliability and validity of the BDI has been well documented (Beck et al., 1988). Handedness was determined with the Edinburgh Handedness Inventory.
Participants were included in the depressed group if they met DSM-IV criteria for MDD, and in the never-disordered control group if they had no current DSM-IV diagnosis and no history of any Axis I disorder. Twenty-two women who met criteria for MDD (mean age: 38.5 years; range 21-55) and 25 never-disordered control women (mean age: 35.3 years; range 23-56) participated in this study.
2.2. Image Acquisition
All structural brain images were acquired using a 1.5 Tesla GE magnetic resonance imaging (MRI) scanner (GE Medical Systems Signa, Milwaukee, WI). A T1-weighted three-dimensional spoiled gradient echo pulse sequence was used to acquire 116 1.5 mm-thick contiguous sagittal images (TR=100 ms, TE=7 ms, flip angle=90°, matrix=256×256, field of view=22 cm, in plane resolution=0.859375×0.859375 mm).
Optimized Voxel-Based Morphometry
VBM analyses were performed with Statistical Parametric Mapping 2 (SPM2) software (The Wellcome Institute of Imaging Neuroscience, London, UK). An optimized version of VBM, originally introduced by Good et al. (2001), was used to assess differences between depressed and control participants in gray and white matter volume. First, brain images of all 47 participants were processed to create a study-specific template image. Using a study-specific template image rather than a standard template image helps mitigate error associated with the normalization process. All 47 of these brain images were segmented into gray and white matter, and normalization parameters were estimated by matching gray matter images to a common gray matter template. The normalization parameters rendered were then applied in warping the original whole brain images. These images were then averaged and smoothed with a Gaussian kernel (full-width at half maximum = 8 mm). All brain images were used to create the template in order to prevent sample bias in the normalization process.
Next, the original whole brain images were segmented into gray and white matter images. Unconnected non-brain voxels (i.e., voxels not containing gray matter, white matter, or cerebrospinal fluid) were then extracted from these segmented images (Good et al., 2001). Because VBM can alias image distortions, brought about by such extra-experimental factors as excessive motion, as changes in white or grey matter (Mechelli et al., 2005), group differences in these extra-experimental factors can result in the detection of spurious group differences in structure volume or density. Consequently, we screened the structural MR images for the presence of artifacts and eliminated any images containing noticeable artifacts from the study.
Extracted and segmented gray and white matter images were then renormalized, preventing any contribution of non-brain voxels to the normalization process and affording optimal spatial normalization of gray and white matter images. Then, optimized normalization parameters derived from the previous step were reapplied to all 47 original whole brain images. They were normalized to the template using a 12 parameter affine transformation and nonlinear warping using 7 × 8 × 7 basis functions to a voxel size of 2 × 2 × 2 mm. These normalized images were then segmented into gray matter, white matter, and cerebrospinal fluid. An additional processing step, using the Jacobian determinants derived from spatial normalization, was applied to all gray and white matter images in order to acquire volume information from the voxels. All images were smoothed using a Gaussian kernel (full-width-half maximum = 12 mm) to increase the signal-to-noise ratio.
2.3. Statistical Analysis
Voxel-wise comparisons were conducted to assess differences between depressed and control participants in both gray- and white-matter volume in structures comprising the CSPT loop. Anatomical ROI masks for the hippocampus, amygdala, subgenual ACC, caudate, putamen, globus pallidus, and thalamus were selected from the Automated Anatomical Labeling system and applied to the data (Tzourio-Mazoyer et al., 2002). An analysis of covariance model was used to examine group differences in gray and white matter with age as a covariate. In addition, voxel-wise correlational analyses were conducted on these images with BDI scores within the MDD group. A family-wise error (FWE) corrected significance level of p < 0.05 was adopted to correct for multiple comparisons across the set of voxels comprising the ROIs.
3. Results
3.1. Demographic and Clinical Characteristics
Demographic and clinical characteristics of the MDD and never-disordered participants are presented in Table 1. There were no significant differences between the two groups with respect to age or years of education (age: t(45) = 1.03, p = 0.31; years of education: t(45) = 0.71, p = 0.48). As expected, the MDD participants had significantly higher BDI scores than did the never-disordered controls, t(45) = 5.91, p < 0.001. Ten of the depressed participants were stabilized on antidepressant medication at the time of assessment.
