Abstract
Voluntary exercise is associated with the prevention and treatment of numerous physical and psychological illnesses, yet the mechanisms by which it confers this protection remain unclear. In contrast, stress, particularly under conditions of prolonged or repeated exposure when glucocorticoid levels are consistently elevated, can have a devastating impact on health. It has been suggested that the benefits of physical exercise may lie in an ability to reduce some of the more deleterious health effects of stress and stress hormones. The present series of experiments provides evidence that voluntary exercise facilitates habituation of corticosterone but not adrenocorticotropin hormone responses to repeated stress presentations. After 6 weeks of running wheel access or sedentary housing conditions, rats were exposed to 11 consecutive daily 30 min presentations of 98 dB noise stress. Similar corticosterone responses in exercised rats and sedentary controls were observed following the first, acute stress presentation. While both groups demonstrated habituation of corticosterone secretory responses with repeated noise stress exposures, the rate of habituation was significantly facilitated in exercised animals. These results suggest that voluntary exercise may reduce the negative impact of prolonged or repeated stress on health by enhancing habituation of hypothalamo-pituitary–adrenocortical axis responses at the level of the adrenal cortex, ultimately reducing the amount of glucocorticoids the body and brain are exposed to.
Keywords: Adaptation, corticosterone, HPA-axis, repeated stress, voluntary exercise
Introduction
Voluntary physical exercise produces many physiological effects that promote both physical (Manson et al. 1992; Wannamethee et al. 1998; Oguma and Shinoda-Tagawa 2004; Shea et al. 2004; Kruk and Aboul-Enein 2006) and psychological (Chodzko-Zajko and Moore 1994; Fox 1999; Dunn et al. 2001) health. Yet in light of the many studies elucidating the numerous health benefits associated with physical exercise, the mechanisms conferring this protection remain poorly understood. Stress, on the other hand, represents a significant risk factor for many of the same health conditions shown to profit from physical activity (Sapolsky 1992, 1996; Hammen et al. 1992; Stratakis and Chrousos 1995; Arborelius et al. 1999; Vanitallie 2002). This has led to the hypothesis that the benefits of physical exercise lie, at least in part, in an ability to provide protection from, or resiliency to, the more damaging health effects of stress (Roth and Holmes 1985; Brown and Lawton 1986; Dishman et al. 1995; Moraska and Fleshner 2001; Salmon 2001).
Organisms have intricate, multi-dimensional systems in place to cope with and adapt to the frequent, unavoidable stressful situations encountered throughout life. An integral component of these systems is the endocrine hypothalamo-pituitary–adrenocortical (HPA) axis response, which ultimately releases glucocorticoids into the bloodstream that participate in metabolic regulation throughout the body, ensuring adequate energy availability to cope with and recover from the threat or challenge (Akil and Morano 1995; Nelson 2000). It is these same hormones (cortisol in humans, corticosterone in rodents), however, that can severely harm the body and brain when present in excess over long periods of time, for example, under conditions of long-term exogenous glucocorticoid treatment (Truhan and Ahmed 1989; Lester et al. 1998; Nelson 2000) or chronic stressor exposure (Sapolsky 1992, 1996; McEwen 2000; Vanitallie 2002; Swaab et al. 2005).
Surprisingly, studies have typically not shown a reliable effect of voluntary physical exercise on glucocorticoid responses to various acute stressor exposures in humans (Sothmann et al. 1988) or animals (Dishman 1995, 1998; Fediuc et al. 2006; Fleshner 2000). While it is the situations involving prolonged or repeated stressor exposures that are typically associated with physical and psychological illness, there has been little investigation into the potential for voluntary exercise to alter the way the body and brain respond to repeated stress. Animals repeatedly exposed to the same, mild to moderate stressor often demonstrate the adaptive phenomenon of habituation, in which their HPA-axis response, as measured by plasma glucocorticoid concentrations, decreases with repeated exposures (Kant et al. 1985; Armario et al. 1986; Spencer and McEwen 1990; Campeau et al. 2002). A dysfunction in the ability to appropriately adapt could result in elevated levels of circulating glucocorticoids, which may then contribute to the development and/or exacerbation of various stress-related disorders.
The present series of experiments were designed to investigate whether voluntary physical exercise, in the form of wheel running, has an effect on HPA-axis response habituation to repeated audiogenic stress presentations in rats. A facilitation of habituation in exercised as compared to sedentary rats would suggest that voluntary exercise might buffer some of the negative physiological impacts of stress by specifically modulating this adaptive process, ultimately reducing the amount of glucocorticoids the body and brain are exposed to.
Materials and methods
Subjects
Subjects in both experiments were young adult (approximately 56 days old), male Sprague-Dawley rats obtained from Harlan (Indianapolis, IN), with weights ranging from 150–200 g upon arrival. Rats were housed in a dedicated colony facility, initially in groups of 4–5 in clear polycarbonate cages (48 × 27 × 20 cm) with ad libitum access to rat chow and water in a temperature, humidity, and light (12:12 h light/dark cycle; lights on at 0700 h) controlled environment. All rats were housed for a period of 7 days after arrival from the supplier before any experimental manipulations were conducted. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Colorado and conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Voluntary exercise
After the appropriate acclimation time, rats in both experiments were divided into two groups matched for body weight and individually housed in clear polycarbonate cages (43 × 22 × 21 cm) under the facility conditions described above for the remainder of the study. Those assigned to the Exercise (Ex) condition were given 24 h unlimited access to a stainless steel running wheel (34.5 cm wheel diameter × 9.7 cm width, Nalge Nunc International) attached to the lid of their cages for the 6 weeks prior to, and for the 11 days of, the experimental testing phase. Running distances were monitored daily by Sigma Sport (BC 400) bicycle odometers directly attached to the wheels in Experiment no. 1, and by computer with the Vital View Automated Data-Acquisition System (Sunny River, OR) in Experiment no. 2. Sedentary (Sed) control rats were individually housed in the same colony room in similar size cages without running wheels. All rats were weighed weekly.
Audiogenic stress
Audiogenic (loud noise) stress has been characterized as a mild to moderate intensity stressor for which the corticosterone response has been shown to habituate almost completely to control levels within 10–15 consecutive daily 30 min exposures in male Sprague-Dawley rats (Campeau et al. 2002). Audiogenic stress presentations consisted of carefully transporting animals to a separate testing room during the early phase of the light cycle (0900–1100 h) and placing them, in their home cages, into 1 of 8 completely enclosed acoustic chambers. Each rat was assigned a specific chamber for all stress (or no stress) presentations. Each acoustic chamber consisted of a ventilated, double wooden (2.5 cm plywood board) enclosure, with the outer enclosure lined internally with 2.5 cm insulation (Celotex™). The internal dimensions of the inner enclosure were 60 cm (w) × 38 cm (d) × 38 cm (h), which allowed placement of a polycarbonate rat home cage with attached running wheel inside. Each inner enclosure was fitted with a single 15.2 × 22.9 cm Optimus speaker (No. 12–769–120 W RMS) fixed in the middle of the ceiling. Lighting was provided by a fluorescent lamp (15 W) covered with red tissue paper located in the upper left corner of the chamber. Noise was produced by a General Radio (No. 1381) solid-state random-noise generator with the bandwidth set at 2 Hz–50 kHz. The output of the noise generator was fed to power amplifiers (Pyramid Studio Pro no. PA-600X), the outputs of which fed the speakers. Noise intensity was measured each morning prior to stress (or no stress) presentations by placing a Radio Shack Realistic Sound Level Meter (A scale; no. 33–2050) in each acoustic chamber at several locations and taking an average of the different readings. The insulated double-enclosure design of the acoustic chambers provided approximately 30 dB of noise attenuation, easily preventing any measurable noise conduction between even adjacent chambers.
Experiment no. 1
Fifty rats weighing 150–175 g upon arrival were used. Daily testing began after 6 weeks of wheel access (n = 25) or sedentary housing conditions (n = 25) had elapsed. For 3 days prior to Day 1 of testing, all rats were transported to the testing room and placed into their respective acoustic chambers for 30 min where they were exposed only to the background noise (60 dB) produced by the ventilation fans. This pre-exposure to the testing conditions was employed in order to minimize the potential stress of what would otherwise be a novel experience on HPA-axis responsiveness to the subsequent testing conditions. Then, for 11 consecutive days, some of the rats from the Ex and Sed groups (designated Ex/Repeated noise and Sed/Repeated noise, n = 17/group) were exposed to 30 min of 98-dB loud noise stress, an intermediate intensity strong enough to allow room for HPA-axis habituation to be observed, but not so strong that adaptation would be prevented (Burow et al. 2005). The remaining rats (Ex/No Noise and Sed/No Noise, n = 8/group) served as no noise controls and were exposed only to the background noise (60 dB) of the chambers for 30 min. All Ex rats had access to running wheels during the 30-min stress or no stress presentations. On Days 1, 4, and 8 of testing, blood samples were obtained via tail bleeds immediately following removal from the acoustic chambers, after which rats were returned to the colony room. On the last day of testing (Day 11), all rats were killed by decapitation immediately following the 30-min stress or no stress exposures and trunk blood was collected.
