Abstract
The novel iboga alkaloid congener 18-methoxycoronaridine (18-MC) is a putative anti-addictive agent that has been shown, in rats, to decrease the self-administration of several drugs of abuse. Previous work has established that 18-MC is a potent antagonist at α3β4 nicotinic receptors. Because high densities of α3β4 nicotinic receptors occur in the medial habenula and the interpeduncular nucleus and moderate densities occur in the dorsolateral tegmentum, ventral tegmental area, and basolateral amygdala, the present study was conducted to determine if 18-MC could act in these brain areas to modulate methamphetamine self-administration in rats. Local administration of 18-MC into either the medial habenula, the interpeduncular area or the basolateral amygdala decreased methamphetamine self-administration. Similar results were produced by local administration into the same brain areas of two other α3β4 nicotinic antagonists, mecamylamine and α-conotoxin AuIB. Local administration of 18-MC, or the other antagonists, into the dorsolateral tegmentum or the ventral tegmental area had no effect on methamphetamine self-administration. In contrast, local administration of 18-MC and the other antagonists decreased sucrose self-administration when administered into the dorsolateral tegmentum or basolateral amygdala but had no effect when infused into the medial habenula, interpeduncular nucleus, or ventral tegmental area. These data are consistent with the hypothesis that 18-MC decreases methamphetamine self-administration by indirectly modulating the dopaminergic mesolimbic pathway via blockade of α3β4 nicotinic receptors in the habenulo-interpeduncular pathway and the basolateral amygdala. The data also suggest that the basolateral amygdala along with a different pathway involving α3β4 receptors in the dorsolateral tegmentum mediate the effect of 18-MC on sucrose self-administration.
Keywords: 18-methoxycoronaridine; α-conotoxin AuIB; mecamylamine; methamphetamine; sucrose; medial habenula; interpeduncular nucleus; basolateral amygdala, ventral tegmental area; dorsolateral tegmentum; α3β4nicotinic receptors; drug self-administration; drug addiction
1. Introduction
18-Methoxycoronaridine (18-MC), an iboga alkaloid congener that has the potential to be useful in treating multiple forms of drug abuse, has been shown, in rats, to decrease the self-administration of several drugs of abuse, including morphine (Glick et al. 1996), cocaine (Glick et al., 1996), methamphetamine (Glick et al., 2000a), nicotine (Glick et al., 2000a) and alcohol (Rezvani et al., 1997). 18-MC's primary mechanism of action appears to be to selectively block α3β4 nicotinic receptors (Glick et al., 2002; Pace et al., 2004). However, it is not simply an open channel blocker like the nonspecific nicotinic antagonist mecamylamine; rather, 18-MC is a non-competitive negative allosteric modulator that acts by stabilizing the ligand bound, desensitized state of the receptor (Pace et al., 2003). 18-MC, as well as several of its congeners, binds with higher affinity to the desensitized than to the resting state of the nicotinic receptor, and may in fact induce the desensitization process (Yuan et al., 2006). This mechanism potentially confers a unique antagonist profile—18-MC should have little or no effect on fast cholinergic transmission, for example in ganglia, and should not produce the peripheral side effects (constipation, hypotension) associated with mecamylamine. Consequently, 18-MC may have a unique spectrum of in vivo effects in multiple models of addictive disorders.
In one way or another, the mechanisms of action of virtually all drugs of abuse appear to involve the dopaminergic mesolimbic system, and new anti-addictive medications are usually designed to affect this system. Although 18-MC also affects this system, it does so in an indirect way via other pathways (cf. Maisonneuve and Glick, 2003). It was in fact the distribution of α3β4 nicotinic receptors in the brain that suggested a role of these other pathways in mediating 18-MC's behavioral effects. That is, in the brain, α3β4 nicotinic receptors are preferentially localized in the medial habenula and interpeduncular nucleus, while lower densities of these receptors reside in the ventral tegmental area (e.g., Klink et al., 2001; Quick et al., 1999) and other brain regions (e.g., dorsolateral tegmentum and basolateral amygdala; Perry et al., 2002; Zhu et al., 2005).
The interpeduncular nucleus receives its main input from the medial habenula, forming the habenulo-interpeduncular pathway in the fasciculus retroflexus. While there are multiple avenues for interaction between this pathway and the mesolimbic pathway in the medial forebrain bundle, it has been known since the 1980's that the habenulointerpeduncular pathway can function as a reward system separate from the mesolimbic pathway (e.g., Sutherland and Nakajima, 1981; Rompre and Miliaressis, 1985; Blander and Wise, 1989; Vachon and Miliaressis, 1992). Indeed, it appears that the two pathways probably modulate each other (Sutherland and Nakajima, 1981; Nishikawa et al., 1986). Based on its α3β4 nicotinic antagonist action, we postulated that 18-MC might act in the habenulo-interpeduncular pathway to dampen the activity of the mesolimbic pathway. Accordingly, we recently reported that local administration of 18-MC into either the medial habenula or interpeduncular nucleus decreases morphine self-administration in rats (Glick et al., 2006) and blocks sensitization of morphine-induced dopamine release in the nucleus accumbens (Taraschenko et al., 2007). In the present study, we have sought to determine if locally administered 18-MC also affects the self-administration of methamphetamine. In addition to the medial habenula and interpeduncular nucleus, we have also included the ventral tegmental area, the basolateral amygdala, and the dorsolateral tegmentum as potential sites of action, inasmuch as each of these latter areas is involved in reward mechanisms and has at least low or moderate densities of α3β4 nicotinic receptors. Lastly, we conducted parallel studies with a non-drug reinforcer, sucrose. While previous findings indicated that systemic 18-MC (5−20 mg/kg, i.p.) decreased drug self-administration but had no effects on responding for water or sucrose (e.g., Glick et al., 1996, 2002; Pace et al., 2004), recent work has shown that a higher dose of 18-MC (40 mg/kg) can also decrease sucrose (but not water) self-administration (Taraschenko et al., in preparation).