Table 1.
Demographic and clinical characteristics of the participants
| MDD participants
(n=22) |
Never-disordered participants
(n=25) |
|||
|---|---|---|---|---|
| mean (n) | SD (%) | mean (n) | SD (%) | |
| Demographic variables | ||||
| Age (years) | 38.5 | 9.70 | 35.3 | 11.25 |
| Education (years) | 16.5 | 2.13 | 15.7 | 4.24 |
| Handedness (right) | 19 | 86.36 | 21 | 84 |
| Clinical variables | ||||
| Years since first depressive episode | 17.4 | 10.01 | - | - |
| Currently on psychotropic medication | 10 | 45.45 | - | - |
| Number of depressive episodes | 6.5 | 3.33 | - | - |
| BDI * | 22.3 | 13.61 | 3.5 | 7.74 |
Handedness data were not available for 3 MDD participants;
p < 0.001; MDD = Major Depressive Disorder; BDI = Beck Depression Inventory-II
3.2. VBM Analyses
Compared with the never-disordered controls, the MDD participants had lower gray matter volume in the bilateral caudate body extending into the anterior nucleus of the thalamus (see Figure 1 and Table 2). The extent of these regions was larger in the left than in the right hemisphere. No gray matter volume reductions were found in the controls relative to the MDD participants, nor were there group differences in white matter volume. Finally, no group differences were obtained within the amygdala, hippocampus, subgenual ACC, globus pallidus, and putamen. Voxel-wise correlational analyses assessing relations between volume estimates within the a priori ROIs and BDI scores did not yield significant results.
Figure 1.
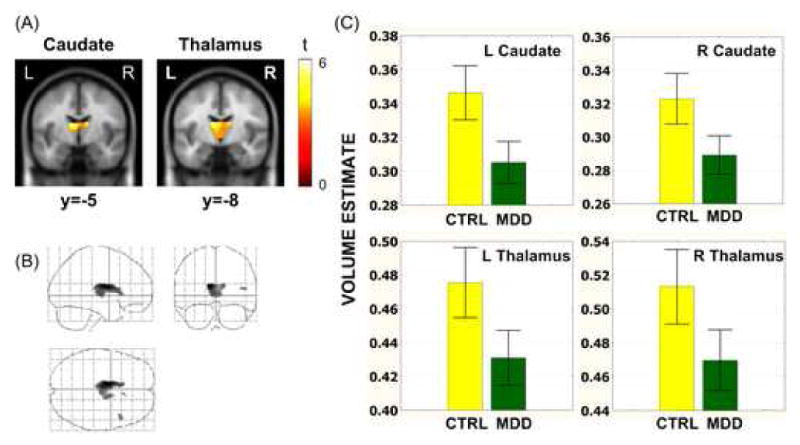
A. Coronal sections showing suprathreshold voxels in comparison of VBM gray matter volume estimates in MDD and CTRL participants. Caudate and thalamus showed significant (p < 0.05, FWE corrected for each search volume) volumetric reduction in MDD participants. B. Group differences across the whole brain showing the results in sagittal, coronal, and axial views. An uncorrected p < 0.001 was used here to show that the group differences were localized in the caudate and thalamus regions even at a relatively liberal threshold. C. Average VBM volume coefficients (+/- 95% SE) of MDD and CTRL participants in suprathreshold voxels in caudate and thalamus.
Notes: MDD = Major Depressive Disorder; CTRL = Never-disordered control; SE = Standard Error; FWE = Family-Wise Error; VBM = Voxel-Based Morphometry.
Table 2.