Experiment no. 2
This experiment investigated the possibility that free access to running wheels during the repeated noise stress presentations in Experiment no. 1 provided a coping strategy not available to Sed rats that may have reduced the overall impact of the stress experience on glucocorticoid release, as previously suggested by Droste et al. (2003). To test this, all running wheels were locked during each of 11 daily 30 min noise or no noise exposures by placing a long metal rod through both sides of the wheel, preventing it from rotating. Further, two additional groups were included in this experiment that were exposed to the noise stress only on the last day (Day 11) of testing as an additional examination of any potential response differences between active and sedentary rats to an acute noise stress presentation.
Forty-eight rats (175–200 g upon arrival)were used in Experiment no. 2. Daily testing began after 6 weeks of wheel access (n = 24) or sedentary housing conditions (n = 24) had elapsed. Pre-exposure to the testing conditions began 3 days prior to Day 1 of testing, during which all rats were transported to the testing room and placed into their respective acoustic chambers for 30 min where they were exposed only to the 60-dB background noise. Then, for 11 consecutive days, some of the rats from the Ex and Sed groups (designated Ex/Repeated Noise and Sed/Repeated Noise, n = 8/group) were exposed to daily presentations of 30 min of 98 dB noise stress. The additional acute stress groups (Ex/Acute Noise and Sed/Acute Noise, n = 8/group) were exposed to daily 30 min exposures to the 60-dB background noise except on the last day of testing (Day 11), upon which they received an acute, 30 min presentation of the 98-dB noise stress. The no stress control groups (Ex/No Noise and Sed/No Noise, n = 8/group) were placed into the acoustic chambers but were exposed only to 30 min of 60 dB background noise for the 11 days of testing. Blood samples were obtained via tail bleeds immediately upon removal from the acoustic chambers on Days 1, 4, and 8 of testing, after which running wheels were unlocked and all rats were returned to the colony room. On the last day of testing, all rats were killed by decapitation, without anaesthesia, immediately following the 30-min stress or no stress exposures and trunk blood was collected.
Blood sampling and tissue collection
On Days 1, 4, and 8 of noise or no noise exposures, blood samples were obtained from each rat by making a small incision into the tail vein with a sterile razor blade immediately upon removal from the acoustic chambers. Blood (approximately 200 μl for Experiment. no. 1 and 400 μl for Experiment no. 2) was collected into ice-chilled 1.5 ml Eppendorf microcentrifuge tubes containing 15 μl of EDTA (20 mg/ml). The entire sampling procedure, from the termination of the noise/no noise exposures to the rats being returned to their home cages, lasted no longer than 3 min. Samples were immediately centrifuged at 6000 rpm for 2 min and resulting plasma was pipetted into chilled microcentrifuge tubes and stored at −80°C until assayed. On the last day, trunk blood was collected into ice-chilled tubes (BD-Vacutainer) coated with EDTA and were immediately centrifuged at 2000 rpm for 10 min at 4°C. The resulting plasma was pipetted into chilled microcentrifuge tubes and stored at −80°C until assayed. Upon sacrifice, adrenals and thymus were immediately excised and weighed while wet.
HPA-axis responsiveness
Corticosterone radioimmunoassay
Plasma samples were allowed to thaw to room temperature and were then diluted 1:100 in 0.05 M sodium phosphate buffer containing 0.25% bovine serum albumin, pH 7.4–7.6. These samples were heated at 70°C for 30 min in a water bath to release corticosterone from plasma binding proteins. Fifty microliters of trace solution (3H-corticosterone, Amersham, Arlington Heights, IL; 560 Ci/mmol, 10,000 cpm/tube) and 50 μl of corticosterone antibody (courtesy of Dr. Stanley Watson, Ann Arbor, MI, final concentration of 1:12,800/tube) were added to duplicate samples of 200 μl plasma/buffer solution. A standard curve was concurrently generated with known amounts of corticosterone, by serial dilution (0–80 μg/dl). Samples were then vortexed and incubated at 4°C overnight.
A mixture of 0.5 ml chilled 1% charcoal/0.1% dextran in buffer solution was then added to each sample and allowed to incubate for 10 min in order to separate antibody-bound from free corticosterone. Samples were then centrifuged at 3000 rpm for 8 min at 4°C (Eppendorf 5810R). The supernatant was poured into 4 ml of scintillation fluid, and bound 3H-corticosterone was counted (disintegrations per minute) on a Packard 1600TR liquid scintillation analyzer and calculated against the standard curve. All samples from a single experiment were measured simultaneously to reduce interassay variability. Variation within the assay duplicates was no more than 8%.
Adrenocorticotropic hormone radioimmunoassay
Plasma adrenocorticotropic hormone (ACTH) was measured in samples obtained from Experiment no. 2 only (not enough plasma was collected in Experiment no. 1) with a kit (ACTH IRMA RIA Ref No. 27130) obtained from DiaSorin Inc. (Stillwater, MN, USA), according to the manufacturer’s protocol. The sensitivity of the assay ranged from 0–1500 pg/ml. All samples from a single experiment were measured simultaneously to reduce interassay variability. Intra-assay variability was no more than 12%.
Statistical analyses
All statistical analyses were performed using the Statistical Analysis Software (SAS) program. Mean body weights were calculated for each experimental group and analyzed using a repeated measures analysis of variance (ANOVA). Adrenal and thymus weights were corrected for body weight (organ weight/100 g body weight) unless otherwise indicated and compared using a two-factor (Activity Status × Stress Treatment) ANOVA. Mean plasma corticosterone concentrations were examined over the 4 sampling days using a repeated measures ANOVA. Additional univariate ANOVAs for each sampling day were used to further elucidate group differences when observed. Mean plasma ACTH concentrations were determined for samples obtained in Experiment no. 2 and were analyzed over the 4 sampling days using a repeated measures ANOVA. Due to incomplete data collected via the bicycle odometers in Experiment no. 1, running distance analyses were only performed on data collected with the more thorough and accurate computerized monitoring equipment used in Experiment no. 2. Mean daily distances for rats in each Stress Treatment group were averaged for each week and were compared using a repeated measures ANOVA. Simple regression analyses were used to investigate possible relationships between various aspects of running behavior (i.e., total distance traveled, mean daily distance traveled at onset or termination of stress presentations) and body weights, organ weights, and HPA-axis responsiveness. Where applicable, post-hoc comparisons were performed using Scheffé’s multiple-comparisons procedure. Statistical significance for all analyses was set at p ≤ 0.05.
Results
Experiment no. 1
Body weights
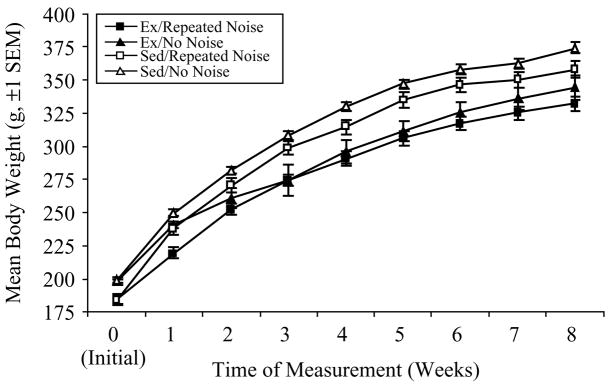
All rats were weighed initially, prior to group assignment, and then once per week for the 8-week duration of the experiment. Mean body weights (±SEM) for each of the four groups are depicted in Figure 1. A repeated measures ANOVA [Time (9 weekly measurements) = within-subjects; Activity Status (Ex or Sed) and Stress Treatment (Repeated or No Noise) = between-subjects] revealed significant Time (F(8, 368) = 1444.91, p < 0.0001), Activity Status (F(1, 46) = 19.35, p < 0.0001), and Time × Activity Status (F(8, 368) = 13.39, p < 0.0001) effects on mean body weights. Thus, while all rats gained weight over the course of the experiment, Ex rats gained weight at a significantly slower rate than Sed controls. These results were further confirmed by individual univariate ANOVAs performed for each weekly time point, which showed significantly lower body weights in Ex as compared to Sed rats at all time points following the initial measurement (Scheffé, all p < 0.05).