2. Materials and Methods
2.1 Treatment Drugs
Treatment drugs included 18-methoxycoronaridine hydrochloride (1−20 μg; Albany Molecular Research, Inc., Albany, NY), mecamylamine hydrochloride (10 μg; Sigma/RBI, St. Louis, MO), and α-conotoxin AuIB (25 pmol; generously provided bv Dr. J. Michael McIntosh, University of Utah). All treatments were injected intracerebrally immediately before behavioral testing.
2.2 Animals
Naïve female Long-Evans derived rats (250 g; Charles River, NY), housed individually, were maintained on a normal 12 h light cycle (lights on at 7:00 a.m., lights off at 7:00 p.m.). For all experiments, the “Guide for the Care and Use of Laboratory Animals” (National Academy of Sciences, 1996) was followed.
2.3 Self-administration procedure
The intravenous self-administration procedure has been described previously (e.g., Glick et al., 1996, 2000a). Briefly, responses on either of two levers (mounted 15 cm apart on the front wall of each operant test cage) were recorded on an IBM compatible computer with a Med Associates, Inc. interface. The intravenous self-administration system consisted of polyethylene-silicone cannulas constructed according to the design of Weeks (1972), Instech harnesses and swivels, and Harvard Apparatus infusion pumps (#55−2222). Shaping of the bar-press response was initially accomplished by training rats to bar-press for water. Cannulas were then implanted in the external jugular vein according to procedures described by Weeks (1972). Self-administration testing began with a 16-h nocturnal session followed by daily 1-h sessions, 5 days (Monday-Friday) a week. A lever-press response produced a 50 μl infusion of drug solution (0.025 mg of d-methamphetamine hydrochloride) in about 1 s. Since all rats generally weighed 250±20 g, each response delivered approximately 0.1 mg/kg of methamphetamine. Surgery to implant cannulae for intracerebral drug administration was performed when baseline self-administration rates stabilized (±20% variation from one day to the next across 5 days), usually after 2 weeks of testing. Each rat typically received two or three different treatments spaced at least one week apart. In order to provide an indication of the specificity of treatment effects on drug self-administration, all treatments were also administered to other rats bar-pressing for sucrose (15% solution; 0.01 ml orally) on a comparable schedule (continuous reinforcement; 1-h sessions).
2.4 Intracerebral Drug Administration
Rats were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and secured in a stereotaxic instrument. A midline incision was made, the bone was exposed, and bilateral holes for the microinjection guide cannulae were drilled. Microinjection guide cannulae (22-gauge; Plastics One, Roanoke, VA, USA) were lowered into place such that, when inserted, the tips of injectors would be located in the medial habenula or interpeduncular nucleus. The coordinates for the medial habenula and interpeduncular nucleus injections were, respectively, as follows: AP −4.2 mm, ML ±2.9 mm, DV −5.0 mm, using a 24° angle; AP −6.3, ML ±2.6 mm, DV −8.7 mm, using a 15° angle (Paxinos and Watson, 1986). Some injections were also made into the ventral tegmental area, basolateral amygdala and dorsolateral tegmentum; coordinates were, respectively, as follows: AP −6.0 mm, ML ±2.6 mm, DV −8.0 mm, using a 14° angle; AP −2.2 mm, ML ±4.8 mm, DV −6.0 mm, using a 0° angle; AP −7.8 mm, ML ±3.5 mm, DV −6.7 mm, using a 14° angle. The microinjection guide cannulae were permanently secured with stainless steel screws and cranioplastic cement. Rats were allowed to recover for approximately 24 hours before resuming self-administration testing. Intracerebral injections were made with the use of microsyringe (Hamilton; Reno, Nevada). Treatment drugs (or vehicle) were administered in a volume of 1 μl over 1 minute to prevent backflow through the microinjection guide; the injection cannula (26 gauge) was kept in place for an additional minute after drug infusion. All intracerebral injections were made bilaterally, immediately before starting a self-administration session.
Upon completion of an experiment, rats were sacrificed, and their brains were removed, frozen, and sliced (40 μm sections) in a cryostat. All sections containing a cannula track were traced and the locus of each track was mapped. The anterior-posterior extent was determined by making a composite of the drawings in which the track was present and referencing its full extent to the position of neuroanatomical landmarks. Intracerebral injection placements were verified without knowledge of the behavioral data.