Regions with significantly smaller gray matter volumes in MDD than in never-disordered participants (p < 0.05, FWE corrected for each search volume)
| Region | MNI coordinates | Number of contiguous voxels | t(45) | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Left Caudate | -10 | -5 | +16 | 1318 | 5.90 |
| Left Thalamus | -10 | -8 | +15 | 3563 | 5.55 |
| Right Caudate | +13 | -5 | +16 | 386 | 4.11 |
| Right Thalamus | +1 | -15 | +4 | 2602 | 4.03 |
MDD = Major Depressive Disorder; FWE = family-wise error; MNI = Montreal Neurological Institute
4. Discussion
In this study we used VBM to conduct voxel-by-voxel volumetric comparisons of structures comprising the CSPT loop in women diagnosed with MDD and a sample of never-disordered control women. We found that, compared to their never-disordered counterparts, women diagnosed with MDD were characterized, most prominently, by significantly smaller volumes in the medial aspect of the bilateral caudate body and a small contiguous portion of the anterior nucleus of the thalamus. These volumetric differences were identified using a technique that is not susceptible to the biases to which both conventional voxel-based morphometric and structural tracing techniques are subject. While it is possible that the automated segmentation process used by VBM techniques does not distinguish tissue types as well as manual segmentation might, it is unlikely that this affected our interpretation of the depression-associated difference in caudate volume found in this study given that automated segmentation of the easily-defined caudate is not as prone to bias as it is in other, less easily defined, structures.
The finding of reduced caudate volume in this sample of currently depressed women is consistent with results from previous studies using structural tracing techniques that reported decreased caudate volume in major depression (Krishnan et al., 1993; Krishnan et al., 1992; Parashos et al., 1998). It is important to note, however, that other studies have not found smaller caudate volume in depressed patients than in nondepressed controls (Greenwald et al., 1997; Lacerda et al., 2003; Pillay et al., 1998). There do not appear to be systematic differences between studies that found caudate volume differences in depression and studies that did not with respect to dimensions relevant to brain structure volume (e.g., age, size, and gender composition of samples, depression severity). The fact that all investigations had relatively small samples (∼25 participants per group) leaves open the possibility that the discrepant findings are a function of variation in population sampling. Our finding of decreased volume in the anterior nucleus of the thalamus in depression is the first report of a difference between depressed and nondepressed participants in thalamus volume obtained using an MRI-derived technique. This both confirms and adds specificity to the findings of decreased total thalamus volume in depression reported by Bielau et al. (2005) using postmortem tissue samples from depressed individuals (Bielau et al., 2005). Indeed, given recent reports linking the anterior portion of the thalamus to the experience of affect (e.g., Young et al., 2000; Zubieta et al., 2001), the localization of thalamic abnormality in the present study to the anterior nucleus of this structure in depression contributes to emerging neural models of depression that highlight the nature of the relation between dorsal and limbic structures (Gotlib and Hamilton, in press).
The finding of a greater spatial extent of left-sided than of right-sided caudate and thalamus volumetric reduction in depression warrants comment. One explanation for this asymmetry involves the greater availability dopamine, a neurotransmitter shown to have neuroprotective effects, in the right than in the left caudate (Bobrov et al., 2006; Shih et al., 2007; Vernaleken et al., 2007). It is possible that the increased dopamine in the right caudate reduces the vulnerability of this structure to the stress-induced neurotoxic damage associated with depression. It would be useful in future research to examine this possibility more explicitly.
We did not replicate findings from previous volume tracing studies that have reported volumetric decreases in the amygdala, hippocampus, and subgenual ACC in depression. Meta-analyses of volume tracing studies of the hippocampus and amygdala in depression provide a potential explanation for these discrepancies. These meta-analyses show that volumetric decreases in the hippocampus (Videbech and Ravnkilde, 2004) and amygdala (Hamilton et al., 2007) correlate with total duration of depressive illness, such that decreased volume appears to be a function of increased number of depressive episodes. It is possible that the 6.5 episodes of depression, on average, experienced by our sample were insufficient to detect changes in amygdala or hippocampus volume. Consistent with this formulation, we found in a post-hoc analysis that the right hippocampus volume (peak voxel: 21, -31, -9; p < 0.05, FWE corrected) was significantly smaller in the depressed individuals in our sample who had experienced 10 or more episodes of depression (n = 9) than it was in those depressed participants who had experienced four or fewer episodes (n = 8), in the absence of any demographic differences between these two subsamples. This finding underscores the importance of carefully assessing history of depression in studies examining structural volume in this disorder.