Figure 1.
Mean body weights (g, ±SEM) for each of the 4 experimental groups (exercised, Ex and sedentary, Sed Repeated Noise, n = 17/group; Ex and Sed No Noise, n = 8/group) over the 8-week duration of Experiment no. 1. Ex rats weighed significantly less than Sed rats at all time points following the initial (Week 0) measurement (p < 0.05).
Organ weights
Mean wet organ weights for all groups, corrected for body weight (organ weight/100 g body weight, + SEM), are presented in Table I. Two-factor ANOVAs revealed no effects of Activity Status, Stress Treatment, or Activity Status × Stress Treatment on mean adrenal or thymus weights.
Table I.
Mean adrenal and thymus weights (g per 100 g body weight, ±SEM) for rats from Experiment no. 1 that were exercising (Ex) or sedentary (Sed) for 6 weeks prior to 11 consecutive daily 30 min presentations of 98-dB noise stress or 60-dB background noise. There were no effects of Activity Status or Stress Treatment on mean adrenal or thymus weights. n = number of rats providing tissue weight per group.
| Treatment group | Mean adrenal weight (g/100 g body weight (SEM)) | Mean thymus weight (g/100 g body weight (SEM)) |
|---|---|---|
| Ex/Repeated noise | 0.0144 (0.0021) | 0.0978 (0.0065) |
| n = 7 | n = 17 | |
| Ex/No noise | 0.0157 (0.0015) | 0.0825 (0.0086) |
| n = 8 | n = 8 | |
| Sed/Repeated noise | 0.0125 (0.0027) | 0.0979 (0.0043) |
| n = 5 | n = 17 | |
| Sed/No noise | 0.0131 (0.0014) | 0.0986 (0.0064) |
| n = 8 | n = 8 |
HPA-axis response
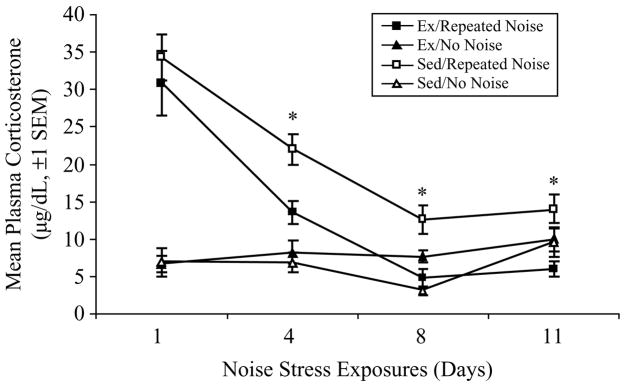
Mean plasma corticosterone concentrations (±SEM) are presented in Figure 2. A repeated measures ANOVA [4 sampling Days = within-subjects; Activity Status and Stress Treatment = between-subjects] revealed significant Days (F(3, 132) = 17.45, p < 0.0001), Stress Treatment (F(1, 44) = 46.70, p < 0.0001), Days × Stress Treatment (F(3, 132) = 21.05, p < 0.0001), and Stress Treatment × Activity Status (F(1, 44) = 8.91, p = 0.0046) effects on mean corticosterone responses. When controlling for Activity Status, the first noise stress exposure on Day 1 elicited a robust corticosterone response in both Repeated Noise groups compared to those groups exposed only to background noise (F(1, 46) = 42.32, p < 0.0001). On Day 4, while mean corticosterone responses for the Repeated Noise groups had decreased to nearly half of the acute Day 1 response, a univariate ANOVA showed that the responses were still significantly higher than No Noise controls (F(1, 46) = 26.13, p < 0.0001). By the eighth consecutive stress exposure, corticosterone responses had decreased to nearly a quarter of the acute Day 1 response, yet were still significantly higher than No Noise controls (F(1, 46) = 5.97, p = 0.049). Most profoundly, on the eleventh and final day of testing, corticosterone responses were equivalent for the Repeated Noise groups and the No Noise controls, indicating that complete habituation had taken place. No differences in corticosterone concentrations were observed between Ex and Sed No Noise control groups on any of the 4 sampling days, implying no effect of 6 weeks of wheel running on basal HPA-axis tone at the trough of the circadian cycle, during which all samples were obtained.
Figure 2.
Mean plasma corticosterone responses (μg/dL, ±SEM) in exercised (Ex) and sedentary (Sed) rats exposed to 11 consecutive daily 30 min presentations of 98-dB noise stress (n = 17/group) or 60-dB background noise (n = 8/group except on Days 8 and 11, where n = 7 for Ex and Sed No Noise groups, respectively, due to missing data) in Experiment no. 1. Asterisks indicate p ≤ 0.05 between Ex and Sed Repeated Noise groups only.
To further investigate the Stress Treatment × Activity Status interaction effect, additional univariate ANOVAs were performed for each sampling day for only those rats in the Repeated Noise groups, as our main interest concerned the effect, if any, of voluntary exercise on HPA-axis response habituation to repeated stress exposures. As indicated by the asterisks in Figure 2, a reliable effect of Activity Status on corticosterone responses was found on Days 4 (F(1, 32) = 10.88, p = 0.0024), 8 (F(1, 32) = 13.46, p = 0.0009), and 11 (F(1, 32) = 14.18, p = 0.0007), with Ex rats responding significantly lower than Sed rats on each day. No differences between groups were observed on Day 1. These results suggest that 6 weeks of voluntary wheel running significantly facilitated the rate and ultimate extent of corticosterone response habituation to repeated loud noise stress in comparison to Sed controls, without affecting the responsiveness to the first, acute noise stress presentation.
Experiment no. 2
Body weights
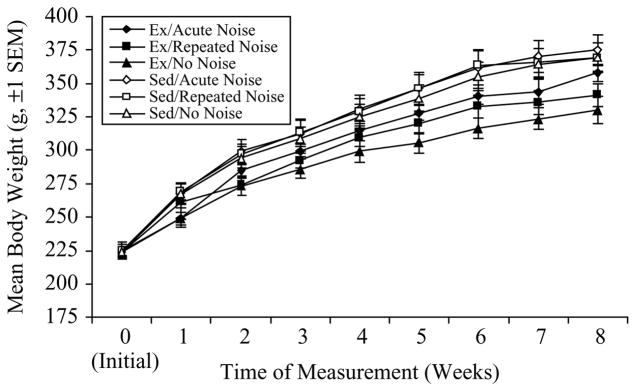
Mean body weights (±SEM) for the 6 experimental groups are presented in Figure 3. A repeated measures ANOVA [Time (9 weekly measurements) = within-subjects; Activity Status (Ex or Sed) and Stress Treatment (Acute, Repeated, or No Noise) = between-subjects] revealed significant Time (F(8, 336) = 877.6, p < 0.0001), Activity Status (F(1, 42) = 11.04, p = 0.0019), and Time × Activity Status (F(8, 336) = 10.30, p < 0.0001) effects on mean body weights. As in Experiment no. 1, Ex rats gained weight at a slower rate than Sed controls, as further evidenced by individual univariate ANOVAs performed for each weekly time point, which showed significantly lower body weights in Ex as compared to Sed rats at all time points following the initial measurement (Scheffé, all p < 0.05).
Figure 3.
Mean body weights (g, ±SEM) for each of the 6 experimental groups (n = 8/group) over the 8-week duration of Experiment no. 2. Exercised (Ex) rats weighed significantly less than sedentary (Sed) rats at all time points following the initial (Week 0) measurement (p < 0.05).
Activity levels and body weights
While Ex rats gained weight significantly slower than Sed rats, a series of simple regression analyses showed that individual activity levels (defined as either the total distance traveled up to the point of weight measurement or the mean daily distance traveled during the week prior to weight measurement) were not reliable predictors of body weights (data not shown).
