Abstract
Purpose
To use Dynamic Light Scattering (DLS) to clinically assess early pre-cataractous lens protein changes.
Methods
We performed a cross sectional study in 380 eyes of 235 subjects aged 7–86 years with AREDS clinical lens nuclear grades ranging from 0–3.8. A DLS device was used to assess α-crystallin, a molecular chaperone protein shown to bind other damaged lens proteins, preventing their aggregation. The outcome measure was the α-crystallin index (ACI), a measure of unbound α-crystallin in each lens. The association of ACI with increasing nuclear opacity and aging was determined.
Results
There was a significant decrease in ACI associated with increasing grades of lens nuclear opacity (p<0.0001). There are significant losses of α-crystallin even in clinically clear lenses associated with aging (p<0.0001). The standard error of measurement was 3%.
Conclusions
DLS clinically detects loss of α-crystallin proteins even in clinically clear lenses. ACI measurements may be useful in identifying patients at high risk for developing cataract, and as an outcome variable in clinical lens studies.
Clinical Relevance
Our studies suggest that the ACI may be a useful measure of the protective α-crystallin molecular chaperone reserve present in a lens, analogous to creatinine clearance in estimating renal function reserve.
Clinical detection of pre-cataractous lens damage in clinically clear lenses and identification of patients at high risk for developing cataracts would be useful for many reasons. It can help alert a patient in advance of functional change and allow timely lifestyle adjustments to reduce cataract risk factors such as sun exposure, cigarette smoking, alcohol intake or poor control of diabetes. It could also help determine subject eligibility for clinical trials of anti cataract drugs, since studies have suggested that once lens opacities develop, it may be too late to intervene.1–12 Exposure to noxious physical agents (such as smoking, x-radiation and sunlight 2) or drug treatments may also result in increased risk of cataracts, and early detection would be important to assess lens damage and avoidance of these agents. For example, long-term studies of drugs that lower intraocular pressure have suggested a possible increased risk of nuclear opacity.13 Since the development of overt nuclear cataract can proceed slowly, a highly sensitive and quantitative means of assessing pre-cataractous changes would also be useful as an outcome variable in clinical trials for assessing treatment effects (protective or toxic) on the lens.
Dynamic Light Scattering (DLS) was developed over 30 years ago and used by numerous researchers since that time to study lens protein changes.14–22 Recently, a new Dynamic Light Scattering (DLS) device developed by Ansari, and colleagues23–29 was tested in animals under a NASA-NEI Inter Agency Agreement and was shown to detect lens protein changes much earlier than conventional optical methods. These studies were conducted using different animal models of cataract, including: cold cataract, radiation-, diabetic-, selenite- and hyperbaric oxygen cataracts.23–29 Following these findings, a clinical device was developed. The DLS probe was mounted on a movable carriage inside a Keratoscope (Keratron, Italy) with a 3-dimensional aiming system to improve repeatability.23–24, 30–32 Preliminary clinical studies with this DLS device demonstrated its safety and repeatability.31 In this paper, we describe further clinical studies using this device to assess early lens changes in a large group of normal and cataractous subjects in a cross sectional study, as well as further laboratory studies to clarify the information obtained with DLS.
Materials and Methods
The Clinical DLS System
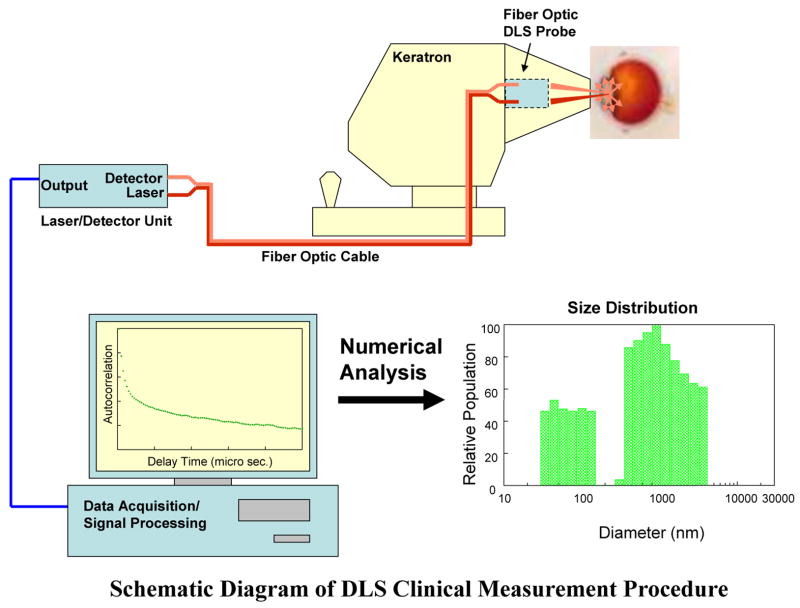
In this new clinical DLS system (Figure 1) a beam of light is directed to a specific area in the lens, and light scattered by randomly moving particles in that area is collected over a 5-second interval.31 The time autocorrelation function of the measurements is then processed to estimate a profile of intensities. Since the intensity is directly related to particle size, this provides an estimate of the frequency distribution of particle sizes. This profile is typically bimodal (see example in Figure 1): the first peak represents scattering from particles in the size range of α-crystallin proteins (see below) and the second peak represents scattering from high molecular weight particles, mainly large lens protein aggregates, cellular organelles, and membrane components.17–18, 20–21, 28
Figure 1.
Schematic Diagram of how the NASA-NEI DLS clinical device works. The DLS device 37 directs a beam of light to the lens nuclear region (see text for method of determination) and the light scattered by randomly moving particles in the lens is collected by a photo detector over a 5-second interval. The time autocorrelation function of the measurements is then processed to estimate a profile of intensities and their corresponding particle sizes, resulting in a frequency distribution of particle sizes.