It is not yet clear whether volume reduction in the caudate plays a causal role in depression or is simply a neural correlate of the disorder. The formulation that caudate pathology can influence the onset of depression is consistent with the results of investigations indicating that damage to the caudate nucleus elicits depressive symptoms beyond those associated with brain damage more generally. For example, damage to the caudate resulting from calcification (Gluck-Vanlaer et al., 1996), gliosis (Bhatia et al., 1993), and cerebrovascular disease (Mendez et al., 1989), in the absence of other neural anomalies, has been found to result in symptoms of depression. Further, other findings suggest that smaller caudate volume in depression is mediated by a specific genotype. Hickie et al. (2007), for example, recently reported that within a sample of depressed participants, the short allele polymorphism of the serotonin transporter gene was associated with smaller caudate volumes, but not with differential amygdala or hippocampal volumes.
Structural and functional abnormalities in the caudate are also apparent in other disorders that are characterized by anomalies in mood. For example, both schizophrenia (e.g., Levitt et al., 2004) and bipolar disorder (e.g., Blumberg et al., 2000) have been found to be associated with atypical caudate structure and/or function. Volumetric studies of the caudate in schizophrenia have yielded mixed results, with some studies reporting smaller caudate and thalamus volumes in schizophrenia (Gaser et al., 2004; Jayakumar et al., 2005; Staal et al., 1998), and others showing larger caudate volume associated with this disorder (Hokama et al., 1995; Hulshoff Pol et al., 2001). Importantly, bipolar disorder has more consistently been found to be associated with increased caudate volume (Aylward et al., 1994; Strakowski et al., 1999).
It is also noteworthy that in Huntington's and Parkinson's Diseases, both of which have been found to be associated with caudate abnormality (e.g., Almeida et al., 2003; Aylward et al., 2004), diagnoses of comorbid unipolar depression are common. Patients with Huntington's Disease, which is associated with cell death in the dorsomedial striatum (Lawrence et al., 1998) (the same part of the striatum that was found to be affected in the present depressed sample), often present with a clinically significant episode of depression that precedes the uncontrollable and repetitive choreoform movements that characterize the disorder (Folstein et al., 1979; Mendez et al., 1989). Similarly, individuals with Parkinson's Disease, another disorder that is associated with striatal pathology, also often report debilitating depression prior to onset of more characteristic motor and cognitive symptoms (Ayd, 1995; Mindham, 1970).
It is important to consider limitations of this study and alternative explanations for the obtained results. It is possible, for example, that the depression-associated decrease in caudate volume found in the present analysis is due, in part, to medication effects, given that several of the depressed participants in this study were taking antidepressant medication. In this context, it is important to note that while antidepressants have been shown to influence metabolism in the caudate (Henry et al., 2003), they have not been found to affect caudate volume. In fact, in structures in which antidepressant medication has been shown to affect volume (Banasr et al., 2006; Malberg et al., 2000), a volumetric increase, rather than a decrease, has been reported. We should also note that only female participants were included in the present study, which limits the generalizability of our findings. Nevertheless, if the decrease in caudate volume in depression is due to hypercortisolemic damage, including males in the study would likely yield similar results given that males are, if anything, more susceptible than females to the neurodegenerative effects of hypercortisolemia (Liu et al., 2006; Wagner et al., 2004).
While the present voxel-based morphometric study does point, along with previous volumetric studies of depression, to structural anomalies associated with this disorder, additional work is required to map connections between the structural pathology noted here and depressive symptomatology. Although studies documenting the onset of depression following damage to the caudate (Bhatia et al., 1993; Gluck-Vanlaer et al., 1996) help to establish the sufficiency of caudate damage for depression, it is still unclear exactly what role the caudate nucleus plays within the CSPT loop such that damage to this structure results in depression. A small literature indicating that lesions of the globus pallidus are associated with depression (Lauterbach et al., 1997a, 1997b) supports the formulation that compromising the affective CSPT loop more generally can lead to depressive symptomatology. Nevertheless, additional and more systematic work, likely using animal models, is necessary to elucidate the precise roles of the components of the CSPT loop in contributing to the development and maintenance of major depressive disorder.