Running distance profile
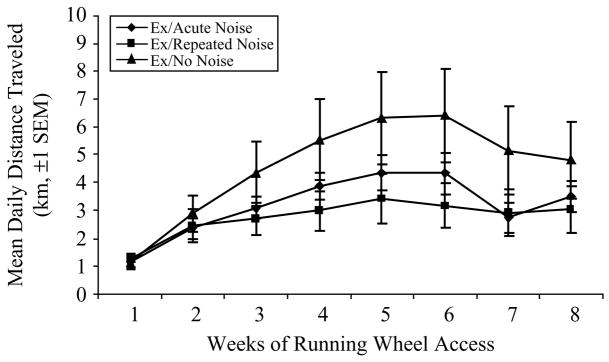
Running distances in Experiment no. 2 were monitored via computer with the VitalView Automated Data-Acquisition System. Daily running behavior patterns were similar for all Ex rats, differing only quantitatively (data not shown). Briefly, all rats were essentially inactive during the “lights on” phase of their light cycle (0700–1900 h), although an increase in activity tended to precede the initiation of the dark phase (around 1700 h). Once the “lights off” phase began, there was a large increase in running activity in most rats. Average 24-h running distances increased weekly from 1.17 ± 0.11 km at week 1 to a maximum of 4.69 ± 0.68 km during the fifth week of wheel access. Following week 5, distances began to gradually decrease and continued to do so for the remainder of the experiment. There was, however, great variability among Ex rats in the actual amount of activity that took place during the dark phase. Figure 4 shows the mean 24-h distance run per week (±SEM) for the duration of the experiment for each Stress Treatment group. A repeated measures ANOVA [Time (8 weekly measurements) = within-subjects; Stress Treatment (Acute, Repeated, or No Noise) = between-subjects] showed a significant Time (F(7, 147) = 14.90, p < 0.0001), but not Stress Treatment or Time × Stress Treatment effects on mean weekly running distances, suggesting no reliable group differences. This was confirmed by individual univariate ANOVAs performed at each weekly time point which showed no effect of Stress Treatment on mean weekly running distances at any time point.
Figure 4.
Mean distance traveled per 24-h period, averaged over each week of running wheel access (km, ±SEM), for the 8-week duration of Experiment no. 2, for each of the three Stress Treatment groups (n = 8/group). While individual variation in activity levels was evident, overall group differences were not reliable.
Organ weights
Mean wet organ weights (corrected for body weight, + SEM) for all groups are presented in Table II. Two-factor ANOVAs revealed no effect of Stress Treatment on mean adrenal or thymus weights. In contrast to Experiment no. 1, however, a significant effect of Activity Status on mean adrenal (F(1, 42) = 8.51, p = 0.006) and thymus (F(1, 42) = 4.63, p = 0.037) weights was found. As a function of body weight, adrenals weighed more and the thymus weighed less (Scheffé, p < 0.05) in Ex as compared to Sed rats. No Stress Treatment × Activity Status effects were observed.
Table II.
Mean adrenal and thymus weights (g per 100 g body weight, ±SEM) for rats from Experiment no. 2 that were exercising (Ex) or sedentary (Sed) for 6 weeks prior to 11 consecutive daily 30 min presentations of 98-dB noise stress, 60-dB background noise, or 10 days of background noise followed by one acute 98-dB noise stress presentation on Day 11 of testing. Only a significant main effect of Activity Status on mean adrenal and thymus weights was found such that, as a function of body weight, adrenals weighed more and the thymus weighed less in Ex as compared to Sed rats (p < 0.05).
| Treatment group
(n = 8/group for each tissue analyzed) |
Mean adrenal weight
(g/100 g body weight (SEM)) |
Mean thymus weight
(g/100 g body weight (SEM)) |
|---|---|---|
| Ex/Acute noise | 0.0138 (0.0004) | 0.0899 (0.0033) |
| Ex/Repeated noise | 0.0145 (0.0011) | 0.0795 (0.0051) |
| Ex/No noise | 0.0143 (0.0004) | 0.0839 (0.0082) |
| Sed/Acute noise | 0.0124 (0.0005) | 0.0933 (0.0059) |
| Sed/Repeated noise | 0.0129 (0.0006) | 0.0981 (0.0077) |
| Sed/No noise | 0.0128 (0.0004) | 0.0939 (0.0047) |
Activity levels and organ weights
To further examine the effect of Activity Status on adrenal and thymus weights, a series of simple regression analyses were performed to determine the possibility of a relationship between activity levels and organ weights (actual weights, not corrected for body weight) in all Ex animals. The mean daily distances traveled during week 8 (at the end of which organs were collected) were found to be reliable predictors of both adrenal (R2 = 0.2297, p = 0.018) and thymus (R2 = 0.2969, p = 0.006) weights, with tendencies toward increased adrenal weights and decreased thymus weights with increasing activity levels. The total distance traveled at the end of week 8 was similarly found to be a reliable predictor of thymus weights (R2 = 0.2089, p = 0.025), with thymus weights tending to decrease with increasing distances traveled.
HPA-axis response
Corticosterone
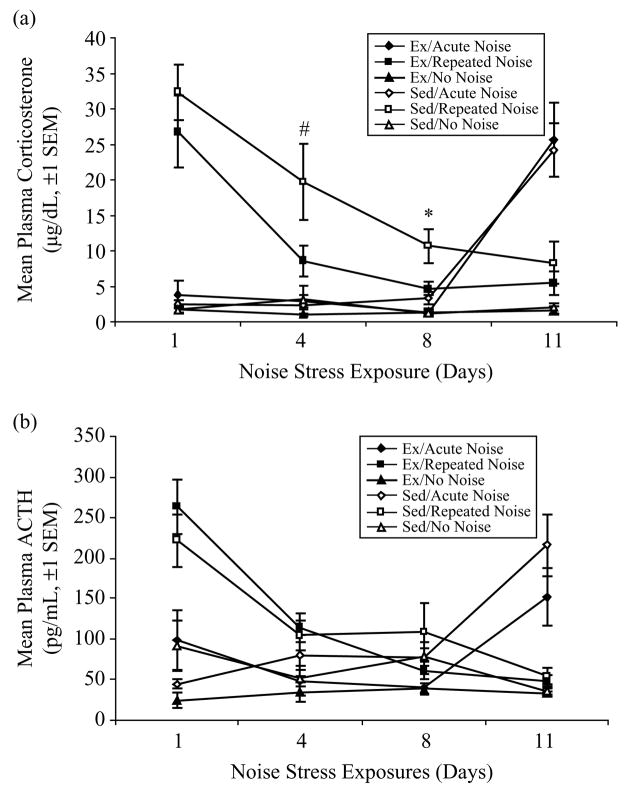
Mean plasma corticosterone concentrations (±SEM) are presented in Figure 5a. A repeated measures ANOVA [4 sampling Days = within-subjects; Activity Status and Stress Treatment (Acute, Repeated, or No Noise) = between-subjects] revealed significant Days (F(3, 111) = 18.25, p < 0.0001), Activity Status (F(1, 37) = 4.38, p = 0.043), Stress Treatment (F(2, 37) = 39.42, p < 0.0001), Days × Stress Treatment (F(6, 111) = 40.34, p < 0.0001), and Activity Status × Stress Treatment (F(2, 37) = 3.79, p = 0.032) effects on mean corticosterone responses. When controlling for Activity Status, the two Repeated Noise groups were exposed to their first, acute noise stress presentation on Day 1 and responded with a significant increase in plasma corticosterone compared to the Acute Noise groups (that received no noise on this day) and No Noise controls (Scheffé, p < 0.05), which did not differ from one another. A separate univariate ANOVA for only the Repeated Noise groups on Day 1 (their first noise stress exposure) showed no effect of Activity Status on corticosterone responses. Similarly, on Day 11, the two Acute Noise groups were exposed to their first, acute presentation of the noise stress. Mean plasma corticosterone responses were greatly elevated in these rats compared to No Noise controls and the habituated, Repeated Noise rats (Scheffé, p < 0.05), which did not differ from one another. A univariate ANOVA for only the Acute Noise groups on Day 11 again showed no effect of Activity Status on corticosterone responses. As in Experiment no. 1, there were no differences in corticosterone concentrations between Ex and Sed No Noise controls on any of the 4 sampling days. Similarly, no differences were observed between Ex and Sed Acute Noise groups on their first, fourth, or eighth exposure to the 60-dB background noise. This again implies no effect of 6 weeks of voluntary wheel running on basal HPA-axis tone at the trough of the circadian cycle.
Figure 5.