Significance of α-crystallins
Results of recent studies have highlighted an important role played by α-crystallins in the lens. The α-crystallins are members of the small heat shock protein family and have been found to act as molecular chaperones that prevent the unfolding and uncontrolled aggregation of damaged lens proteins.28, 33–45 α-Crystallins have been shown to be highly efficient in recognizing, and binding to, proteins in the early stages of unfolding. Since formation of large protein aggregates in the lens causes light scatter and leads to clinical cataract, the net effect of the chaperone activity of α-crystallin is the maintenance of lens transparency. As an individual ages, there is a loss of α-crystallins, diminishing the capacity to prevent uncontrolled protein aggregation, due to the irreversible binding of damaged proteins and to the fact that the bulk of the lens lacks the capacity to synthesize new proteins. Previous biochemical analyses of lens extracts have shown that while young clear lenses have abundant unbound α-crystallins, eyes with nuclear cataracts have little or none. 34–38, 46–48 Thus, it has been proposed that lenses remain clear as long as there is available α-crystallin binding capacity. When that capacity is saturated, there is no further chaperone reserve left to prevent protein aggregation, and lens proteins aggregate in an uncontrolled manner, resulting in light scattering and opacity. Thus, a measure of the α-crystallin remaining in the lens may reflect the level of protective reserve, much as an assessment of creatinine clearance allows us to estimate the renal functional reserve.
We conducted a prospective cross-sectional study of normal subjects with clear lenses as well as patients with varying ages and grades of nuclear lens opacity using the DLS device at the National Eye Institute (NEI), approved by the NEI Institutional Review Board. All tenets of the Declaration of Helsinki on Human Subjects were followed, and all subjects gave informed consent. Subjects who had tear film disorders, corneal opacities or disorders, uveitis, or glaucoma were excluded. Subjects who were thought to be at risk for or who had a history of allergic or adverse reaction to one of the dilating or anesthetic agents used, were also excluded. No subject was excluded based on gender or race.
All subjects underwent a comprehensive dilated eye examination by one of the authors (MBD) including AREDS clinical lens nucleus grading, and lens photography 49. AREDS photographs were masked and sent for grading to the University of Wisconsin Reading Center (Madison, WI). DLS testing was performed using the clinical DLS device by one of the authors (MBD), as previously described.31 DLS measurements of the lens nucleus were repeated 2–3 times. After the first measurement, the subject disengaged from the DLS device and after several minutes the device was re-focused for a repeat measurement. The lens nucleus location was determined by performing an A-scan ultrasound measurement of each eye. The DLS probe depth (as an offset from the corneal vertex) was set to the anterior chamber depth plus one half of the lens thickness.
The DLS autocorrelation data, with masked identifiers, was sent to NASA-Glenn Research Center (Cleveland, OH) for processing and analysis. The autocorrelation curve from each measurement was analyzed using an exponential sampling method 50 (software developed at the Brookhaven National Laboratory (Upton, NY) to produce 18 separate exponential terms corresponding to 18 particle size intervals (see Figure 1). The final output of the processing is the set of 18 particle size intervals, and the scattering intensity for each particle size interval, scaled relative to the highest intensity. The output resembles a histogram of particle size, divided into low- and high-molecular-weight particles, as seen in Figure 1.
Because of the biological significance of α-crystallins as chaperone molecules, we derived the α-Crystallin Index (ACI) as our DLS main summary parameter and primary outcome variable. The ACI is the percentage of scatter from small particles (the first peak) relative to scatter from all particles, weighted by their corresponding intensities. The ACI was computed as the sum of intensities of the first six particle size intervals (corresponding to the first peak, representing mostly unbound α-crystallins) of the DLS output, divided by the sum of the intensities of all eighteen particle size intervals, expressed as a percentage (ranging from 0–100%). Other candidate indices were also explored extensively. Parameters reflecting the higher molecular weight proteins (second peak) 17–20 represent the complement of the ACI in the pre-cataractous and early stages of lens opacification. No other parameters were more highly associated with age or lens opacity than the ACI. The ACI also had the biologic characteristic of being directly associated with the unbound α-Crystallin.
We focused on lenses with clinically non significant nuclear opacities and normal visual acuity (Nuclear Grades 0–1), hence visual acuity was not a primary outcome variable.
We applied linear regression and graphical techniques to examine the association between the ACI values and lens grades and aging. The repeatability of the ACI value was estimated as the square root of the pooled within-eye variances 51, divided by the mean value of the ACI.
Laboratory Study
A parallel laboratory lens protein study was carried out on fetal calf lenses to determine which lens proteins accounted for the first and second peaks of the DLS bimodal scattering distribution. DLS measurements were obtained on: (a) purified fetal calf - crystallin, (b) a known mixture of β- and γ-crystallins, and (c) a complete mixture of soluble lens proteins. Lenses were homogenized in 0.05 M Tris buffer, pH 7.4 containing 0.1 M KCl and 0.02% NaN3. The homogenate was centrifuged at 25,000 ×g to remove the insoluble material. The α- and β, γ-crystallin fractions were then separated by gel exclusion chromatography on a Superose 6 column (Amersham Biosciences, Uppsala, Sweden). Isolated fractions were concentrated using Centriprep YM-10 concentrators (Millipore Corp., Bedford, MA) and kept at 4 degrees C until the DLS analyses were complete.
Results
Laboratory Study
To determine if the first peak in the DLS distribution could be attributed to scatter from unbound α-crystallin, we chose a fetal calf lens model to ensure that the crystallins would be in their nascent state without aging effects. Figure 2 shows the results of DLS analysis of the soluble proteins from fetal calf lenses as well as the purified α-crystallin and the mixture of β- and γ-crystallin proteins. The profile of the complete mixture of soluble proteins showed two peaks (similar to the human distributions), with the relative proportion of scattering from the first peak being particularly high. The α-crystallin protein scattered only in the first DLS peak; the mixture of β- and γ-crystallin proteins also scattered light in the first peak, as would be expected for these relatively small molecules. These in vitro measurements were made at much higher light intensities than are used clinically because the β- γ-fraction did not yield a detectable peak at the intensities used clinically (limited to <100 microwatts for patient safety). For comparison, the DLS profile for a 28 yr. old volunteer with a clear lens nucleus (N=0) is shown in the bottom panel.