2.5 Statistical Analysis
Statistical analyses of 18-MC effects initially employed one way analyses of variance across doses, separately for each brain region and reinforcer (e.g., effect of interpeduncular 18-MC on methamphetamine self-administration, effect of interpeduncular 18-MC on responding for sucrose etc.); subsequently, paired t-tests were employed to compare effects at individual doses with each animal's baseline performance. Only the latter analyses were conducted for assessing the effects of mecamylamine and α-conotoxin AuIB as only single dosages of these agents were tested. The outcomes of the analyses of variance are reported in the text of the results section while the outcomes of the paired t-tests are included in the figure and table legends.
3. Results
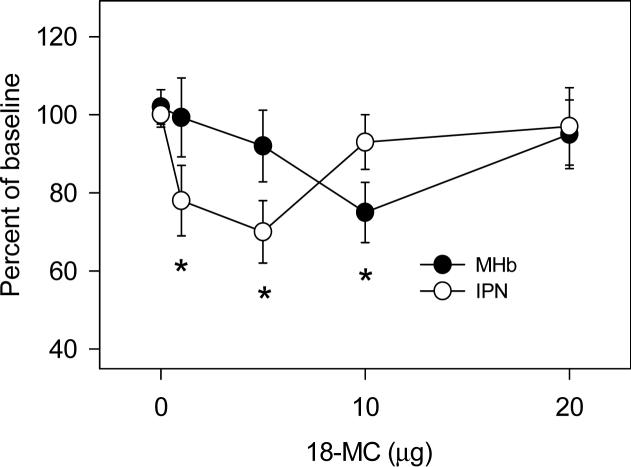
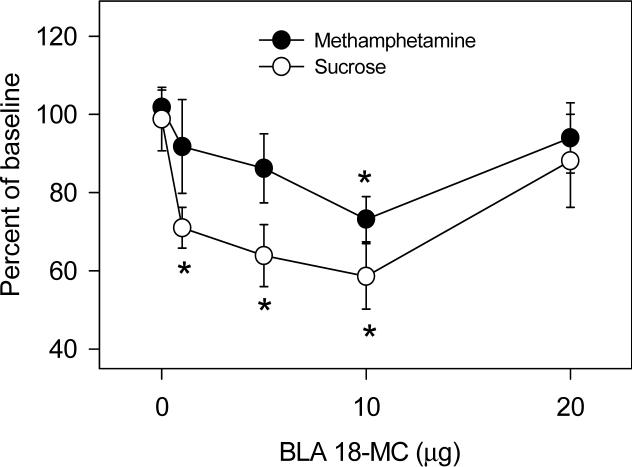
Figures 1 and 2 show the effects of local administration of 18-MC into the medial habenula, interpeduncular nucleus, and basolateral amygdala on methamphetamine self-administration. Infusion of 18-MC into either of these three sites decreased methamphetamine self-administration [interpeduncular nucleus: F (4,28)= 3.11, P<0.05; medial habenula: F (4,28)= 2.81, P<0.05; basolateral amygdala: F (4,28)= 2.85, P<0.05]. 18-MC appeared to be more potent in the interpeduncular nucleus than in the medial habenula and basolateral amygdala: 1 μg and 5 μg had significant effects in the interpeduncular nucleus while 10 μg was required to produce significant effects in the medial habenula and basolateral amygdala. In all three sites, the dose-response function was U-shaped, with the higher dosages (10−20 μg in the interpeduncular nucleus; 20 μg in the medial habenula and basolateral amygdala) having no effect. While 18-MC had no effect on sucrose self-administration when infused into either the medial habenula or interpeduncular nucleus (Glick et al., 2006), infusion into the basolateral amygdala had significant effects [Figure 2; F (4,28)= 4.11, P<0.01], decreasing sucrose responding even with the 1 and 5 μg dosages of 18-MC.
1.
Effects of local infusion of 18-MC into the medial habenula (MHb) and interpeduncular nucleus (IPN) on methamphetamine self-administration. Baseline methamphetamine infusions averaged (±S.E.M.) 21.4±1.0 and 20.5±1.2 for rats in the MHb and IPN groups, respectively. Each data point represents the mean (±S.E.M.) percent of baseline of 6−7 rats. *Significant difference between drug and baseline (paired t-test, P<0.05−0.02).
2.
Effects of local infusion of 18-MC into the basolateral amygdala on methamphetamine self-administration and on responding for sucrose. Baseline methamphetamine infusions averaged (±S.E.M.) 20.2±0.9 while baseline responding for sucrose averaged (±S.E.M.) 27.1±2.1. Each data point represents the mean (±S.E.M.) percent of baseline of 6−9 rats. *Significant difference between drug and baseline (paired t-tests, P<0.05−0.01).