(a) Mean plasma corticosterone responses (μg/dL, ±SEM) in exercised (Ex) and sedentary (Sed) rats exposed to 11 consecutive days of 30-min 98-dB noise stress, 60-dB background noise, or 10 days of background noise exposures followed by an acute 98-dB noise stress presentation on Day 11 in Experiment no. 2 (n = 8/group except for Ex/Acute Noise and Ex/No Noise groups on Day 11, and Sed/Repeated Noise on Days 4 and 8, where n = 7 due to missing data). Asterisk indicates p ≤ 0.05 and 5# indicates p ≤ 0.10 between Ex and Sed Repeated Noise groups only. (b) Mean plasma ACTH responses (pg/mL, ±SEM) for each of the 6 experimental groups (n = 8/group except for Sed/Repeated Noise on Day 1, where n = 7 due to missing data). No reliable differences were observed between Ex and Sed Repeated Noise groups on any of the 4 sampling days.
To further investigate the Stress Treatment × Activity Status interaction effect, additional univariate ANOVAs were performed for Days 4, 8, and 11, for only those rats in the Repeated Noise groups. As indicated in Figure 5a, the effect of Activity Status on corticosterone responses was nearly significant on Day 4 (F(1, 13) = 4.08, p = 0.064) and was statistically significant on Day 8 (F(1, 13) = 6.26, p = 0.026), with Ex rats having lower responses on both days as compared to Sed controls. In contrast to Experiment no. 1, there were no reliable differences in corticosterone responses between Ex and Sed Repeated Noise groups on Day 11, suggesting that 6 weeks of voluntary wheel running facilitated the rate, but not the ultimate extent, of corticosterone response habituation to repeated loud noise stress in comparison to Sed rats.
ACTH
Figure 5b depicts mean plasma ACTH concentrations (±SEM) in Ex and Sed rats. A repeated measures ANOVA [4 sampling Days = within-subjects; Activity Status (Ex or Sed) and Stress Treatment (Acute, Repeated, or No Noise) = between-subjects] revealed significant Days (F(3, 123) = 9.85, p < 0.0001), Stress Treatment (F(2, 41) = 16.29, p < 0.0001), and Days × Stress Treatment (F(6, 123) = 20.45, p < 0.0001) effects on mean plasma ACTH responses. There was a near significant Days × Activity Status × Stress Treatment effect (F(6, 123) = 2.13, p = 0.055). The ACTH responses in both Ex and Sed Repeated Noise groups did habituate with repeated noise stress exposures, however, no effect of Activity Status was found on the rate or extent of habituation. Further, Ex and Sed rats responded with equivalent ACTH concentrations to the acute noise stress presentations (Day 1 for Ex and Sed/Repeated Noise groups and Day 11 for Ex and Sed/Acute Noise groups).
Activity levels and HPA-axis responsiveness
To investigate potential relationships between activity levels both prior to and during the testing phase and HPA-axis responsiveness, a series of simple regression analyses were performed. The Day 1 (acute) corticosterone responses in the Ex/Repeated Noise group could not be predicted by the total distance run at the time of the first stress exposure (through week 6, R2 = 0.0003) or by the mean 24-h distance run in week 6 (R2 = 0.0217), the week just before noise stress presentations commenced. Similarly, the Day 11 (acute) corticosterone responses in the Ex/Acute Noise group could not be predicted by the total distance run over the full 8 weeks of the experiment (R2 = 0.0585) or by the mean 24-h distance run in week 8 (R2 = 0.07), the week in which these rats received their first noise stress presentation. No relationship was observed between the total distance traveled at the time of stress exposure onset (through week 6) and the rate of habituation in the Ex/Repeated Noise group, whether defined as the difference in corticosterone responses between Days 1 and 4 (R2 = 0.017) or between Days 1 and 8 (R2 = 0.0065). Further, no relationship was found between the overall distance run at the end of week 8 and the ultimate extent of habituation, defined as the difference between the Day 1 and Day 11 corticosterone responses (R2 = 0.033).
Discussion
The results of these experiments provide evidence that voluntary physical exercise facilitates habituation of HPA-axis responses to repeated stress presentations. Specifically, 6 weeks of voluntary wheel running was found to facilitate corticosterone response habituation to repeated loud noise stress presentations in male Sprague-Dawley rats. In Experiment no. 1, both the exercised and sedentary Repeated Noise groups demonstrated corticosterone response habituation with repeated stressor exposures. Interestingly, on Days 4, 8, and 11, exercised rats had significantly lower corticosterone responses than sedentary controls, suggesting that the rate and ultimate extent of habituation was enhanced in these rats. One potential explanation for this effect was that access to running wheels during each of the 11 noise stress presentations may have provided active rats with a distraction or a coping mechanism not available to sedentary rats that could contribute to the differences observed in corticosterone responses between groups. To eliminate this as a possible mechanism, all running wheels were locked during each of the 30-min stress (or no stress) exposures in Experiment no. 2. Under these modified conditions, exercised rats still demonstrated lower corticosterone responses on Days 4 (near statistical significance on Day 4; p = 0.064) and 8 as compared to sedentary, Repeated Noise controls. Thus, consistent with Experiment no. 1, these results suggest that the rate of corticosterone response habituation was facilitated in exercised rats, and further, that this effect was not due to differential coping abilities between groups during the noise stress exposures.
Of note, plasma ACTH concentrations did not differ reliably between the repeatedly stressed exercised and sedentary rats, as measured in the second experiment. Taken together with the corticosterone response habituation results, this may suggest a putative regulatory mechanism at the level of the adrenal gland, similar to that suggested by the results of a recent study in acutely stressed rats (Droste et al. 2007). We plan to investigate the possibility that voluntary exercise may regulate adrenal sensitivity to ACTH by testing the effects of exogenous ACTH injections on plasma corticosterone concentrations in exercised and sedentary, stressed and non-stressed rats. However, while overall glucocorticoid regulation could certainly be an important target in the beneficial effects of voluntary exercise, the above results do not limit putative regulation to peripheral mechanisms, as central control of adrenal sensitivity to ACTH is beginning to be defined (Ulrich-Lai et al. 2006). Interpretations of the ACTH results obtained presently are made cautiously, however, as it will be important first to determine if the dynamics of corticosterone and/or ACTH release and recovery differ between exercised and sedentary rats by sampling blood more frequently during and after stressor exposures, as this could be another explanation for the disparity between the effects of voluntary exercise on corticosterone and ACTH response habituation. Similarly, it should be reiterated that all blood samples in the present experiments were obtained 30 min after noise stress onset. While it has been previously demonstrated by Patz et al. (2006) that acute audiogenic (105 dB) stress-induced increases in ACTH and corticosterone plasma concentrations peak at or near this time-point, it remains unclear whether manipulations such as voluntary exercise or repeated stressor exposure can modify the release and recovery dynamics of either or both of these hormones. Future studies employing more frequent blood sampling both within and between repeated stressor exposures will therefore provide a more accurate assessment of the effects of these manipulations, if any, on ACTH response habituation, which simply may have been missed under the present experimental conditions.
No differences were observed in plasma corticosterone or ACTH concentrations on any of the 4 sampling days between exercised and sedentary No Noise controls, which suggests little effect of 6 weeks of voluntary wheel running on basal HPA-axis tone at the trough of the circadian cycle. This is consistent with previous findings in which 4 weeks of wheel running had no effect on corticosterone concentrations at either the trough or the peak of the circadian cycle in male Sprague-Dawley rats (Fediuc et al. 2006), but in contrast to other studies reporting increased basal corticosterone concentrations with respect to sedentary controls after 3 weeks of wheel access in male Lewis rats at the trough (Makatsori et al. 2003) and C57BL/6 mice at the peak (Droste et al. 2003) of their circadian cycles. Thus it appears that the duration of running wheel access, rat strain, species type, and phase of the circadian cycle in which hormone concentrations are measured are all important contributors to basal HPA-axis tone, and should therefore be carefully considered when comparing physically active and more sedentary animals on this particular measure.