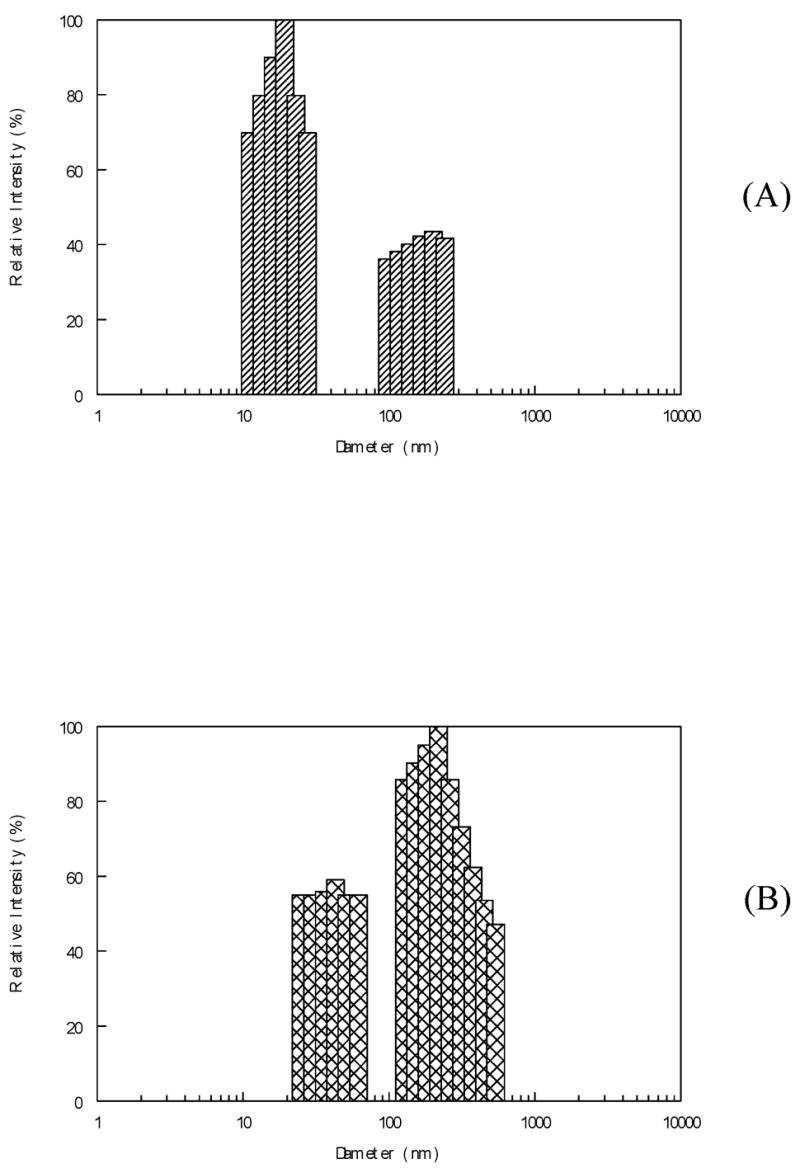
Figure 2.

Panel A shows the bi-modal DLS distribution for the total soluble protein isolated from a fetal calf lens. In panel B is shown the DLS profile for α-crystallin isolated from the same soluble lens protein preparation. Note that there is only a single scattering peak which coincides with the first peak in A. The DLS profile for the purified β,γ-crystallins (C) is also a single peak at the leading edge of the first peak in A. This is to be expected since these proteins are much smaller than α-crystallin. For comparison, the DLS profile for the clear lens of a 28 year old human subject (nuclear opacity N=0) is shown in D. The two peaks in the profile indicate higher particle sizes as is the case for all species tested. This is in part due to the increased age of the tissue, but also reflects interactions among the protein species in the very protein dense milieu of the intact lens and the fact that the second peak also includes scattering from cellular constituents such as membranes and organelles that are removed in the soluble protein preparations.
In a further experiment, we modelled the effects of aging on the crystallins by exposing solutions of total lens soluble proteins to elevated temperatures (50–60 °C) inducing denaturation of β, γ-crystallins. We found that this stress caused the first peak to diminish, with a concomitant increase in the second scattering peak, indicating an increase in protein aggregates. This is demonstrated in Figure 3 by comparison of a representative sample before heating and after 60 minutes at 55°C. Note the shift of both scattering peaks to higher molecular size and the decrease in area for the first and increase in the second.
Figure 3.
The effect of heating on the DLS profile of fetal calf soluble protein. In A is shown the initial profile obtained before the sample was heated to 55°C. In B is shown the profile of the same sample after 60 minutes at 55°C. Note the shift of scattering intensity from the first to the second peak and the increased particle size seen in both peaks after heating.
Clinical Study
We examined 235 subjects (380 eyes), ranging in age from 7–86 years. AREDS clinical lens nuclear grades ranged from 0 (clear) – 3.8 (opaque). Table 1 shows the distribution of age, lens nuclear grade, and ACI values for study participants. ACI values ranged from 0 to 41.4%. Figure 4 shows a statistically significant decrease in ACI with increasing lens nuclear opacity grade. For each 1-unit increase in nuclear grade, ACI decreased by 8.9 units, p<0.0001. Results were very similar for photographic grades (data not shown).
Table 1. Patient demographics: shows the distribution of age, lens nuclear grade, and DLS ACI values for study participants.
Table 1. Characteristics of participants.