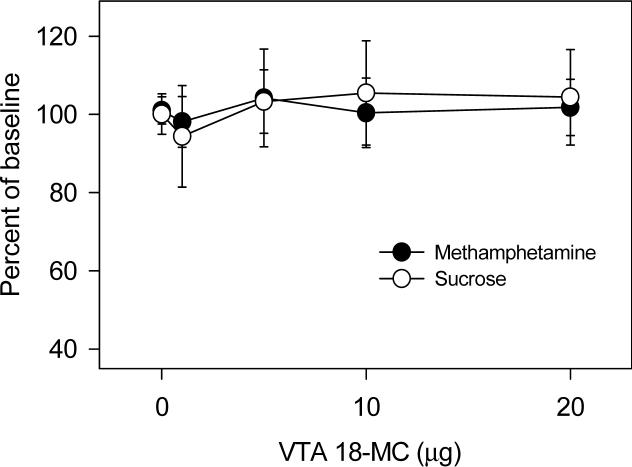
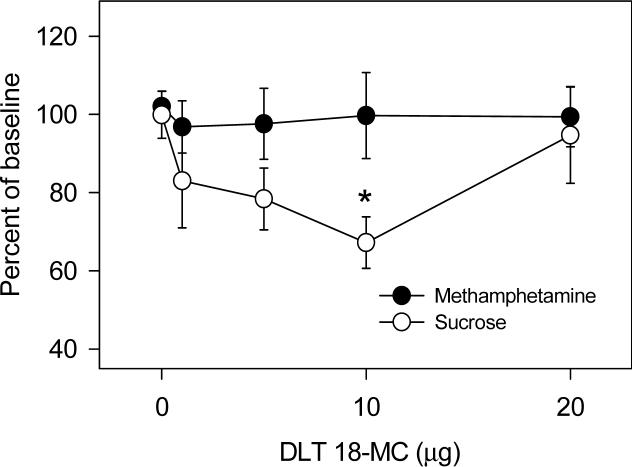
As shown in Figure 3, when infused into the ventral tegmental area, 18-MC had no effect on methamphetamine self-administration or on responding for sucrose. However, as shown in Figure 4, when infused into the dorsolateral tegmentum, 18-MC decreased responding for sucrose [F (4,28)= 3.21, P<0.05] while having no effect on methamphetamine self-administration.
3.
Effects of local infusion of 18-MC into the ventral tegmental area on methamphetamine self-administration and on responding for sucrose. Baseline methamphetamine infusions averaged (±S.E.M.) 22.5±1.8 while baseline responding for sucrose averaged (±S.E.M.) 23.7±3.2. Each data point represents the mean (±S.E.M.) percent of baseline of 6−10 rats.
4.
Effects of local infusion of 18-MC into the dorsolateral tegmentum on methamphetamine self-administration and on responding for sucrose. Baseline methamphetamine infusions averaged (±S.E.M.) 21.2±1.9 while baseline responding for sucrose averaged (±S.E.M.) 24.7±3.1. Each data point represents the mean (±S.E.M.) percent of baseline of 6−8 rats. *Significant difference between drug and baseline (paired t-test, P<0.05).
Table 1 shows effects of locally administered mecamylamine (10 μg) and α-conotoxin AuIB (25 pmol). Both agents significantly decreased methamphetamine self-administration when infused into either the interpeduncular nucleus or medial habenula, while having no effects on responding for sucrose. Both agents significantly decreased responding for methamphetamine and sucrose when infused into the basolateral amygdala. Both agents significantly decreased responding for sucrose when infused into the dorsolateral tegmentum while having no effect on methamphetamine self-administration. When infused into the ventral tegmental area, there was no effect of either agent on methamphetamine self-administration or on responding for sucrose.
Table 1.
Effects of locally administered mecamylamine (10 μg) and α-conotoxin AuIB (25 pmol) on rates (mean responses per hour ± S.E.M.; Ns= 6−8) of methamphetamine self-administration and responding for sucrose
| Reinforcer | Sitea | Treatment | ||
|---|---|---|---|---|
| vehicle | mecamylamine | α-conotoxin uIB | ||
| methamphetamine | MHb | 21.7±1.5 | 18.0±0.6b | 16.8±0.8b |
| sucrose | MHb | 23.9±2.4 | 23.0±2.5 | 23.6±2.7 |
| methamphetamine | IPN | 20.8±1.7 | 15.2±1.1b | 13.3±1.4b |
| sucrose | IPN | 23.1±0.9 | 25.3±2.3 | 22.8±1.5 |
| methamphetamine | BLA | 23.7±1.4 | 19.3±1.6b | 16.4±1.5b |
| sucrose | BLA | 25.1±2.6 | 18.0±2.8b | 15.8±2.2b |
| methamphetamine | DLT | 23.5±2.1 | 22.8±1.8 | 22.3±2.2 |
| sucrose | DLT | 24.8±1.6 | 19.7±2.2b | 19.1±2.4b |
| methamphetamine | VTA | 21.7±1.8 | 20.8±1.6 | 21.8±2.1 |
| sucrose | VTA | 24.8±2.3 | 25.5±2.9 | 27.1±3.2 |
MHB= medial habenula; IPN= interpeduncular nucleus; BLA= basolateral amygdala; DLT= dorsolateral tegmentum; VTA= ventral tegmental area
significantly different from baseline, p<0.05−0.02, paired t-test
4. Discussion
The results of this study suggest that 18-MC acts in both the medial habenula and interpeduncular nucleus as well as the basolateral amygdala to modulate methamphetamine self-administration. The comparable effects of mecamylamine and α-conotoxin AuIB in all three regions are consistent with the premise that 18-MC's primary mode of action is to block α3β4 nicotinic receptors (Glick et al., 2002a; Pace et al., 2004). While mecamylamine blocks all nicotinic receptor subtypes and has some selectivity for the α3β4 subtype (Papke et al., 2001), α-conotoxin AuIB is a specific antagonist of α3β4 nicotinic receptors (Luo et al., 1998).