The results of both experiments showed little effect of 6 weeks of voluntary wheel running on the amount of corticosterone released in response to an acute noise stress exposure during the trough of the circadian cycle. This replicates previous work in rats showing no effect of physical activity on corticosterone responses to acute cage switch (Dishman et al. 1998), tail shock (Fleshner 2000), or restraint (Fediuc et al. 2006) stress presentations. Figures 2 and 5a show robust yet equivalent corticosterone responses in exercised and sedentary Repeated Noise groups to their first loud noise exposure. Figure 5a similarly shows large but equivalent corticosterone responses in the exercised and sedentary Acute Noise groups to their first presentation of the noise stress (Day 11) in Experiment no. 2. These results imply that HPA-axis responsiveness to the experience of a relatively strong and novel stressor is not reduced by voluntary exercise, which is important in the sense that appropriate responses to challenging or threatening situations can be critical for survival. Interestingly, there is recent evidence that 6 weeks of voluntary wheel running attenuates the corticosterone response 30 minutes following a single intraperitoneal (i.p.) 0.9% saline injection in male Sprague-Dawley rats in comparison to sedentary controls (Day et al. 2006), suggesting that voluntary exercise may reduce HPA-axis responsiveness to acute exposure to a relatively mild stressor. Indeed, this same study found no difference in corticosterone responses between exercised and sedentary rats following a single 30-min, moderate-intensity restraint stress exposure. Thus the effect of voluntary exercise on HPA-axis responsiveness to acute stressor exposure may be quite sensitive to the intensity of the stressor. Similarly, a recent study by Droste et al. (2007) suggests a distinction in the effects of voluntary exercise on HPA-axis responsiveness to mild, psychologically stressful stimuli (novelty) and stronger, more physically demanding stressors (forced swimming).
Free access to running wheels resulted in a significant reduction in the rate of body weight gain over time in comparison to sedentary controls. This divergence in body weight was apparent by the end of the first week of wheel access and remained for the duration of the experiments, which is similar to previous findings in Sprague-Dawley rats (Looy and Eikelboom 1989; Afonso and Eikelboom 2003; Greenwood et al. 2003a,b; Fediuc et al. 2006) but not mice (Harri et al. 1999; Droste et al. 2003).
The running behaviors observed in the present experiments are consistent with previous characterizations of male Sprague-Dawley rats (Looy and Eikelboom 1989; Afonso and Eikelboom 2003; Greenwood et al. 2003a,b; Fediuc et al. 2006), in that rats given unlimited access to running wheels voluntarily ran between 1–5 km/24 h period, almost entirely during the dark phase of the light cycle. Exercised rats were not initially matched for running activity when assigned to Stress Treatment groups. However, average 24-h distances were calculated for each week for each Stress Treatment group and subsequent analyses suggested that although individual variation in activity levels was present, overall group differences were not reliable. Further, no significant relationships were observed between running distances and any measure of HPA-axis responsiveness analyzed. Total and mean daily distances at 6 weeks (just prior to the onset of stress presentations) and 8 weeks (at the end of stress presentations) did not reliably predict acute or habituated corticosterone responses to the audiogenic stress presentations, nor the rate or ultimate extent of the response habituation. Similarly, Greenwood et al. (2003a) have reported that the protective effects of 6 weeks of wheel running on the acute stress-induced expression of learned helplessness behaviors were not correlated with activity levels. Interestingly, however, the duration of access to running wheels was found to be important (Greenwood et al. 2005), that is, 6 but not 3 weeks of voluntary wheel running was sufficient to prevent the behavioral consequences of uncontrollable stress. This may indicate that meeting some minimum threshold amount of activity is required in order to reap some of the protective benefits of physical exercise (Greenwood et al. 2003a; 2005).
It is quite possible that any amount of physical activity may be better than none at all, and, as previously suggested (Fleshner 2005), what is really being demonstrated here may be an inhibitory effect of a prolonged, isolated, and sedentary lifestyle on the ability of the HPA-axis to adapt to repeated stress rather than a facilitation of adaptation due to exercise training per se. Subsequent investigations will address these possibilities through the inclusion of group-housed animals and/or animals housed in enriched environments (e.g. with access to toys, nesting materials in their home cages). How the HPA-axis responds and adapts to repeated stress presentations in these groups as compared to voluntarily exercised and individually housed, sedentary animals will provide important insight regarding these issues.
It has been argued that exercise training in and of itself is stressful and therefore caution is advised when interpreting behavioral or endocrine response adaptations (or the lack thereof) to subsequent challenges or stressors, as they could be masked or altered by the chronic stress inherent in the training (Moraska et al. 2000; Burghardt et al. 2004). Indeed, certain forced exercise training paradigms in controlled animal studies have resulted in physiological adaptations, including adrenal hypertrophy and/or thymic involution, that are typically associated with chronic activation of the HPA-axis system (Selye 1936). For example, 2 and 4 weeks of treadmill training in male Wistar rats (Kawashima et al. 2004) and 8 weeks of treadmill training in male Sprague-Dawley rats (Moraska et al. 2000) resulted in significantly increased adrenal and decreased thymus weights in comparison to sedentary controls.
Whether voluntary exercise is inherently stressful remains less clear. There is little evidence that voluntary wheel running is perceived as stressful by the rat. Lett et al. (2000) have reported, for example, that voluntary wheel running is an inherently rewarding behavior leading to a conditioned place preference in Sprague-Dawley rats. In a subsequent study (Lett et al. 2001), this same group showed that naloxone attenuated the conditioned place preference induced by wheel running, implying that the rewarding effect of wheel running is mediated by endogenous opioids. Pierce et al. (1986) have reported that rats will bar press vigorously to gain even brief access to running wheels.
It is less apparent if voluntary exercise manifests itself physiologically as stressful. One group (Griesbach et al. 2007) recently compared plasma corticosterone concentrations before and at 1, 7, and 14 days after commencement of daily 20 min sessions of forced (motorized) wheel running or during the active period of voluntary (unlimited access) wheel running, and reported that, except for baseline values (before commencement of exercise), corticosterone concentrations were significantly elevated in the forced exercise group compared to the voluntary exercise group at all subsequent time-points. Further, although an increase in corticosterone was observed after the first day of voluntary wheel running, it was not significantly higher than baseline (pre-exercise) levels, indicating that even acute exposure to voluntary wheel running did not elicit a marked HPA-axis response.
If voluntary wheel running does elevate corticosterone concentrations, it is also entirely possible that this response would habituate with repeated running events. After 6 weeks of running wheel access in the present series of experiments, there was no evidence that voluntarily exercised rats had increased basal levels of corticosterone, as the exercised and sedentary No Noise groups were never observed to differ reliably on this measure. Even if there is a corticosterone response evoked by voluntary exercise that habituates with repeated experiences, this would be expected to have little effect on HPA-axis responsiveness to a subsequent noise stress presentation, as habituation to repeated stress has been demonstrated to be stressor specific (Kant et al. 1985; Armario et al. 1986).
With regard to physiological adaptations at the level of chronic stress-responsive tissues, Fediuc et al. (2006) did not observe any increase in adrenal mass following 5 weeks of voluntary wheel running in male Sprague-Dawley rats relative to sedentary controls. In the present experiments, the adrenals and thymus were removed and weighed immediately after the rats were killed on the eleventh and last day of noise or no noise exposures. Overall, eight weeks of voluntary wheel running did not affect mean adrenal or thymus weights in Experiment no. 1, but an effect of Activity Status was found in Experiment no. 2, with reliably increased adrenal and decreased thymus weights in exercised as compared to sedentary rats. A series of simple regression analyses on data collected in the second experiment showed that adrenal and thymus weights were related to the mean daily distances traveled during the week these organs were collected (week 8), with tendencies for adrenal weights to increase and thymus weights to decrease with increasing activity levels. It is somewhat surprising that with or without the presence of what are typically considered as chronic stress-induced physiological adaptations, the exercised rats still displayed a facilitated rate of corticosterone response habituation compared to sedentary controls. These results imply that these particular indices of what is often referred to as a “chronic stress state” may be relatively independent of, or at least poor predictors of, stress-induced HPA-axis responsiveness.
Due to potential idiosyncrasies between audiogenic stress and glucocorticoid responses, future studies will investigate whether exercise-induced facilitation of HPA-axis habituation will generalize across stressor modalities, such as repeated restraint and/or predator odor exposures. Along these lines, other stress response systems, such as the sympathetic-adrenomedullary response, as measured by catecholamine levels in the blood, have been shown to habituate to repeated stress presentations (Dobrakovova et al. 1993). Thus it is plausible that exercise-induced facilitation of stress response adaptation generalizes across stress response systems as well as stressor modalities. In fact, Greenwood et al. (2003) reported that 6 weeks of voluntary wheel running produced adaptations in acute stress-induced activation of the sympathetic system. A more detailed characterization of the exercise training paradigm required to produce the observed effects on HPA-axis response habituation, including the exercise apparatus (running wheel, treadmill), the scheduling of apparatus access (daily, alternating days, once weekly), and the training duration (longer or shorter than 6 weeks), is also needed. Experiments on training duration requirements can also serve to analyze the extent to which the observed effects of voluntary exercise on stress response adaptation generalize across the rat life span. Rats reach sexual maturity between 40–60 days of age (Quinn 2005). Rats in the present experiments were approximately 65 days old and are thus considered young adults upon gaining access to running wheels. In the light of reports that early (Meaney et al. 1996) and pubertal (Romeo et al. 2006) life events can affect HPA-axis tone, responsiveness, and plasticity in adulthood, it will be important to determine whether introducing rats to running wheels pre-pubertally or in old age will similarly facilitate HPA-axis response habituation to repeated stressor exposures. Detailed analyses of brain and pituitary tissues collected in the present set of experiments will be necessary in order to begin to elucidate the neurocircuitry and neurochemical mechanisms underlying the observed effect of voluntary physical exercise on HPA-axis response habituation.