| Characteristic | N (%) | Mean (SD) [range] |
|---|---|---|
| Age (years) | 52.7 (19.6) [7.5–86] | |
| 7–25 | 38 (16.2%) | |
| 26–35 | 11 (4.7%) | |
| 36–45 | 22 (9.4%) | |
| 46–55 | 36 (15.3%) | |
| 56–65 | 55 (23.4%) | |
| 66–75 | 50 (21.3%) | |
| 76+ | 23 (9.8%) | |
| Total (# persons) | 235 (100%) | |
| Gender | ||
| Female | 140 (59.6%) | |
| Male | 95 (40.4%) | |
| Total (# persons) | 235 (100%) | |
| Clinical nuclear lens opacity grade | 0.68 (0.73) [0.0–3.8] | |
| 0.0 | 66 (17.4%) | |
| 0.1–0.2 | 80 (21.0%) | |
| 0.3–0.5 | 86 (22.6%) | |
| 0.6–0.8 | 20 (5.3%) | |
| 0.9–1.4 | 63 (16.6%) | |
| 1.5–1.9 | 26 (6.8%) | |
| 2.0+ | 39 (10.3%) | |
| Total (#eyes) | 380 (100%) | |
| Photo nuclear lens opacity grade | 2.62 (1.36) [0.9–6.0] | |
| 0.9 – 1.9 | 97 (40.6%) | |
| 2.0 – 2.9 | 56 (23.4%) | |
| 3.0 – 3.9 | 36 (15.1%) | |
| 4.0 – 4.9 | 37 (15.5%) | |
| 5.0 + | 13 (5.4%) | |
| Total (# eyes with photos) | 239 (100%) | |
| Alpha-Crystallin Index (%) | 15.77 (10.55) [0–41.41] | |
| 0.0 | 46 (12.1%) | |
| >0.0 – <10.0 | 74 (19.5%) | |
| >=10 – <20 | 136 (35.8%) | |
| >=20 – <30 | 80 (21.0%) | |
| >=30 | 44 (11.6%) | |
| Total (# eyes w/information) | 380 (100%) |
Figure 4.
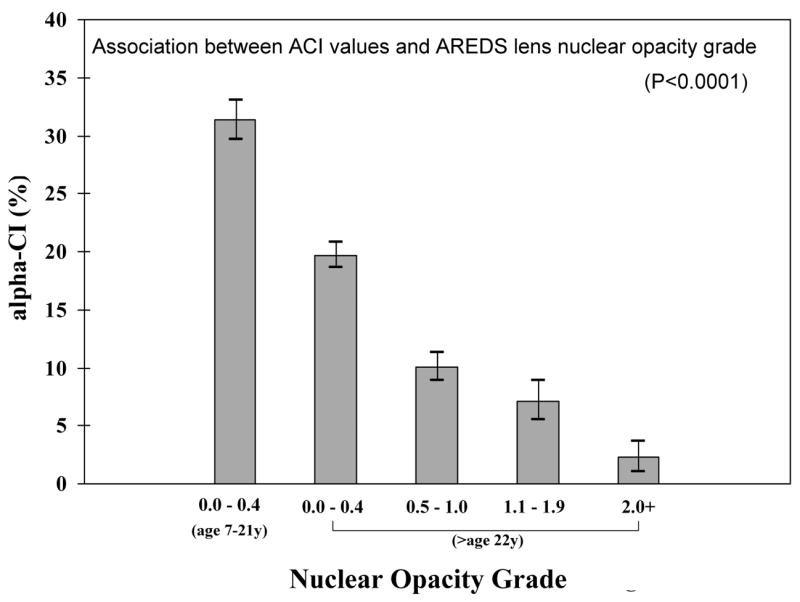
Association between ACI values and AREDS lens nuclear opacity grade, showing highly significant (P<0.0001) inverse relationship. The first bar on the left represent ACI values for young normal individuals (7–21 yrs, N=48 eyes). The rest of the bars are from those 22 yrs or older (N=332 eyes). For each category of lens opacity, the height of the bar represents the mean ACI and the vertical line with serifs indicates the 95% confidence interval for the mean.
In a multiple regression model, ACI values significantly decreased with increasing nuclear grade even after adjusting for age. For each 1-unit increase in nuclear opacity grade, ACI decreased by 4.0 units, p<0.0001. The mean value of ACI in clear lenses from normal subjects younger than age 22 was 31%, whereas the mean value of ACI in eyes with nuclear grades of 2 or greater (significant nuclear opacity) was 2%. Two-thirds (67%) of persons who were 70 years of age or older with clinical nuclear grade of 2 or more had an ACI of 0%. This finding also suggests that ACI measurements may be most useful and meaningful when used for lenses with nuclear grades of less than 2.
Among subjects with clinically clear nuclear regions (nuclear grade 0–1), there was a statistically significant decrease in the ACI associated with increase in age (p<0.0001) (Figure 5). The average amount of unbound α-crystallin (ACI) is highest (31%) among those aged less than 22 years and declines at each successive decade approaching zero for persons over age 75. Thus, even in clinically clear lenses, there are demonstrable losses of α-crystallin associated with aging.
Figure 5.
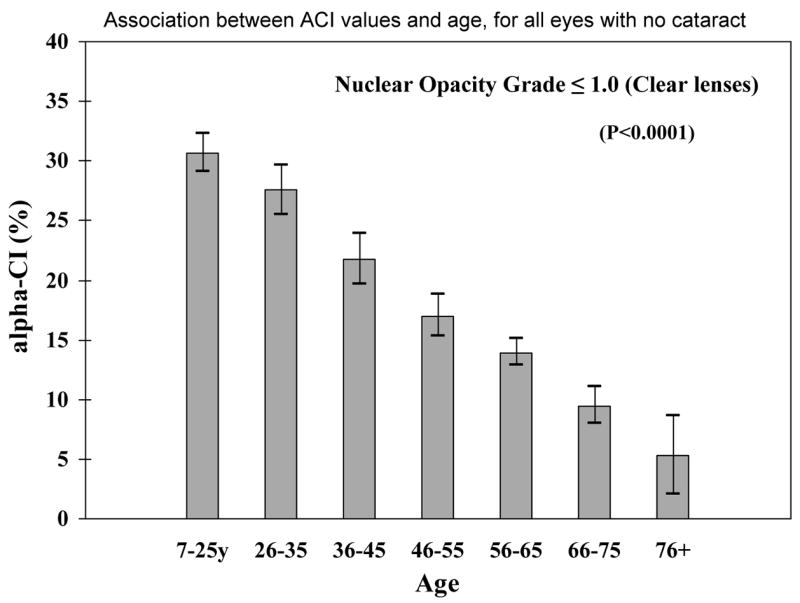
Association between ACI values and age, for all eyes with no cataract (lens nuclear grade 1.0), showing that the DLS detect early protein changes even while the lenses remain clear by clinical evaluation (p<0.0001). The labels on the x axis indicate the midpoint of each age category. For each age group, the height of the bar represents the mean ACI value and the vertical line with serifs indicates the 95% confidence interval for the mean.