The results of this study also suggest that 18-MC's modulation of sucrose self-administration is mediated by pathways that are both similar and dissimilar from those mediating 18-MC's effects on methamphetamine self-administration. While intrahabenular and intra-interpedunculuar administration of 18-MC had no effect on responding for sucrose, infusion of 18-MC into the basolateral amgydala or dorsolateral tegementum was effective. The common involvement of the basolateral amygdala is consistent with other data implicating the basolateral amygdala in reward-related phenomena associated with both stimulants (e.g., Hiroi and White, 1991; Whitelaw et al., 1996) and sucrose (e.g., Everitt et al., 1991). Previous work has also indicated that the dorsolateral tegmentum, which includes the pedunculopontine nucleus as well as the laterodorsal tegmental nucleus, is involved in consumption of sucrose (e.g., Ainge et al., 2006). Thus 18-MC appears to act in three circuits: medial habenula-interpeduncular nucleus, basolateral amygdala-nucleus accumbens, and dorsolateral tegmentum-ventral tegmental area; although all three circuits potentially modulate the dopaminergic mesolimbic pathway (e.g., Maisonneuve and Glick, 2003), their importance for the actions of 18-MC appears to vary with the particular reward, i.e., whether it be methamphetamine or sucrose. Interestingly, the basolateral amygdala is apparently much less important for opioid reward than for stimulant reward (e.g., Olmstead and Franklin, 1997; Alderson et al., 2000), and preliminary data from this laboratory indicate that infusion of 18-MC into the basolateral amygdala has no effect on morphine self-administration.
The dose-effect relationship for 18-MC was non-monotonic in all regions in which it had an effect; similar results were observed on morphine self-administration (Glick et al., 2006). The lack of effect of higher doses suggests that 18-MC has an opposing but less potent action at another receptor, i.e., possibly at the 5-HT3 serotonin or M1 muscarinic receptor (Glick et al., 2000b; Glick et al., 2002). Such opposing actions, along with regional differences in receptor densities, might be responsible for the regional selectivity of 18-MC's effects on methamphetamine versus sucrose self-administration; however, the comparable effects produced by mecamylamine and α-conotoxin AuIB would argue in favor of α3β4 antagonism being the primary mechanism involved.
When infused into the ventral tegmental area, 18-MC had no effect on either methamphetamine or sucrose self-administration. Aside from showing that 18-MC does not directly influence the ascending mesolimbic pathway, this lack of effect is important in that it rules out the possibility that, when injected into the interpeduncular nucleus, 18-MC might have diffused to the ventral tegmental area to produce its effect. How 18-MC alters the interactions between each of the three circuits noted above and the mesolimbic pathway may depend on the activity of these pathways and on how drugs of abuse exert their effects; that is, an action of 18-MC in each of these circuits may modulate rather than simply inhibit the activity of the mesolimbic pathway. Thus, for example, 18-MC interacts differently with drugs of abuse (cocaine, methamphetamine, morphine) depending on whether the latter are administered acutely or repeatedly (e.g., Szumlinski et al., 2000a, 2000b, 2000c). Interestingly, one modulatory mechanism common to all of these circuits might involve acetylcholine-induced activation of GABAergic neurotransmission (e.g., Zhu and Chiapinelli, 1999; Dani and Bertrand, 2007). At least in the interpeduncular nucleus and basolateral amygdala, acetylcholine appears to preferentially activate presynaptic α3β4 receptors on GABA neurons, releasing GABA (Lena et al., 1993; Zhu et al., 2005). 18-MC should promote desensitization of these receptors (Pace et al., 2004; Yuan et al., 2007) and reduce an inhibitory GABAergic influence, thereby altering the output of these circuits and their influence on mesolimbic responses involved in reward.
There are several potential routes, both direct and indirect, by which mesolimbic activity could be altered by each of the three circuits affected by 18-MC. The habenulo-interpeduncular and dopaminergic mesolimbic pathways appear to be reciprocally related, with the former inhibiting the latter and vice-versa (e.g., Nishikawa et al., 1986; Ellison, 1994). Such effects may be mediated by inputs from the interpeduncular nucleus to the raphe nuclei, which in turn provide input to the ventral tegmental area; or inputs from the interpeduncular nucleus to the medial dorsal thalamic nucleus may be involved, as the latter projects to the prefrontal cortex, which has connections to the nucleus accumbens and ventral tegmental area (cf. Groenewegen et al., 1986; Maisonneuve and Glick, 2003). Alternatively, the medial habenula projects to the lateral habenula (but not vice-versa; Kim and Chang, 2005), and connections from the lateral habenula to the ventral tegmental area (Christoph et al., 1986) appear to be directly involved in reward mechanisms (Ji and Shepard, 2007; Matsumoto and Hikosaka, 2007).