Acknowledgments
This research was supported by grant MH59259 from the National Institute of Mental Health awarded to Ian H. Gotlib. We thank Lauren Atlas for her help with analyses. M. Justin Kim is now at the Department of Psychological and Brain Sciences, Dartmouth College.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Almeida OP, Burton EJ, McKeith I, Gholkar A, Burn D, O'Brien JT. MRI study of caudate nucleus volume in Parkinson's disease with and without dementia with Lewy bodies and Alzheimer's disease. Dementia and Geriatric Cognitive Disorders. 2003;16:57–63. doi: 10.1159/000070676. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Why voxel-based morphometry should be used. Neuroimage. 2001;14:1238–1243. doi: 10.1006/nimg.2001.0961. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. American Psychiatric Press; Washington DC: 1994. [Google Scholar]
- Ayd FJ. Lexicon of Psychiatry, Neurology, and the Neurosciences. Williams & Wilkins; Baltimore: 1995. [Google Scholar]
- Aylward EH, Roberts-Twillie JV, Barta PE, Kumar AJ, Harris GJ, Geer M, Peyser CE, Pearlson GD. Basal ganglia volumes and white matter hyperintensities in patients with bipolar disorder. Am J Psychiatry. 1994;151:687–693. doi: 10.1176/ajp.151.5.687. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Sparks BF, Field KM, Yallapragada V, Shpritz BD, Rosenblatt A, Brandt J, Gourley LM, Liang K, Zhou H, Margolis RL, Ross CA. Onset and rate of striatal atrophy in preclinical Huntington disease. Neurology. 2004;63:66–72. doi: 10.1212/01.wnl.0000132965.14653.d1. [DOI] [PubMed] [Google Scholar]
- Banasr M, Soumier A, Hery M, Mocaer E, Daszuta A. Agomelatine, a new antidepressant, induces regional changes in hippocampal neurogenesis. Biol Psychiatry. 2006;59:1087–1096. doi: 10.1016/j.biopsych.2005.11.025. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1988;8:77–100. [Google Scholar]
- Bell-McGinty S, Butters MA, Meltzer CC, Greer PJ, Reynolds CF, 3rd, Becker JT. Brain morphometric abnormalities in geriatric depression: long-term neurobiological effects of illness duration. American Journal of Psychiatry. 2002;159:1424–1427. doi: 10.1176/appi.ajp.159.8.1424. [DOI] [PubMed] [Google Scholar]
- Bhatia KP, Daniel SE, Marsden CD. Familial parkinsonism with depression: a clinicopathological study. Annals of Neurology. 1993;34:842–847. doi: 10.1002/ana.410340614. [DOI] [PubMed] [Google Scholar]
- Bielau H, Trubner K, Krell D, Agelink MW, Bernstein HG, Stauch R, Mawrin C, Danos P, Gerhard L, Bogerts B, Baumann B. Volume deficits of subcortical nuclei in mood disorders A postmortem study. European Archives of Psychiatry and Clinical Neuroscience. 2005;255:401–412. doi: 10.1007/s00406-005-0581-y. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Stern E, Martinez D, Ricketts S, de Asis J, White T, Epstein J, McBride PA, Eidelberg D, Kocsis JH, Silbersweig DA. Increased anterior cingulate and caudate activity in bipolar mania. Biological Psychiatry. 2000;48:1045–1052. doi: 10.1016/s0006-3223(00)00962-8. [DOI] [PubMed] [Google Scholar]
- Bobrov MY, Lyzhin AA, Andrianova EL, Gretskaya NM, Zinchenko GN, Frumkina LE, Khaspekov LG, Bezuglov VV. Antioxidant and neuroprotective properties of N-docosahexaenoyl dopamine. Bull Exp Biol Med. 2006;142:425–427. doi: 10.1007/s10517-006-0383-x. [DOI] [PubMed] [Google Scholar]
- Bookstein FL. “Voxel-based morphometry” should not be used with imperfectly registered images. Neuroimage. 2001;14:1454–1462. doi: 10.1006/nimg.2001.0770. [DOI] [PubMed] [Google Scholar]