Figure 6 shows the distribution of DLS measurements in a patient with Grade 3 cataract. In this lens there is a complete disappearance of the first peak representing α-crystallins (ACI = 0), and a single large particle size peak, representing high-molecular-weight particles (mainly large lens protein aggregates, cellular organelles, and membrane components 17–18, 20–21, 28).
Figure 6.
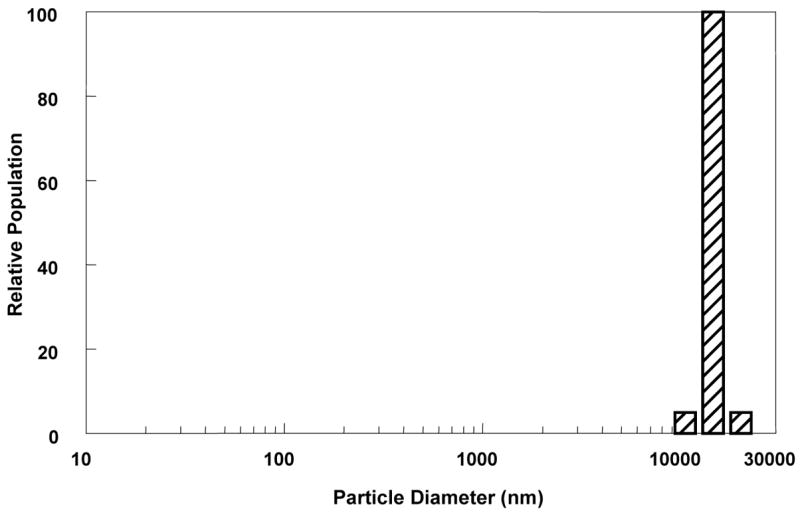
The profile from a DLS measurement of an eye with advanced cataract (Lens nuclear opacity grade N=3) shows a unimodal distribution of lens protein sizes, with an absent first peak (corresponding to an undetectable unbound α-crystallin fraction).
Figure 7 shows that within the group of older persons aged 60 to 70 years, a significant decrease in ACI is associated with increasing nuclear lens grade. For each 1-unit increase in nuclear opacity grade, ACI decreased by 4.8 units (p<0.0001). This decrease in ACI remained statistically significant even after adjusting for age within the 60–70-year interval: for each 1-unit increase in nuclear opacity grade, ACI decreased by 3.9 units (p<0.0001). Thus, the ACI may be useful for identifying persons at higher risk for cataract development in older-age populations who still have clear lenses.
Figure 7.
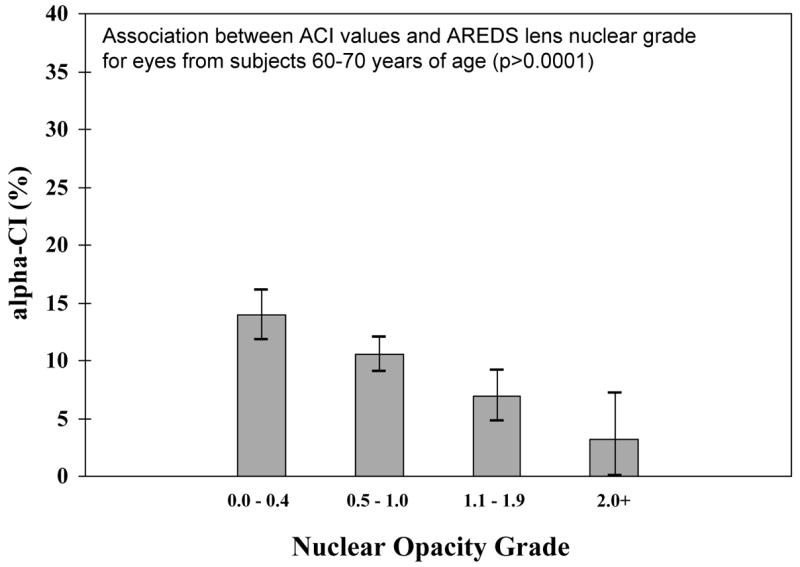
Association between ACI values and AREDS lens nuclear grade for eyes from subjects 60–70 years of age, showing that the DLS can detect lens opacity grade differences independently of age within age group of elderly subjects (P<0.0001). For each category of lens opacity, the height of the bar represents the mean ACI value and the vertical line with serifs indicates the 95% confidence interval for the mean.
For measurement of DLS system error, since there were 2 or 3 independent ACI measurements per eye, a variance components model51 was fit to the data in order to estimate the within-eye variance, the square root of which is the standard error of measurement. This estimate was 3%, regardless of the size of the mean ACI.
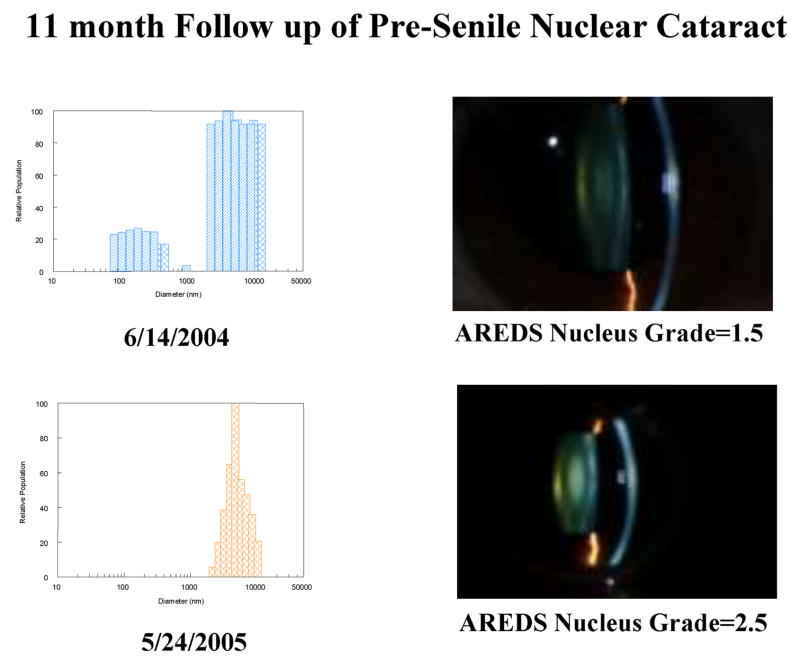
Figure 8 shows baseline and 11 month follow up ACI measurements for a 42 year old patient who had rapid development of pre-senile nuclear cataract, providing an example of the potential of this system for use in longitudinal clinical studies of nuclear cataracts.