In conclusion, the present studies provide evidence that 6 weeks of voluntary wheel running facilitates the adaptive process of HPA-axis response habituation to repeated loud noise stress presentations in male Sprague-Dawley rats, without affecting responsiveness to acute stressor exposure. In light of the finding that consistently elevated levels of circulating glucocorticoids have been specifically indicated in the mechanism by which stress negatively affects physical and/or psychological health, these results suggest that voluntary physical exercise might buffer some of the more damaging effects of stress by enhancing HPA-axis response habituation to situations of repeated or prolonged stress exposures, ultimately reducing the amount of glucocorticoids the body and brain are exposed to.
Acknowledgments
This work was supported by a grant from the National Institutes of Health R03 NS054358. We would like to thank Dr Robert Spencer for his constructive comments on earlier versions of this manuscript.
Abbreviations
- ACTH
adrenocorticotropin hormone
- HPA
hypothalamo-pituitary–adrenocortical axis
- Sed
sedentary
- Ex
exercised
References
- Afonso VM, Eikelboom R. Relationship between wheel running, feeding, drinking, and body weight in male rats. Physiol Behav. 2003;80:19–26. doi: 10.1016/s0031-9384(03)00216-6. [DOI] [PubMed] [Google Scholar]
- Akil H, Morano M. Stress. In: Bloom F, Kupfer D, editors. Psychopharmacology: The fourth generation of progress. New York, NY: Raven Press; 1995. pp. 773–785. [Google Scholar]
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- Armario A, Lopez-Calderon A, Jolin T, Balasch J. Response of anterior pituitary hormones to chronic stress. The specificity of adaptation. Neurosci Biobehav R. 1986;10(3):245–250. doi: 10.1016/0149-7634(86)90011-4. [DOI] [PubMed] [Google Scholar]
- Brown JD, Lawton M. Stress and well-being in adolescence: The moderating role of physical exercise. J hum stress. 1986;12:125–131. doi: 10.1080/0097840X.1986.9936777. [DOI] [PubMed] [Google Scholar]
- Burghardt PR, Fulk LJ, Hand GA, Wilson MA. The effects of chronic treadmill and wheel running on behavior in rats. Brain Res. 2004;1019(12):84–96. doi: 10.1016/j.brainres.2004.05.086. [DOI] [PubMed] [Google Scholar]
- Burow A, Day HE, Campeau S. A detailed characterization of loud noise stress: Intensity analysis of hypothalamo-pituitary–adrenocortical axis and brain activation. Brain Res. 2005;1062(12):63–73. doi: 10.1016/j.brainres.2005.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau S, Dolan D, Akil H, Watson SJ. c-fos mRNA induction in acute and chronic audiogenic stress: Possible role of the orbitofrontal cortex in habituation. Stress. 2002;5(2):121–130. doi: 10.1080/10253890290027895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodzko-Zajko WJ, Moore KA. Physical fitness and cognitive functioning in aging. Exerc Sport Sci Rev. 1994;22:195–220. [PubMed] [Google Scholar]
- Day HE, Wolf EM, Herlihy L, Campeau S. Program No. 563.20.2006 Neuroscience Meeting Planner. Atlanta GA: Society for Neuroscience; 2006. The effect of voluntary exercise on the acute HPA axis response to mild stress in rats. [Google Scholar]
- Dishman RK, Warren JM, Youngstedt SD, Yoo H, Bunnell BN, Mougey EH, Meyerhoff JL, Jaso-Friedmann L, Evans JS. Activity wheel running attenuates suppression of natural killer cell activity after footshock. J Appl Physiol. 1995;78(4):1547–1554. doi: 10.1152/jappl.1995.78.4.1547. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Bunnell BN, Youngstedt SD, Yoo HS, Mougey EH, Meyerhoff JL. Activity wheel running blunts increased plasma adrenocorticotropin hormone (ACTH) after footshock and cage-switch stress. Physiol Behav. 1998;63(5):911–917. doi: 10.1016/s0031-9384(98)00017-1. [DOI] [PubMed] [Google Scholar]
- Dobrakovova M, Kvetnansky R, Oprsalova Z, Jezova D. Specificity of the effect of repeated handling on sympathetic-adrenomedullary and pituitary-adrenocortical activity in rats. Psychoneuroendocrinology. 1993;18(3):163–174. doi: 10.1016/0306-4530(93)90001-2. [DOI] [PubMed] [Google Scholar]
- Droste SK, Gesing A, Ulbricht S, Muller MB, Linthorst ACE, Reul JMHM. Effects of long-term voluntary exercise on the mouse hypothalamic–pituitary–adrenocortical axis. Endocrinology. 2003;144(7):3012–3023. doi: 10.1210/en.2003-0097. [DOI] [PubMed] [Google Scholar]
- Droste SK, Chandramohan Y, Hill LE, Linthorst AC, Reul JM. Voluntary exercise impacts on the rat hypothalamic–pituitary–adrenocortical axis mainly at the adrenal level. Neuroendocrinology. 2007;86(1):26–37. doi: 10.1159/000104770. [DOI] [PubMed] [Google Scholar]
- Dunn AL, Trivedi MH, O’Neal HA. Physical activity dose-response effects on outcomes of depression and anxiety. Med Sci Sport Exerc. 2001;33:587–597. doi: 10.1097/00005768-200106001-00027. [DOI] [PubMed] [Google Scholar]
- Fediuc S, Campbell JE, Riddell MC. Effect of voluntary wheel running on circadian corticosterone release and on HPA axis responsiveness to restraint stress in Sprague-Dawley rats. J Appl Physiol. 2006;100:1867–1875. doi: 10.1152/japplphysiol.01416.2005. [DOI] [PubMed] [Google Scholar]
- Fleshner M. Exercise and neuroendocrine regulation of antibody production: Protective effects of physical activity on stress-induced suppression of the specific antibody response. Int J Sports Med. 2000;21:S14–S19. doi: 10.1055/s-2000-1454. [DOI] [PubMed] [Google Scholar]
- Fleshner M. Physical activity and stress resistance: Sympathetic nervous system adaptations prevent stress-induced immunosuppression. Exerc Sport Sci Rev. 2005;33(3):120–126. doi: 10.1097/00003677-200507000-00004. [DOI] [PubMed] [Google Scholar]
- Fox KR. The influence of physical activity on mental well-being. Public Health Nutr. 1999;2(3A):411–418. doi: 10.1017/s1368980099000567. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Day HEW, Campisi J, Hammack SH, Campeau S, Maier SF, Fleshner M. Freewheel running prevents learned helplessness/behavioral depression: Role of dorsal raphe serotonergic neurons. J Neurosci. 2003a;23(7):2889–2898. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Kennedy S, Smith TP, Campeau S, Day HEW, Fleshner M. Voluntary freewheel running selectively modulates catecholamine content in peripheral tissue and c-fos expression in the central sympathetic circuit following exposure to uncontrollable stress in rats. Neuroscience. 2003b;120:269–281. doi: 10.1016/s0306-4522(03)00047-2. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Burhans D, Maier SF, Fleshner M. The consequences of uncontrollable stress are sensitive to duration of prior wheel running. Brain Res. 2005;1033:164–178. doi: 10.1016/j.brainres.2004.11.037. [DOI] [PubMed] [Google Scholar]
- Griesbach GS, Taylor AN, Tio DL, Hovda DA. Program No. 522.25. Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience; 2007. Increases in corticosterone following voluntary or forced exercise. [Google Scholar]
- Hammen C, Davila J, Brown G, Ellicott A, Gitlin M. Psychiatric history and stress: Predictors of severity of unipolar depression. J Abnormal Psychol. 1992;101:45–52. doi: 10.1037//0021-843x.101.1.45. [DOI] [PubMed] [Google Scholar]
- Harri M, Lindbloom J, Malinen H, Lapvetelainen T, Eskola S, Helminen HJ. Effect of access to a running wheel on behavior of C57BL/6J mice. Lab Anim Sci. 1999;49:401–405. [PubMed] [Google Scholar]