- Botteron KN, Raichle ME, Drevets WC, Heath AC, Todd RD. Volumetric reduction in left subgenual prefrontal cortex in early onset depression. Biol Psychiatry. 2002;51:342–344. doi: 10.1016/s0006-3223(01)01280-x. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157:115–118. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- Caetano SC, Kaur S, Brambilla P, Nicoletti M, Hatch JP, Sassi RB, Mallinger AG, Keshavan MS, Kupfer DJ, Frank E, Soares JC. Smaller cingulate volumes in unipolar depressed patients. Biological Psychiatry. 2006;59:702–706. doi: 10.1016/j.biopsych.2005.10.011. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview (SCID) for DSM-IV Axis 1 Disorders. American Psychiatric Press; Washington DC: 1997. [Google Scholar]
- Folstein SE, Folstein MF, McHugh PR. Psychiatric syndromes in Huntington's disease. Advances in Neurology. 1979;23:281–289. [Google Scholar]
- Gaser C, Nenadic I, Buchsbaum BR, Hazlett EA, Buchsbaum MS. Ventricular enlargement in schizophrenia related to volume reduction of the thalamus, striatum, and superior temporal cortex. Am J Psychiatry. 2004;161:154–156. doi: 10.1176/appi.ajp.161.1.154. [DOI] [PubMed] [Google Scholar]
- Gluck-Vanlaer N, Fallet A, Plas J, Chevalier JF. Depression et calcifications des noyaux gris centraux: a propos d'un cas. Encephale. 1996;22:127–131. [PubMed] [Google Scholar]
- Gold JI. Linking reward expectation to behavior in the basal ganglia. Trends in Neurosciences. 2003;26:12–14. doi: 10.1016/s0166-2236(02)00002-4. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Hamilton JP. Neuroimaging and depression: Current status and unresolved issues. Current Directions in Psychological Science in press. [Google Scholar]
- Gotlib IH, Krasnoperova E, Yue DN, Joormann J. Attentional biases for negative interpersonal stimuli in clinical depression. Journal of Abnormal Psychology. 2004;113:121–135. doi: 10.1037/0021-843X.113.1.121. [DOI] [PubMed] [Google Scholar]
- Greenwald BS, Kramer-Ginsberg E, Bogerts B, Ashtari M, Aupperle P, Wu H, Allen L, Zeman D, Patel M. Qualitative magnetic resonance imaging findings in geriatric depression. Possible link between later-onset depression and Alzheimer's disease? Psychol Med. 1997;27:421–431. doi: 10.1017/s0033291796004576. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Siemer M, Cooeny RE, Gotlib IH. Amygdala volume in depression: A meta-analysis of MRI studies. Manuscript under editorial review 2007 [Google Scholar]
- Henry ME, Kaufman MJ, Hennen J, Michelson D, Schmidt ME, Stoddard E, Vukovic AJ, Barreira PJ, Cohen BM, Renshaw PF. Cerebral blood volume and clinical changes on the third day of placebo substitution for SSRI treatment. Biol Psychiatry. 2003;53:100–105. doi: 10.1016/s0006-3223(02)01441-5. [DOI] [PubMed] [Google Scholar]
- Hickie IB, Naismith SL, Ward PB, Scott EM, Mitchell PB, Schofield PR, Scimone A, Wilhelm K, Parker G. Serotonin transporter gene status predicts caudate nucleus but not amygdala or hippocampal volumes in older persons with major depression. J Affect Disord. 2007;98:137–142. doi: 10.1016/j.jad.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Hokama H, Shenton ME, Nestor PG, Kikinis R, Levitt JJ, Metcalf D, Wible CG, O'Donnell BF, Jolesz FA, McCarley RW. Caudate, putamen, and globus pallidus volume in schizophrenia: a quantitative MRI study. Psychiatry Res. 1995;61:209–229. doi: 10.1016/0925-4927(95)02729-h. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Schnack HG, Mandl RC, van Haren NE, Koning H, Collins DL, Evans AC, Kahn RS. Focal gray matter density changes in schizophrenia. Arch Gen Psychiatry. 2001;58:1118–1125. doi: 10.1001/archpsyc.58.12.1118. [DOI] [PubMed] [Google Scholar]