Figure 8.
Example of follow up data from a 42 year old patient with early pre senile nuclear cataract, showing the loss of the unbound α-crystallin protein fraction (first peak) when a cataract developed 11 months later. Note that the DLS profile on 6/14/2004 represents 2 measurements superimposed on each other to show the repeatability of the system as discussed in the text.
Discussion
Our clinical data show that the Clinical DLS device can detect early pre-cataractous protein changes in the living lens nucleus even as the lens remains clinically clear by slit lamp examination (Grade 0 to 1). Support for our claim that the DLS detects the alpha crystallins is provided by our biochemical studies, which demonstrate that the low molecular weight component (first peak in the DLS measurement profile, i.e., the ACI) (Figure 2) represents unbound α-crystallin19, 21, 26–29. Based on these clinical and laboratory results, we propose the ACI as a clinical measure of unbound crystallin that indirectly reflects the molecular chaperone reserve of the lens (much as creatinine clearance indirectly reflects the renal functional reserve of the kidney) and can be used as a predictor of future cataract development or progression. We believe that a simple DLS clinical assessment can be used in patients to reliably estimate the amount of α-crystallins available as molecular chaperones in the lens nucleus (α-crystallin reserve), and will be useful both as a screening tool as well as an outcome variable.
In this study, we found a strong association between clinical DLS measurements, represented by the ACI, with lens nuclear opacity grading and with aging. The association between ACI and nuclear lens grade remains even after adjusting for age.
However, our data provide evidence that the ACI changes may precede the appearance of clinically observable cataract, since the ACI was zero for clinical cataract stages over Grade 2. Thus, DLS should be most useful in studies of pre cataractous lens changes when the lens is still clear by slit lamp exam (<Grade 2). It should be useful in detecting individuals at high risk for developing cataracts while the lens still remains clear. Beyond a nuclear cataract grade of 2, conventional optical imaging techniques, such as slit lamp clinical and photographic grading, and Scheimpflug and retro-illumination photography remain the best way to assess progression.22
Previous studies have suggested that once lens opacities appear, it may be too late to prevent or stop cataract progession.1–12 This may indicate that persons with clinically clear lenses who are at high risk for cataract will be ideal candidates for future clinical cataract studies. To test anti cataract drugs, the ACI should be useful both in patient selection and as a “proof of principle” outcome variable. The finding that DLS measurements have good repeatability confirms a previous report31, and indicates that the ACI could be used as a reliable, sensitive outcome variable for future longitudinal clinical studies of the lens.
Loss of α-crystallins have recently been found associated with presbyopia.52 ACI may therefore also be useful in studying presbyopia and other bothersome visual symptoms in middle-aged patients undetectable by slit lamp and other optical exams, at the molecular level.
DLS measurements may be also helpful in a number of clinical situations. For example, in glaucoma, cornea transplant or retinal surgery patients with zero ACI in their lenses, a combined cataract procedure may be preferred. When deciding between LASIK versus lens exchange surgery, if the patient’s ACI is low or close to zero, lens exchange may be preferred since cataract surgery may be needed in the near future anyway. For our NASA collaborators, ACI measurements may help assess the effects of cosmic radiation in outer space, aid in developing methods to prevent lens damage from radiation, and help select those at lowest risk for long missions.
In summary, this study demonstrates the usefulness of DLS measurements in assessing early pre-cataractous lens changes. The ACI may be useful in future studies both as a screening tool to detect those at high risk for developing cataract, and as a reproducible, sensitive outcome variable in clinical trials or epidemiologic studies. We present data supporting the applicability of the α-crystallin Index (ACI) as a measure of α-crystallin chaperone reserve in the lens nucleus, reflecting the risk of an individual in developing cataract.
Acknowledgments
We thank Dr. Emily Chew for allowing us to enroll some of her patients into this study; Mss. Rita Hiller, Linda Goodman and Cheryl Perry for serving as clinic coordinators for the study; Mr. James King, for technical help; and Dr. Joram Piatigorsky, for helpful discussions. Dr. Manuel Datiles III had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Supported by The NASA-NEI Inter Agency Agreement.
Footnotes
This paper has been partially presented at the 2007 ARVO Annual meeting in Ft. Lauderdale, Fl.