- Kant GJ, Eggleston T, Landman-Roberts L, Kenion CC, Driver GC, Meyerhoff JL. Habituation to repeated stress is stressor specific. Pharmacol Biochem Behav. 1985;22(4):631–634. doi: 10.1016/0091-3057(85)90286-2. [DOI] [PubMed] [Google Scholar]
- Kawashima H, Saito T, Yoshizato H, Fujikawa T, Sato Y, McEwen BS, Soya H. Endurance treadmill training in rats alters CRH activity in the hypothalamic paraventricular nucleus at rest and during acute running according to its period. Life Sci. 2004;76:763–774. doi: 10.1016/j.lfs.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Kruk J, Aboul-Enein HY. Physical activity in the prevention of cancer. Asian Pac J Cancer Prev. 2006;7(1):11–21. [PubMed] [Google Scholar]
- Lester RS, Knowles SR, Shear NH. The risks of systemic corticosteroid use. Dermatol Clin. 1998;16(2):277–287. doi: 10.1016/s0733-8635(05)70010-3. [DOI] [PubMed] [Google Scholar]
- Lett BT, Grant VL, Byrne MJ, Koh MT. Pairings of a distinctive chamber with the aftereffect of wheel running produce conditioned place preference. Appetite. 2000;34:87–94. doi: 10.1006/appe.1999.0274. [DOI] [PubMed] [Google Scholar]
- Lett BT, Grant VL, Koh MT. Naloxone attenuates the conditioned place preference induced by wheel running in rats. Physiol Behav. 2001;72:355–358. doi: 10.1016/s0031-9384(00)00427-3. [DOI] [PubMed] [Google Scholar]
- Looy H, Eikelboom R. Wheel running, food intake, and body weight in male rats. Physiol Behav. 1989;45:403–405. doi: 10.1016/0031-9384(89)90147-9. [DOI] [PubMed] [Google Scholar]
- Makatsori A, Duncko R, Schwendt M, Moncek F, Johansson BB, Jezova D. Voluntary wheel running modulates glutamate receptor subunit gene expression and stress hormone release in Lewis rats. Psychoneuroendocrinology. 2003;28:702–714. doi: 10.1016/s0306-4530(02)00062-8. [DOI] [PubMed] [Google Scholar]
- Manson JE, Nathan DM, Krolewski AS, Stampfer MJ, Willett WC, Hennekens CH. A prospective study of exercise and incidence of diabetes among US male physicians. J Am Med Assoc. 1992;268:63–67. [PubMed] [Google Scholar]
- McEwen BS. Allostasis and allostatic load: Implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22:108–124. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Diorio J, Francis D, Widdowson J, LaPlante P, Caldji C, Sharma S, Seckl JR, Plotsky PM. Early environmental regulation of forebrain glucocorticoid receptor gene expression: Implications for adrenocortical responses to stress. Dev Neurosci. 1996;18(12):49–72. doi: 10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- Moraska A, Fleshner M. Voluntary physical activity prevents stress-induced behavioral depression and anti-KLH antibody suppression. Am J Physiol-Reg I. 2001;281:R484–R489. doi: 10.1152/ajpregu.2001.281.2.R484. [DOI] [PubMed] [Google Scholar]
- Moraska A, Deak T, Spencer RL, Roth D, Fleshner M. Treadmill running produces both positive and negative physiological adaptations in Sprague-Dawley rats. Am J Physiol-Reg I. 2000;279:R1321–R1329. doi: 10.1152/ajpregu.2000.279.4.R1321. [DOI] [PubMed] [Google Scholar]
- Nelson RJ. Stress. In: Farley P, editor. An introduction to behavioral endocrinology. 2. Inc/Sunderland, MA: Sinauer Associates; 2000. pp. 557–591. [Google Scholar]
- Oguma Y, Shinoda-Tagawa T. Physical activity decreases cardiovascular disease risk in women: Review and meta-analysis. Am J Prev Med. 2004;26:407–418. doi: 10.1016/j.amepre.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Patz MD, Day HEW, Burow A, Campeau S. Modulation of the hypothalamo-pituitary–adrenocortical axis by caffeine. Psychoneuroendocrinology. 2006;31(4):493–500. doi: 10.1016/j.psyneuen.2005.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce WD, Epling WF, Boer DP. Deprivation and satiation: The interrelations between food and wheel running. J Exp Anal Behav. 1986;46:199–210. doi: 10.1901/jeab.1986.46-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn R. Comparing rat’s to human’s age: How old is my rat in people years? Nutrition. 2005;21:775–777. doi: 10.1016/j.nut.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Bellani R, Karatsoreos IN, Chhua N, Vernov M, Conrad CD, McEwen BS. Stress history and pubertal development interact to shape hypothalamic–pituitary–adrenal axis plasticity. Endocrinology. 2006;147(4):1664–1674. doi: 10.1210/en.2005-1432. [DOI] [PubMed] [Google Scholar]
- Roth DL, Holmes DS. Influence of physical fitness in determining the impact of stressful life events on physical and psychological health. Psychosom Med. 1985;47:164–173. doi: 10.1097/00006842-198503000-00008. [DOI] [PubMed] [Google Scholar]
- Salmon P. Effects of physical exercise on anxiety, depression, and sensitivity to stress: A unifying theory. Clin Psychol Rev. 2001;21:33–61. doi: 10.1016/s0272-7358(99)00032-x. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Neuroendocrinology of the stress-response. In: Becker JB, Breedlove SM, Crews D, editors. Behavioral endocrinology. Cambridge, MA: MIT Press; 1992. pp. 287–324. [Google Scholar]
- Sapolsky RM. Why stress is bad for your brain. Science. 1996;273:749–750. doi: 10.1126/science.273.5276.749. [DOI] [PubMed] [Google Scholar]
- Selye H. A syndrome produced by diverse nocuous agents. Nature. 1936;138:32. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- Shea B, Bonaiuti D, Iovine R, Negrini S, Robinson V, Kemper HC, Wells G, Tugwell P, Cranney A. Cochrane review on exercise for preventing and treating osteoporosis in postmenopausal women. Eura Medicophys. 2004;40(3):199–209. [PubMed] [Google Scholar]
- Sothmann MS, Gustafson AB, Garthwaite TL, Horn TS, Hart BA. Cardiovascular fitness and selected adrenal hormone responses to cognitive stress. Endocr Res. 1988;14(1):59–69. doi: 10.3109/07435808809036340. [DOI] [PubMed] [Google Scholar]
- Spencer RL, McEwen BS. Adaptation of the hypothalamic–pituitary–adrenal axis to chronic ethanol stress. Neuroendocrinology. 1990;52(5):481–489. doi: 10.1159/000125632. [DOI] [PubMed] [Google Scholar]
- Stratakis CA, Chrousos GP. Neuroendocrinology and pathophysiology of the stress system. Ann NY Acad Sci. 1995;771:1–18. doi: 10.1111/j.1749-6632.1995.tb44666.x. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Bao AM, Lucassen PJ. The stress system and the human brain in depression and neurodegeneration. Ageing Res Rev. 2005;4(2):141–194. doi: 10.1016/j.arr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Truhan AP, Ahmed AR. Corticosteroids: A review with emphasis on complications of prolonged systemic therapy. Ann Allergy. 1989;62(5):375–390. [PubMed] [Google Scholar]
- Ulrich-Lai YM, Arnhold MM, Engeland WC. Adrenal splanchnic innervation contributes to the diurnal rhythm of plasma corticosterone in rats by modulating adrenal sensitivity to ACTH. Am J Physiol-Reg I. 2006;290:R1128–R1135. doi: 10.1152/ajpregu.00042.2003. [DOI] [PubMed] [Google Scholar]
- Vanitallie TB. Stress: A risk factor for serious illness. Metabolism. 2002;51:40–45. doi: 10.1053/meta.2002.33191. [DOI] [PubMed] [Google Scholar]
- Wannamethee SG, Shaper AG, Walker M. Changes in physical activity, mortality, and incidence of coronary heart disease in older men. Lancet. 1998;351:1603–1608. doi: 10.1016/S0140-6736(97)12355-8. [DOI] [PubMed] [Google Scholar]