- Jayakumar PN, Venkatasubramanian G, Gangadhar BN, Janakiramaiah N, Keshavan MS. Optimized voxel-based morphometry of gray matter volume in first-episode, antipsychotic-naive schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:587–591. doi: 10.1016/j.pnpbp.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Kawagoe R, Takikawa Y, Hikosaka O. Expectation of reward modulates cognitive signals in the basal ganglia. Nature Neuroscience. 1998;1:411–416. doi: 10.1038/1625. [DOI] [PubMed] [Google Scholar]
- Kessler RC. Epidemiology of depression. In: Gotlib IH, Hammen CL, editors. Handbook of Depression. Guilford Press; New York: 2002. [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) Journal of the American Medical Association. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Krishnan KR, McDonald WM, Doraiswamy PM, Tupler LA, Husain M, Boyko OB, Figiel GS, Ellinwood EH., Jr Neuroanatomical substrates of depression in the elderly. European Archives of Psychiatry and Clinical Neuroscience. 1993;243:41–46. doi: 10.1007/BF02191522. [DOI] [PubMed] [Google Scholar]
- Krishnan KR, McDonald WM, Escalona PR, Doraiswamy PM, Na C, Husain MM, Figiel GS, Boyko OB, Ellinwood EH, Nemeroff CB. Magnetic resonance imaging of the caudate nuclei in depression. Preliminary observations. Archives of General Psychiatry. 1992;49:553–557. doi: 10.1001/archpsyc.1992.01820070047007. [DOI] [PubMed] [Google Scholar]
- Lacerda AL, Nicoletti MA, Brambilla P, Sassi RB, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC. Anatomical MRI study of basal ganglia in major depressive disorder. Psychiatry Res. 2003;124:129–140. doi: 10.1016/s0925-4927(03)00123-9. [DOI] [PubMed] [Google Scholar]
- Lauterbach EC, Jackson JG, Price ST, Wilson AN, Kirsh AD, Dever GE. Clinical, motor, and biological correlates of depressive disorders after focal subcortical lesions. Journal of Neuropsychiatry and Clinical Neurosciences. 1997a;9:259–266. doi: 10.1176/jnp.9.2.259. [DOI] [PubMed] [Google Scholar]
- Lauterbach EC, Jackson JG, Wilson AN, Dever GE, Kirsh AD. Major depression after left posterior globus pallidus lesions. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 1997b;10:9–16. [PubMed] [Google Scholar]
- Lawrence AD, Sahakian BJ, Robbins TW. Cognitive functions and corticostriatal circuits: insights from Huntington's disease. Trends in Cognitive Sciences. 1998;2:379–388. doi: 10.1016/s1364-6613(98)01231-5. [DOI] [PubMed] [Google Scholar]
- Liu HH, Payne HR, Wang B, Brady ST. Gender differences in response of hippocampus to chronic glucocorticoid stress: role of glutamate receptors. J Neurosci Res. 2006;83:775–786. doi: 10.1002/jnr.20782. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A, Price CJ, Friston KJ, Ashburner J. Voxel-based morphometry of the human brain: methods and applications. Current Medical Imaging Reviews. 2005;1:105–113. [Google Scholar]
- Mendez MF, Adams NL, Lewandowski KS. Neurobehavioral changes associated with caudate lesions. Neurology. 1989;39:349–354. doi: 10.1212/wnl.39.3.349. [DOI] [PubMed] [Google Scholar]
- Mindham RH. Psychiatric symptoms in Parkinsonism. Journal of Neurology, Neurosurgery & Psychiatry. 1970;33:188–191. doi: 10.1136/jnnp.33.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parashos IA, Tupler LA, Blitchington T, Krishnan KR. Magnetic-resonance morphometry in patients with major depression. Psychiatry Research. 1998;84:7–15. doi: 10.1016/s0925-4927(98)00042-0. [DOI] [PubMed] [Google Scholar]