The dorsolateral tegmentum has direct cholinergic projections to the ventral tegmental area (e.g., Oakman et al., 1995; Blaha et al., 1996), but multiple indirect routes via several basal ganglia structures may also modulate mesolimbic activity (e.g., Mena-Segovia et al., 2004). Similarly, the basolateral amygdala has direct projections to the nucleus accumbens (e.g., McDonald, 1991; Petrovich et al., 1996; French and Totterdell, 2003), but indirect connections via the prefrontal cortex (e.g., Shinonaga et al., 1994; Berendse et al.,. 1992; Gray, 1999) also occur. Exactly how 18-MC alters the flow of information in these circuits, by which routes, remains to be determined.
In summary, along with other data from this laboratory (Glick et al., 2006; Taraschenko et al., 2007), the present data indicate that 18-MC acts in one or more of three circuits to decrease drug (morphine, methamphetamine) and sucrose self-administration. Although there is overlap among rewards in terms of which circuits are involved, there also appears to be some specificity. In all cases, however, 18-MC appears to act via antagonism of α3β4 nicotinic receptors to ultimately alter dopaminergic mesolimbic activity mediating reward.
Acknowledgements
This study was supported by NIDA grant DA 016283. We wish to thank Dr. J. Michael McIntosh for providing the α-conotoxin AuIB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ainge JA, Keating GL, Latimer MP, Winn P. The pedunculopontine tegmental nucleus and responding for sucrose reward. Behav. Neurosci. 2006;120:563–570. doi: 10.1037/0735-7044.120.3.563. [DOI] [PubMed] [Google Scholar]
- Berendise HW, Gallis-de Graaf Y, Groenewegen HJ. Topographic organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J. Comp. Neurol. 1992;316:314–347. doi: 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- Alderson HL, Robbins TW, Everitt BJ. The effects of excitotoxic lesions of the basolateral amygdala on the acquisition of heroin-seeking behaviour in rats. Psychopharmacology. 2000;153:111–119. doi: 10.1007/s002130000527. [DOI] [PubMed] [Google Scholar]
- Blaha CD, Allen LF, Das S, Inglis WL, Latimer MP, Vincent SR, Winn P. Modulation of dopamine efflux in the nucleus accumbens after cholinergic stimulation of the ventral tegmental area in intact, pedunculopontine tegmental nucleus-lesioned, and laterodorsal tegmental nucleus-lesioned rats. J. Neurosci. 1996;15:714–722. doi: 10.1523/JNEUROSCI.16-02-00714.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blander A, Wise RA. Anatomical mapping of brain stimulation reward sites in The anterior hypothalamic area: special attention to the stria medullaris. Brain Res. 1989;483:12–16. doi: 10.1016/0006-8993(89)90029-2. [DOI] [PubMed] [Google Scholar]
- Christoph GR, Leonzio RJ, Wilcox KS. Stimulation of the lateral habenula inhibits dopamine-containing neurons in the substantia nigra and ventral tegmental area of the rat. J. Neurosci. 1986;6:613–619. doi: 10.1523/JNEUROSCI.06-03-00613.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu. Rev. Pharmacol. Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- Ellison G. Stimulant-induced psychosis, the dopamine theory of schizophrenia, and the habenula. Brain Res. Rev. 1994;19:223–239. doi: 10.1016/0165-0173(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Morris KA, O'Brien A, Robbins TW. The basolateral amygdala-ventral striatal system and conditioned place preference: further evidence of limbic-striatal interactions underlying reward-related processes. Neuroscience. 1991;42:1–18. doi: 10.1016/0306-4522(91)90145-e. [DOI] [PubMed] [Google Scholar]
- French SJ, Totterdell S. Individual nucleus accumbens-projection neurons receive both basolateral amygdala and ventral subicular afferents in rats. Neuroscience. 2003;119:19–31. doi: 10.1016/s0306-4522(03)00150-7. [DOI] [PubMed] [Google Scholar]
- Glick SD, Kuehne ME, Maisonneuve IM, Bandarage UK, Molinari HH. 18-Methoxycoronaridine, a non-toxic iboga alkaloid congener: effects on morphine and cocaine self-administration and on mesolimbic dopamine release in rats. Brain Res. 1996;719:29–35. doi: 10.1016/0006-8993(96)00056-x. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Dickinson HA. 18-MC reduces methamphetamine and nicotine self-administration in rats. NeuroReport. 2000a;11:2013–2015. doi: 10.1097/00001756-200006260-00041. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Kitchen BA, Fleck MW. Antagonism of α3β4 nicotinic receptors as a strategy to reduce opioid and stimulant self-administration. Europ. J. Pharmacol. 2002;438:99–105. doi: 10.1016/s0014-2999(02)01284-0. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Szumlinski KK. 18-Methoxycoronaridine (18-MC) and ibogaine: comparison of anti-addictive efficacy, toxicity and mechanisms of action. Ann. N.Y. Acad. Sci. 2000b;914:369–387. doi: 10.1111/j.1749-6632.2000.tb05211.x. [DOI] [PubMed] [Google Scholar]