Financial Interest Disclosure: Manuel B. Datiles III, M.D. -none; Rafat R. Ansari, Ph. D. and Kwang I. Suh, Ph. D. Patent No. 5973779; Susan Vitale, Ph. D. - none; George Reed, Ph. D. - none; J. Samuel Zigler, Ph. D. none; Frederick Ferris III, M.D. none
References
- 1.Kupfer C. Bowman Lecture: The conquest of cataract: a global challenge. Transactions of the Ophthalmological Society, U.K. 1985;104:1–10. [PubMed] [Google Scholar]
- 2.Congdon NG. Prevention strategies for age related cataract: present limitations and future possibilities. Br J Ophthalmol. 2001;85:516–520. doi: 10.1136/bjo.85.5.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark JI, Livesey JC, Steele JE. Delay or inhibition of rat lens opacification using pantethine and WR-77913. Exp Eye Res. 1996;62:75–84. doi: 10.1006/exer.1996.0009. [DOI] [PubMed] [Google Scholar]
- 4.Hiraoka T, Clark JI, Li XY, Thurston GM. Effect of selected anti-cataract agents on opacification in the selenite cataract model. Exp Eye Res. 1996;62:11–9. doi: 10.1006/exer.1996.0002. [DOI] [PubMed] [Google Scholar]
- 5.Hiraoka T, Clark JI. Inhibition of lens opacification during the early stages of cataract formation. Invest Ophthalmol Vis Sci. 1995;36:2550–5. [PubMed] [Google Scholar]
- 6.Zigler JS, Qin C, Kamiya T, Krishna MC, Cheng Q, Tumminia S, Russell P. Tempol-H inhibits opacification of lenses in organ culture. Free Radical Biology and Medicine. 2003;35:1194–1202. doi: 10.1016/s0891-5849(03)00505-7. [DOI] [PubMed] [Google Scholar]
- 7.Yan H, Harding J. Carnosine inhibits modifications and decreased chaperone activity of lens alpha crystallin induced by ribose and fructose 6 phosphate. Mol Vis. 2006;12:205–214. [PubMed] [Google Scholar]
- 8.Klein BEK, Klein R, Lee KE, Grady LM. Statin use and incident nuclear cataract. Journal of the American Medical Association. 2006;295(23):2752–2758. doi: 10.1001/jama.295.23.2752. [DOI] [PubMed] [Google Scholar]
- 9.Kador PF, Betts D, Wyman M, Blessing K, Randazzo J. Effects of topical administration of an aldose reductase inhibitor on cataract formation in dogs fed a diet high in galactose. American Journal of Veterinary Research. 2006;67:1783–1787. doi: 10.2460/ajvr.67.10.1783. [DOI] [PubMed] [Google Scholar]
- 10.Datiles MB, Fukui H. Cataract Prevention in Diabetic Octodon degus with Sorbinil. Curr Eye Res. 1989;8:233–237. doi: 10.3109/02713688908997564. [DOI] [PubMed] [Google Scholar]
- 11.Benedek G, Pande J, Thurston G, Clark JI. Theoretical and experimental basis for the inhibition of cataract. Prog In Retinal and Eye Res. 1999;18:391–402. doi: 10.1016/s1350-9462(98)00023-8. [DOI] [PubMed] [Google Scholar]
- 12.Chylack L, Toma-Bstaendig S. Prospects for a medical treatment of age related cataract. Ch. 2.7.9. In: Easty DL, Sparrow JM, editors. Oxford Textbook of Ophthalmology. Oxford, UK: Oxford University Press; 1999. pp. 487–499. [Google Scholar]
- 13.Herman DC, Gordon MO, Beiser JA, Chylack LT, Jr, Lamping KA, Schein OD, Soltau JB, Kass MA. Topical Ocular Hypotensive Medication and Lens Opacification: Evidence From the Ocular Hypertension Treatment Study. American Journal of Ophthalmology. 2006;142(5):800.e1–800.e12. doi: 10.1016/j.ajo.2006.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benedek GB. Theory of transparency of the eye. Applied Optics. 1971;10:459–473. doi: 10.1364/AO.10.000459. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka T, Benedek GB. Observation of protein diffusivity in intact human and bovine lenses with application to cataract. Invest Ophthalmol Vis Sci. 1975;14(6):449–456. [PubMed] [Google Scholar]
- 16.Berne BJ, Pecora R. “Dynamic Light Scattering” with applications to chemistry, biology, and physics. Hoboken, N.J: Wiley Interscience Publications, John Wiley & Sons, Inc; 1976. [Google Scholar]
- 17.Benedek GB, Chylack L, Libondi T, Magnante P, Pennett M. Quantitative detection of the molecular changes associated with early cataractogenesis in living human lenses using Dynamic light scattering. Curr Eye Res. 1987;6:1421–1432. doi: 10.3109/02713688709044506. [DOI] [PubMed] [Google Scholar]
- 18.Magnante P, Chylack LT, Benedek GB, Libondi T. In vivo measurements in human lens using quasielastic light scattering. Proc SPIE-The Internat Soc Optical Eng. 1987;712:172–174. [Google Scholar]
- 19.Datiles M, Podgor M, Edwards P. Reproducibility of the early cataract detector (Kowa ECD) Ophthalmic Surgery. 1988;19(9):664–666. [PubMed] [Google Scholar]
- 20.Thurston GM, Hayden DL, Burrows P, et al. Quasielastic light scattering study of the living lens as a function of age. Curr Eye Res. 1997;16:197–207. doi: 10.1076/ceyr.16.3.197.15410. [DOI] [PubMed] [Google Scholar]
- 21.Dhadwal HS, Whitpenn J. In vivo dynamic light scattering characterization of a human lens: Cataract Index. Curr Eye Res. 2000;20:502–510. [PubMed] [Google Scholar]
- 22.Bettelheim FA. Light scattering in lens research: an essay on promises and accomplishments. Exp Eye Res. 2004;79:747–752. doi: 10.1016/j.exer.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Ansari RR. Ocular static and dynamic light scattering a non invasive diagnostic tool of eye research and clinical practice. J Biomed Optics. 2004;9:22–27. doi: 10.1117/1.1626663. [DOI] [PubMed] [Google Scholar]
- 24.Ansari RR, Datiles MB. Use of quasielastic light scattering and Scheimpflug imaging for the early detection of cataracts. Diabetes Technology & Therapeutics. 1999;1:159–168. doi: 10.1089/152091599317378. [DOI] [PubMed] [Google Scholar]
- 25.Ansari RR, Giblin F, King JF, Lambert LM. Non-invasive and remote detection of cataracts during space exploration with dynamic light scattering. Proceedings of SPIE - The International Society for Optical Engineering. 2001;4245:129–134. [Google Scholar]
- 26.Chenault VM, Ediger MN, Ansari RR. In vivo assessment of diabetic lenses using dynamic light scattering. Diabetes Technology and Therapeutics. 2002;4(5):651–659. doi: 10.1089/152091502320798295. [DOI] [PubMed] [Google Scholar]