- Pillay SS, Renshaw PF, Bonello CM, Lafer BC, Fava M, Yurgelun-Todd D. A quantitative magnetic resonance imaging study of caudate and lenticular nucleus gray matter volume in primary unipolar major depression: relationship to treatment response and clinical severity. Psychiatry Res. 1998;84:61–74. doi: 10.1016/s0925-4927(98)00048-1. [DOI] [PubMed] [Google Scholar]
- Shah PJ, Ebmeier KP, Glabus MF, Goodwin GM. Cortical grey matter reductions associated with treatment-resistant chronic unipolar depression. Controlled magnetic resonance imaging study. British Journal of Psychiatry. 1998;172:527–532. doi: 10.1192/bjp.172.6.527. [DOI] [PubMed] [Google Scholar]
- Sheline YI. 3D MRI studies of neuroanatomic changes in unipolar major depression: the role of stress and medical comorbidity. Biological Psychiatry. 2000;48:791–800. doi: 10.1016/s0006-3223(00)00994-x. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Price JL. Amygdala core nuclei volumes are decreased in recurrent major depression. Neuroreport. 1998;9:2023–2028. doi: 10.1097/00001756-199806220-00021. [DOI] [PubMed] [Google Scholar]
- Shih AY, Erb H, Murphy TH. Dopamine activates Nrf2-regulated neuroprotective pathways in astrocytes and meningeal cells. J Neurochem. 2007;101:109–119. doi: 10.1111/j.1471-4159.2006.04345.x. [DOI] [PubMed] [Google Scholar]
- Skre I, Onstad S, Torgersen S, Kringlen E. High interrater reliability for the Structured Clinical Interview for DSM-III-R Axis I (SCID-I) Acta Psychiatrica Scandinavica. 1991;84:167–173. doi: 10.1111/j.1600-0447.1991.tb03123.x. [DOI] [PubMed] [Google Scholar]
- Staal WG, Hulshoff Pol HE, Schnack H, van der Schot AC, Kahn RS. Partial volume decrease of the thalamus in relatives of patients with schizophrenia. Am J Psychiatry. 1998;155:1784–1786. doi: 10.1176/ajp.155.12.1784. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, DelBello MP, Sax KW, Zimmerman ME, Shear PK, Hawkins JM, Larson ER. Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Arch Gen Psychiatry. 1999;56:254–260. doi: 10.1001/archpsyc.56.3.254. [DOI] [PubMed] [Google Scholar]
- Taki Y, Kinomura S, Awata S, Inoue K, Sato K, Ito H, Goto R, Uchida S, Tsuji I, Arai H, Kawashima R, Fukuda H. Male elderly subthreshold depression patients have smaller volume of medial part of prefrontal cortex and precentral gyrus compared with age-matched normal subjects: a voxel-based morphometry. Journal of Affective Disorders. 2005;88:313–320. doi: 10.1016/j.jad.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vernaleken I, Weibrich C, Siessmeier T, Buchholz HG, Rosch F, Heinz A, Cumming P, Stoeter P, Bartenstein P, Grunder G. Asymmetry in dopamine D(2/3) receptors of caudate nucleus is lost with age. Neuroimage. 2007;34:870–878. doi: 10.1016/j.neuroimage.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. American Journal of Psychiatry. 2004;161:1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- Wagner AK, Bayir H, Ren D, Puccio A, Zafonte RD, Kochanek PM. Relationships between cerebrospinal fluid markers of excitotoxicity, ischemia, and oxidative damage after severe TBI: the impact of gender, age, and hypothermia. J Neurotrauma. 2004;21:125–136. doi: 10.1089/089771504322778596. [DOI] [PubMed] [Google Scholar]
- Young KA, Manaye KF, Liang C, Hicks PB, German DC. Reduced number of mediodorsal and anterior thalamic neurons in schizophrenia. Biological Psychiatry. 2000;47:944–953. doi: 10.1016/s0006-3223(00)00826-x. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293:311–315. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]