- Glick SD, Ramirez RL, Livi JM, Maisonneuve IM. 18-Methoxycoronaridine acts in the medial habenula and/or interpeduncular nucleus to decrease morphine self-administration in rats. Europ. J. Pharmacol. 2006;537:94–98. doi: 10.1016/j.ejphar.2006.03.045. [DOI] [PubMed] [Google Scholar]
- Gray TS. Ffunctional and anatomical relationships among the amygdala, basal forebrain, ventral striatum, and cortex: an integrative discussion. Ann. N.Y. Acad. Sci. 1999;877:439–444. doi: 10.1111/j.1749-6632.1999.tb09281.x. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Ahlenius S, Haber SN, Kowall NW, Nauta WJ. Cytoarchitecture, fiber connections, and some histochemical aspects of the interpeduncular nucleus in the rat. J. Comp. Neurol. 1986;249:65–102. doi: 10.1002/cne.902490107. [DOI] [PubMed] [Google Scholar]
- Hiroi N, White NM. The lateral nucleus of the amygdala mediates expression of the amphetamine-produced conditioned place preference. J. Neurosci. 1991;11:2107–2116. doi: 10.1523/JNEUROSCI.11-07-02107.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Shepard PD. Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABAA receptor–mediated mechanism. J. Neurosci. 2007;27:6923–6930. doi: 10.1523/JNEUROSCI.0958-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim U, Chang S-Y. Dendritic morphology, local circuitry, and intrinsic electrophysiology of neurons in the rat medial and lateral habenujlar nuclei of the epithalamus. J. Comp. Neurol. 2005;483:236–250. doi: 10.1002/cne.20410. [DOI] [PubMed] [Google Scholar]
- Klink R, de Kerchove d'Exaerde A, Zoli M, Changeux J-P. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J. Neurosci. 2001;21:1452–1463. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lena C, Changeux JP, Mulle C. Evidence for “preterminal” nicotinic receptors on GABAergic axons in the rat interpeduncular nucleus. J. Neurosci. 1993;13:2680–2688. doi: 10.1523/JNEUROSCI.13-06-02680.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo SQ, Kulak JM, Cartier GE, Jacobsen RB, Yoshikami D, Olivera BM, McIntosh JM. Conotoxin AuIB selectively blocks α3β4 nicotinic acetylcholine receptors and nicotine-evoked norepinephrine release. J. Neurosci. 1998;18:8571–8579. doi: 10.1523/JNEUROSCI.18-21-08571.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonneuve IM, Glick SD. Anti-addictive actions of an iboga alkaloid congener: a novel mechanism for a novel treatment. Pharmacol. Biochem. Behav. 2003;75:607–618. doi: 10.1016/s0091-3057(03)00119-9. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Topographical organization of amygdaloid projections to the caudatoputamen, nucleus accumbens, and related striatal-like areas of the rat brain. Neuroscience. 1991;14:15–33. doi: 10.1016/0306-4522(91)90248-m. [DOI] [PubMed] [Google Scholar]
- Mena-Segovia J, Bolam JP, Magill PJ. Pedunculopontine nucleus and basal ganglia: distant relatives or part of the same family? Trends Neurosci. 2004;10:585–588. doi: 10.1016/j.tins.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Fage D, Scatton B. Evidence for, and nature of, the tonic inhibitory influence of habenulointerpeduncular pathways upon cerebral dopaminergic transmission in the rat. Brain Res. 1986;373:324–336. doi: 10.1016/0006-8993(86)90347-1. [DOI] [PubMed] [Google Scholar]
- Oakman SA, Faris PL, Kerr PE, Cozzari C, Hartman BK. Distribution of pontomesencephalic cholinergic neurons projecting to substantia nigra differs significantly from those projecting to the ventral tegmental area. J. Neurosci. 1995;15:5859–5869. doi: 10.1523/JNEUROSCI.15-09-05859.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmstead MC, Franklin KBJ. The development of a conditioned place preference to morphine: effects of lesions of various CNS sites. Behav. Neurosci. 1997;111:1313–1323. doi: 10.1037//0735-7044.111.6.1313. [DOI] [PubMed] [Google Scholar]
- Pace CJ, Glick SD, Maisonneuve IM, Fleck MW. Mechanism of inhibitory action of ibogaine and 18-MC at nicotinic acetylcholine receptors. Soc. Neurosci. 2003 Abstr. #683.2. [Google Scholar]
- Pace CJ, Glick SD, Maisonneuve IM, He LW, Jokiel PA, Kuehne ME, Fleck MW. Novel iboga alkaloid congeners block nicotinic receptors and reduce drug self-administration. Europ. J. Pharmacol. 2004;492:159–167. doi: 10.1016/j.ejphar.2004.03.062. [DOI] [PubMed] [Google Scholar]