- 27.Ansari RR, King JF, Seeberger T, Clark JI. Early detection of cataract and response to pantethine therapy in non invasive static and dynamic light scattering. Proc SPIE, The International Society for Optical Engineering. 2003;4951:168–176. [Google Scholar]
- 28.Simpanya MF, Ansari RR, Suh KI, Leverenz VR, Giblin FJ. Aggregation of lens proteins on an in vivo hyperbaric oxygen guinea pig model of nuclear cataract: Dynamic Light Scattering and HPLC Analysis. Invest Ophthalmol Vis Sci. 2005;46:4641–4651. doi: 10.1167/iovs.05-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ansari RR, Suh KI, Dunker S, Kitaya N, Sebag J. Quantitative molecular characterization of bovine vitreous and lens with non-invasive dynamic light scattering 2001. Exp Eye Res. 73(6):859–866. doi: 10.1006/exer.2001.1097. [DOI] [PubMed] [Google Scholar]
- 30.Ansari RR, Datiles MB, King JF. A new clinical instrument for the early detection of cataracts using dynamic light scattering and corneal topography. Proceedings of SPIE - J Biomed Optics. 2000;3908:30–49. [Google Scholar]
- 31.Datiles MB, Ansari RR, Reed GF. A clinical study of the human lens with a Dynamic Light Scattering device. Exp Eye Res. 2002;74:93–102. doi: 10.1006/exer.2001.1106. [DOI] [PubMed] [Google Scholar]
- 32.Datiles MB, Ansari RR. Clinical evaluation of cataracts. In: Tasman W, Jaeger E, editors. Duane’s Clinical Ophthalmology. Vol. 1. Ch 72A. Philadelphia: Lipincott Co., Inc; 2004. pp. 1–20. [Google Scholar]
- 33.Horwitz J. Alpha Crystallin can function as a molecular chaperone. Proc Nat Acad Sci USA. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horwitz J. Alpha crystallin. Exp Eye Res. 2003;76:145–153. doi: 10.1016/s0014-4835(02)00278-6. [DOI] [PubMed] [Google Scholar]
- 35.Wang K, Spector A. The chaperone activity of bovine alpha crystalline. Interaction with other lens crystallins in native and denatured states. J Biol Chem. 1994;269:13601–13607. [PubMed] [Google Scholar]
- 36.Boyle D, Takemoto L. Characteristics of the alpha-gamma and alpha beta complex: evidence for an in vivo functional role of alpha crystalline as a molecular chaperone. Exp Eye Res. 1994;58:9–16. doi: 10.1006/exer.1994.1190. [DOI] [PubMed] [Google Scholar]
- 37.Rao PV, Huang QL, Horwitz J, Zigler JS. Evidence that alpha crystallin prevents non specific protein aggregation in the intact eye lens. Biochem Biophys Acta. 1995;1245:439–447. doi: 10.1016/0304-4165(95)00125-5. [DOI] [PubMed] [Google Scholar]
- 38.Carver JA, Nicholls KA, Aquilina JA, Truscott RJW. Age related changes in bovine alpha crystallin and high molecular weight protein. Exp Eye Res. 1996;63:639–647. doi: 10.1006/exer.1996.0158. [DOI] [PubMed] [Google Scholar]
- 39.Hanson SRA, Hasa A, Smith DL, Smith JB. The major in vivo modifications of human water insoluble lens crystallins are disulfide bonds, deamidation, methionine oxidation, and backbone cleavage. Exp Eye Res. 2000;71:195–207. doi: 10.1006/exer.2000.0868. [DOI] [PubMed] [Google Scholar]
- 40.Goishi K, Shimizu A, Najarro G, et al. Alpha crystallin expression prevents gamma crystallin insolubility and cataract formation in the zebrafish cloche mutant lens. Development and Disease. 2006;133:2585–2593. doi: 10.1242/dev.02424. [DOI] [PubMed] [Google Scholar]
- 41.Blakytny R. Harding Bovine and human alpha crystallins as molecular chaperone: prevention of the inactivation of glutathione reductase by fructation. Exp Eye Res. 1997;64:1051–1058. doi: 10.1006/exer.1997.0299. [DOI] [PubMed] [Google Scholar]
- 42.Valasco PT, Lukas TJ, Murthy SNP, et al. Hierarchy of lens proteins requiring protection against heat induced precipitation by the alpha crystallin chaperone. Exp Eye Res. 1997;65:497–505. doi: 10.1006/exer.1997.0358. [DOI] [PubMed] [Google Scholar]
- 43.Muchowski PJ, Valdez MM, Clark JI. Alpha B crystallin selectively targets intermediate filament proteins during thermal stress. Invest Ophthalmol Vis Sci. 1999;40:951–958. [PubMed] [Google Scholar]
- 44.Rachdan D, Lou M, Harding JJ. Glutathione Reductase from human cataract lenses can be revived by reducing agents and by a molecular chaperone, α-crystallin. Curr Eye Res. 2005;30:919–925. doi: 10.1080/02713680590953110. [DOI] [PubMed] [Google Scholar]
- 45.Benedek G. Proctor Lecture: Cataract as a protein condensation disease. Invest Ophthalmol Vis Sci. 1997;38:1911–1921. [PubMed] [Google Scholar]
- 46.Roy D, Spector A. Absence of low molecular weight alpha crystallin in the nuclear region of old human lenses. Proc Nat Acad Sci USA. 1976;76:3484–3487. doi: 10.1073/pnas.73.10.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McFall-Ngai MJ, Ding LL, Takemoto LJ, Horwitz J. Spatial and temporal mapping of the age related changes in human lens crystallins. Exp Eye Res. 1985;41:745–758. doi: 10.1016/0014-4835(85)90183-6. [DOI] [PubMed] [Google Scholar]
- 48.Harding J, Crabbe MJC. The lens: development, protein, metabolism and cataract. In: Davson H, editor. The Eye. 3. 1B. Orlando, FL: Academic Press; 1984. pp. 207–492. [Google Scholar]
- 49.The Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study (AREDS) system for classifying cataracts from photographs: AREDS Report No. 4. Am J Ophthalmol. 2001;131:167–175. doi: 10.1016/s0002-9394(00)00732-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stock RS, Ray WHJ. Interpretation of photon correlation spectroscopy data: a comparison of analysis methods. J Polym Sci. 1985;23:1393–1447. [Google Scholar]
- 51.Fleiss JL. The design and analysis of clinical experiments. Hoboken, N.J: Wiley Interscience Publications, John Wiley & Sons, Inc; 1986. [Google Scholar]
- 52.Heys KR, Friedrich MG, Truscott RJ. Presbyopia and heat: changes associated with aging of the human lens suggest a functional role for the small heat shock protein, a-crystallin, in maintaining lens flexibility. Aging Cell. 2007;6:807–815. doi: 10.1111/j.1474-9726.2007.00342.x. [DOI] [PubMed] [Google Scholar]