- Papke RL, Sanberg PR, Shytle RD. Analysis of mecamylamine stereoisomers on human nicotinic receptor subtypes. J. Pharmacol. Exp. Ther. 2001;297:646–656. [PubMed] [Google Scholar]
- Paxinos G, Watson C. Second ed. Academic Press Inc.; San Diego, CA: 1986. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- Perry DC, Xiao Y, Nguyen HN, Musachio JL, Davila-Garcia MI, Kellar KJ. Measuring nicotinic receptors with characteristics of alpha4beta2, alpha3beta2 and alpha3beta4 subtypes in rat tissues by autoradiography. J. Neurochem. 2002;82:468–481. doi: 10.1046/j.1471-4159.2002.00951.x. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Risold PY, Swanson LW. Organization of projections from the basomedial nucleus of the amygdala: a PHAL study in the rat. J. Comp. Neurol. 1996;374:387–420. doi: 10.1002/(SICI)1096-9861(19961021)374:3<387::AID-CNE6>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Quick MW, Ceballos RM, Kasten M, McIntosh JM, Lester RAJ. A3β4 subunit-containing nicotinic receptors dominate function in rat medial habenula neurons. Neuropharmacology. 1999;38:769–783. doi: 10.1016/s0028-3908(99)00024-6. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Overstreet DH, Yang Y, Maisonneuve IM, Bandarage UK, Kuehne ME, Glick SD. Attenuation of alcohol consumption by a novel non-toxic ibogaine analog (18-methoxycoronaridine) in alcohol preferring rats. Pharmacol. Biochem. Behav. 1997;58:615–619. doi: 10.1016/s0091-3057(97)10003-x. [DOI] [PubMed] [Google Scholar]
- Rompre PP, Miliaressis E. Pontine and mesencephalic substrates of self-stimulation. Brain Res. 1985;359:246–259. doi: 10.1016/0006-8993(85)91435-0. [DOI] [PubMed] [Google Scholar]
- Shinonaga Y, Takada M, Mizuno N. Topographic organization of collateral projections from the basolateral amygdaloid nucleus to both the prefrontal cortex and nucleus accumbens in the rat. Neuroscience. 1994;58:389–397. doi: 10.1016/0306-4522(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Nakajima S. Self-stimulation of the habenular complex in the rat. J. Comp Physiol Psychol. 1981;95:781–791. doi: 10.1037/h0077833. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Balogun MY, Maisonneuve IM, Glick SD. Interactions between iboga agents and methamphetamine sensitization: studies of locomotion and stereotypy. Psychopharmacology. 2000a;151:234–241. doi: 10.1007/s002130000478. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Maisonneuve IM, Glick SD. The potential anti-addictive agent, 18-methoxycoronaridine (18-MC), blocks the sensitized locomotor and dopamine responses produced by repeated morphine treatment. Brain Res. 2000b;864:13–23. doi: 10.1016/s0006-8993(00)02069-2. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, McCafferty CA, Maisonneuve IM, Glick SD. Interactions between 18-methoxycoronaridine (18-MC) and cocaine: dissociation of behavioural and neurochemical sensitization. Brain Res. 2000c;871:245–258. doi: 10.1016/s0006-8993(00)02424-0. [DOI] [PubMed] [Google Scholar]
- Taraschenko OD, Shulan JM, Maisonneuve IM, Glick SD. 18-MC acts in the medial habenula and interpeduncular nucleus to attenuate dopamine sensitization to morphine in the nucleus accumbens. Synapse. 2007;61:547–560. doi: 10.1002/syn.20396. [DOI] [PubMed] [Google Scholar]
- Vachon MP, Miliaressis E. Dorsal diencephalic self-stimulation: a movable electrode mapping study. Behav. Neurosci. 1992;106:981–991. doi: 10.1037//0735-7044.106.6.981. [DOI] [PubMed] [Google Scholar]
- Weeks JR. Long-term intravenous infusion. In: Myers RD, editor. Methods in Psychobiology. Vol. 2. Academic Press; New York: 1972. pp. 155–168. [Google Scholar]
- Whitelaw RB, Markou A, Robbins TW, Everitt BJ. Excitotoxic lesions of the basolateral amygdala impair the acquisition of cocaine-seeking behaviour under a second-order schedule of reinforcement. Psychopharmacology. 1996;127:213–224. [PubMed] [Google Scholar]
- Yuan XJ, Arias HR, Glick SD, Kuehne ME. Characterization of the 18-methoxycoronaridine binding site on the nicotinic acetylcholine receptor. J. Neurochem. 2007;102(Suppl 1):271. [Google Scholar]
- Zhu PJ, Chiappinelli VA. Nicotine modulates evoked GABAergic transmission in the brain. J. Neurophysiol. 1999;82:3041–3045. doi: 10.1152/jn.1999.82.6.3041. [DOI] [PubMed] [Google Scholar]
- Zhu PJ, Stewart RR, McIntosh JM, Weight FF. Activation of nicotinic acetylcholine receptors increases the frequency of spontaneous GABAergic IPSCs in rat basolateral amygdala neurons. J. Neurophysiol. 2005;84:3081–3091. doi: 10.1152/jn.00974.2004. [DOI] [PubMed] [Google Scholar]